Professional Documents
Culture Documents
Hematopoietic System
Uploaded by
applesncoreOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hematopoietic System
Uploaded by
applesncoreCopyright:
Available Formats
Undercover Professor
Dr. Dexter MD, FRC Path
Hematopathology
Department of Pathology
SGUSOM, Grenada (W.I.)
2
Hematopoiesis
Starts in yolk sac
After 3
rd
week mainly in liver, spleen
At term bone marrow is established site of
hematopoiesis
3
How to examine bone marrow ?
Aspiration Biopsy
Posterior superior iliac spine
Sternum
Anterior tibia (infants)
Posterior superior iliac spine
Anterior superior iliac spine
Cell morphology better Architecture details are better
4
Bone marrow
Cords of cells
Sinusoids
Hematopoietic
Cells
Myeloid
Erythroid
Lymphoid
Plasma cells
Stromal cells
Adipocytes
Macrophages
Endothelial cells
fibroblasts
5
How to examine bone marrow?
Cellularity
Myeloid : Erythroid ratio
Number of megakaryocytes
Number of blasts and other cells
Iron stores (Prussian blue stain)
Any other abnormalities
6
Normal Bone Marrow Indices
Normal cellularity varies with age
At birth 100%
At 50 years (approx) 50%
Normal percentages of marrow precursors:
granulocytic = 65%
erythroid = 25%
monocytic = 5 - 10%
lymphocytic = 5 - 10%
Normal M:E ratio 2-3:1
7
Normal Peripheral Blood Smear
Stained with Wright Giemsa stain
Red blood cells
White blood cells
Platelets
8
Normal erythropoiesis
Erythroid precursors mature through
various stages of nucleated cells
(normoblasts), lose their nucleus, then
are released into the circulation as
reticulocytes (5 - 7 days)
Reticulocytes give rise to mature RBCs
(1-2 days)
Depends upon erythropoietin (EPO)
Average life span of RBCs - 120 days
9
Stages of erythropoiesis
Proeryhthroblast
Basophilic normoblast
Polychromatic
normoblast (Hb
appears)
Orthochromatic
normoblast
Reticulocyte
Erythrocyte
Reference - Clinical Hematology and Fundamentals of Hemostasis, 3rd ed, 1997
10
Morphologic Evaluation of Red
Blood Cells
Normocytic RBC -
Normal diameter is
about 7.2 m
Size is comparable to
nucleus of a mature
lymphocyte
Central pallor
around one third
(Normochromic)
Lymphocyte RBC
11
Abnormalities of RBC Morphology
Anisocytosis:
Variation in size of
RBCs
Poikilocytosis:
Variation in shape of
RBCs
Anisopoikilocytosis
12
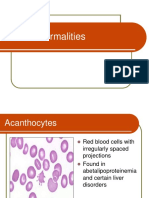
Abnormalities of RBC Morphology
Microcytes
Macrocytes
Target cells
Spherocytes
Schistocyte
(fragmented
RBCs)
Normocytic RBC Microcytic RBC
Macrocytic RBC
Target Cell
Spherocyte
Schistocytes
13
Microcytic Hypochromic cells
Microcytes are small red
blood cells (MCV < 80 fL)
with decreased amounts
of hemoglobin.
Morphology
Increased central pallor
Smaller than nucleus of a
mature lymphocyte
Important causes include:
Iron deficiency
anemia
Thalassemia
Anemia of chronic
disorder
Sideroblastic anemia
14
Macrocytes
large red blood cells
(> 8.5 mm) with an
elevated MCV (> 100)
Important causes
include:
Vitamin B12 or folic acid
deficiency
Liver diseases
Alcoholism
Myeloproliferative
disorders
Myelodysplastic
disorders
Macro-ovalocyte
Macrocytes
15
Target cells
Thin, hypochromatic
cells with a round area
of central area of
hemoglobinization
Increased surface area
to cell volume ratio
Causes:
Hemoglobinopathies
(thalassemia)
Iron deficiency anemia
Liver disease
Familial LCAT deficiency
Splenectomy
16
Spherocytes
Spherocytes are small
(< 6.5 mm), dense
spheroidal RBCs with
normal or decreased
MCV and absent central
pallor.
Increased MCHC
Seen in:
Hereditary
spherocytosis
Autoimmune
hemolytic anemia
Burns
17
Schistocyte (fragmented RBCs)
Damaged red blood
cells.
Seen in
Microangiopathic
hemolytic anemia
(MAHA)
DIC
Thrombotic
thrombocytopenic
purpura
Hemolytic uremic
syndrome
Prosthetic
heart valves
18
Nucleated red blood cells (NRBCs,
normoblasts)
immature red blood
cells.
indicate markedly
accelerated
erythropoiesis and/or
severe bone marrow
stress.
Seen in
acute bleeding, severe
hemolysis, myelofibro-
sis, leukemia
19
Reticulocyte
Immature RBC,
slightly larger than
mature RBC and
contains remnants
of ribosomal RNA
Stained with
supravital stains
(new methylene
blue, brilliant cresyl
blue)
Exhibit polychro-
masia with
routinely stains
Polychromasia
on Giemsa stain
supravital
stain
20
RBC inclusions
Howell Jolly bodies
Heinz bodies
Pappenhiemer
bodies
Basophilic stippling
21
Howell jolly bodies:
Purple-blue nuclear
remnants
Post-splenectomy
Hemolytic anemia
Megaloblastic anemia
Heinz bodies:
Composed of denatured
hemoglobin
Precipitate on RBC
membrane
Well stained with
supravital stains
G-6-PD deficiency Heinz bodies
Stain : brilliant cresyl fast violet
22
Basophilic stippling:
Due to persistence
of ribosomes
Lead poisoning,
Thalassemia
Megaloblastic
anemia
Pappenheimer
bodies
Aggregate in small
cluster near
periphery
Stain with Prussian
blue
23
Disorder of red cells
Anemia- decreased red cells circulating
mass leading to decreased oxygen
carrying capacity of blood
Polycythemia- increased red cell
circulating mass
24
RBC Parameters
Mean Cell Volume (MCV): average
volume of a RBC (femtoliters)
Mean Cell Hemoglobin (MCH): average
content of Hb per RBC (picograms)
PCV
x 10
RBC
Hb
x 10
RBC
25
Mean Cell Hemoglobin Concentration
(MCHC): average concentration of Hb in
a given volume of packed RBCs (g/dL)
Hb
x 100
PCV
26
Red Cell Distribution
Width (RDW):
Indicates variation in
size and shape;
coefficient of variation
of RBC volume
27
RBC Parameters
32%36% Mean corpuscular hemoglobin concentration
2734 pg Mean corpuscular hemoglobin
82100 m
3
Mean corpuscular volume
Indices
0.5%2.5% Reticulocytes
Male, 4.56 10
6
/L
Female, 45.5
10
6
/L
Red blood cell (RBC) count
Male, 40%54%
Female, 35%47%
Hematocrit
Male, 1418 g/dL
Female, 1216 g/dL
Hemoglobin
28
Clinical features of anemia
Easy fatigability, tiredness
Pallor
Decreased appetite
Dyspnea on exertion
Lightheadedness
Increased cardiac output (High output
failure)
29
Diagnosis of Anemia
Complete blood count
Hemoglobin level
RBC count
RBC indices (MCV,MCH,MCHC)
Reticulocyte count
Peripheral blood film
Bone marrow examination
Iron studies
Special tests
30
Iron studies
Serum iron levels
Serum ferritin levels
Total Iron Binding capacity (TIBC)
Indicates iron bound to the transferrin sites
Percentage saturation
Bone marrow iron staining with Prussian
blue
Serum iron X 100
TIBC
31
Morphological Classification of anemias -
Based on MCV
Normocytic
(80-100)
Aplastic anemia
Anemia of
chronic disorder
Anemia of renal
disease
Acute blood loss
Microcytic
(< 80)
Iron deficiency anem
Thalassemia
Anemia of chronic diso
Sideroblastic anemia
Macrocytic
(>100)
Megaloblastic
B12 and folate deficiency
Non-megaloblastic
Alcoholism
Liver disease
Hypothyroidism
Myelodysplastic syndrome
32
Classification based on
underlying mechanism
Blood loss
Impaired red cell
Production
Stem cell defects
Defects of hemoglobin
synthesis
Defects of DNA
synthesis
Unknown or multiple
mechanisms
Increased
destruction
(Hemolytic
anemias)
Intrinsic (intracorpuscular)
Extrinsic (extracorpuscular)
Acute Chronic
33
Anemias secondary to blood
loss
34
Anemia of acute blood loss
Immediate hemodilution
Earliest change is leucocytosis
Initially normocytic normochromic
blood picture
Increased erythropoietin levels
Reticulocytosis after 5-7 days
Reticulocytes are seen as
polychromatic macrocytes
Often accompanied by thrombocytosis
35
Anemia of chronic blood loss
Gastrointestinal tract lesions
Ulcers, Neoplasms
Gynecological disturbances
Menorrhagia - leiomyomas
Leads to iron deficiency anemia
(discussed later)
Hemolytic anemias
37
Hemolytic anemia
Characterized by
shortened red cell survival (< 120 days)
Increased compensatory erythropoiesis
Accumulation of products of hemoglobin
breakdown
38
Causes of hemolytic anemia
Intracorpuscular Extracorpuscular
1. Membrane defects e.g.
Herediatry spherocytosis
2.Enzyme deficiencies
3.Hemoglobinopathies
4.Paroxysmal nocturnal
hemoglobinouria
1. Immune mediated
Mismatched
transfusions
3. Infections e.g. malaria
2. Mechanical
DIC, MAHA*, Prosthetic heart
valves, stenosis
*MAHA- microangiopathic hemolytic anemia
Autoimmune
hemolytic
anemia
39
General features of hemolytic
anemias
Increased reticulocyte count
Nucleated RBCs (NRBCs) in peripheral
blood aka normoblastemia
PBF is usually normocytic
normochromic especially after
acute hemolysis
Increased bone marrow iron (ferritin)
Unconjugated hyperbilirubinemia
40
Decreased haptoglobulin levels
Hemoglobinemia
(free Hb in blood)
Hemoglobinuria
LDH
Hemosiderinuria
Pigment gall stones
Systemic hemosiderosis
Intravascular
hemolysis
41
Site of hemolysis
RBC destruction
secondary
to external causes
e.g. toxins, trauma,
complement
Defect in RBCs
Defective RBCs removed
by spleen
Splenomegaly
Intravascular Extravascular
42
Hereditary Spherocytosis
Inherited disorder caused by intrinsic
defect in red blood cell membrane
(defect in ankyrin, spectrin, band 4.1)
Autosomal dominant disorder
Highest incidence in Northern Europe
43
Mutations in genes coding ankyrin & spectrin
Decreased membrane stability
Loss of membrane fragments
Spherical RBCs
Sequestered in spleen
Destroyed by macrophages
Elevated unconjugated
bilirubin
Splenomegaly
44
Clinically
Commonly presents as chronic hemolytic
anemia of mild to moderate degree
Jaundice
Splenomegaly
Pigment gall stones
Treatment by spleenectomy
Aplastic crisis:
Transient cessation of erythropoiesis
caused by parvovirus B19
Rapid worsening of anemia
Decreased reticulocyte count
45
Diagnosis
Hb low
Increased MCHC
PBF reveals spherocytes,
microspherocytes
Increased reticulocyte count (Not in
aplastic crisis!)
Osmotic fragility test
increased osmotic fragility of the RBCs
46
Sickle cell anemia
Structurally abnormal hemoglobin
Point mutation in globin chain (valine
in place of glutamic acid at position 6)
Homozygotes HbS > 60 %
Heterozygotes HbS < 40%
About 30% of native Africans are
heterozygotes
Common in areas with high prevalence
of Plasmodium falciparum
47
Young RBCs with HbS
(normal shape)
Sickling
Deoxygenation
(polymerization of
HbS)
Ireversibly sickled cells
Repeated episodes
(accumulate Ca and
lose K, H2O)
Factors promoting sickling
HbC
Acidosis
Infections, inflammations
Hypoxia
Factors preventing
Sickling
HbF
thalassemia
(reduced Hb
concentration) Reversible
on reoxygenation
Removed
By Spleen
Microvascular
obstruction
Ischemia Pain crises
Intravascular hemolysis
48
Ireversibly sickled cells (ISC)
Reduced life span Microvascular obstruction
Chronic hemolytic
anemia
Vascular
stasis
Ischemia
Infarction
Spleen
(autosplenectomy)
Repeated
attacks
Erythropoiesis
Fatty change in
Liver, Heart,
kidneys
Crew cut
appearance
Pain crises
49
Clinically
Clinical picture is dominated by features
of chronic hemolysis and ischemic
damage
Homozygous children present six
months after birth
Anemia, jaundice, dactylitis, gallstones
Abdominal pain, leg ulcers
50
Types of crises
Vaso-occlusive
Aplastic crisis: parvo virus
Sequestration crisis
Hemolytic crisis
51
Vaso-occlusive crisis
(pain crises)
Bones
Dactylitis
(Hand-foot
syndrome)
Lungs
Fever, cough,
chest pain
(acute chest
syndrome)
Skin
Ulcers
Brain
Seizures,
stroke
Spleen
Abdominal pain
Infarction
(Autosplenectomy) 52
Complications
Aseptic necrosis of head of femur
Pigmented gall stones
Renal papillary necrosis (presents as
hematuria)
Increased susceptibility to infections
by encapsulated organisms and
Salmonella osteomyelitis
Require prophylactic penicillin and
folic acid
53
Diagnosis
PBF reveals presence of sickle cells
especially in homozygotes
Reduction test: sodium metabisulphite
reduces O2 tension and induces
sickling
Hemoglobin electrophoresis: HbS
moves slower than HbA
Prenatal screening: amniocentesis,
chorionic villous sampling
54
Thalassemias
Genetic disorder of Hb synthesis
Absent or decreased synthesis of
globin chain (either alpha chain or beta
chain)
Alpha thalassemia
Beta thalassemia
High incidence in South East Asia,
Middle East countries
55
Beta thalassemia:
Absent or decreased synthesis of beta
chains 0 or +
Two beta chain genes (chromosome 11)
Point mutations leading to aberrant
splicing (0); promoter mutation (+)
Alpha thalassemia:
Absent or decreased synthesis of alpha
chains
Four alpha chain genes (chromosome 16)
Deletions are more common
56
Absent or Reduced Hb A synthesis
Decreased Hb
Decreased MCHC
Hypochromia
Beta Thalassemia
Excess chains
aggregate
Damage to RBC
membrane
Erythroblasts die in marrow
(Ineffective erythropoiesis)
Defective RBCs removed
By spleen
Splenomegaly
Increased bone
marrow iron
57
Beta Thalassemia major
0/+ or 0/0
Manifests after first 5-6 months of life
Growth retardation, failure to thrive
Pallor
Hepatosplenomegaly, lymphadenopathy
Maxillary prominence, frontal bossing
Skull X ray - crew cut appearance
Iron overload, hemosiderosis and heart
failure
58
Investigations
PBF: severe anisopoikilocytosis,
microcytosis and hypochromia, target
cells, tear drop cells, basophilic stippling,
polychromasia
Increased reticulocyte count,
normoblastemia
Marked increase in RDW
Serum bilirubin (unconjugated) increased
59
Serum iron, serum ferritin - elevated
Bone Marrow - erythroid hyperplasia,
increased bone marrow iron
Hb electrophoresis
HbF markedly increased
HbA2 normal or increased
HbA decreased
60
Beta Thalassemia minor
/+ or /0
Usually asymptomatic, mild anemia
Discovered during routine investigations
Often confused with iron deficiency anemia
Serum iron increased
Free erythrocyte protoporphyrin normal
Hb electrophoresis
HbA2 increased
HbA decreased
HbF can be normal or slightly increased
61
Alpha Thalssemia
One deletion Silent carrier
( / - )
Two deletions Thalassemia trait
( / - - or - / -)
Three deletions HbH disease
( - / - -) (4)
Four deletions Hb Bart disease
(- - / - -) (4)
62
Alpha thalassemia trait:
Clinical picture usually resembles
beta thalassemia minor
HbH disease:
Causes severe transfusion dependent
anemia
HbH gets precipitated on oxidation and
HbH inclusions can be demonstrated on
supravital staining
Hb Barts disease:
Severe disease usually leads to
intrauterine death (hydrops fetalis)
63
Glucose-6-Phosphate
Dehydrogenase deficiency
X linked recessive disorder
Normally oxidative damage to RBC
membrane is prevented by reduced
glutathione (GSH)
G6PD deficiency leads to impaired
regeneration of reduced glutathione
Causes episodic intravascular and
extravascular hemolysis
64
Oxidants
H2O2
H2O
GSH
GSSG
G6PD
Accumulate
Denaturation
of globin
Heinz bodies
Damage to
RBC
Membrane
Intravascular
Hemolysis
Removed by
spleen
Bite cells
X
NADPH
NADP
Glucose-6-
phosphate
6-phospho-
gluconate
Glutathione
reductase
Glutathione
peroxidase
65
Clinically
Usually asymptomatic
Hemolysis precipitated by
ingestion of certain drugs (sulfa drugs,
antimalarials),
Poisoning by napthalene balls
Infections
ingestion of fava beans
Within 2-3 days of oxidative damage
patient presents with
Reddish or dark colored urine
(hemoglobinuria)
Jaundice
66
Hemolysis is followed by rapid
reticulocytosis
Older RBCs are severely deficient in
enzyme
Young RBCs have increased amount of
enzyme as compared to older RBCs, so
during stress older RBCs are lysed first
and levels of G-6-PD s may be normal
following hemolysis
67
Investigations
PBF reveals presence of bite cells
Supra vital staining for Heinz bodies
(best marker in acute hemolytic stage)
Hemoglobinuria in acute stage
G6PD levels can be estimated
Since hemolysis is episodic,
splenomegaly and gall stones are
uncommon
68
Paroxysmal Nocturnal
Hemoglobinuria (PNH)
Acquired membrane defect secondary to
mutation in PIGA gene (Phosphatidyl
Inositol Glycan A) which codes for GPI
(glycosyl-phosphatidylinositol ) anchor
in myeloid stem cells
Red cells, platelets and white cells
deficient in GPI linked proteins
CD55 (Decay accelerating factor)
CD59 (membrane inhibitor of lysis by MAC)
69
PIGA deficient precursors
RBCs
Susceptible to
complement
Platelets
Thrombosis
(main cause
of death)
WBCs
Susceptibility to
infections
Aggregation of platelets (thrombocytosis)
due to release of ADP, thromboxane from
lysed platelets
70
Clinically: Can be triad of hemolytic
anemia, thrombosis and pancytopenia
Chronic hemolysis with hemoglobinuria and
hemosiderinuria
Paroxysmal and nocturnal episodes of
hemolysis seen in 25% cases
Nocturnal due to increased hypoxia at
night causing acidosis and activation of
complement
RBCs are destroyed by acidified serum
which activate complement (Ham test)
Flow cytometry reveals reduced
expression of CD55 and CD 59
71
Immune hemolytic anemias
Mediated by antibodies against RBCs
Warm antibody type
Cold antibody type
These antibodies arise spontaneously
or induced by certain external agents
Demonstrated by antiglobulin test
(Coombs test)
72
Direct Coombs test
(DCT)
Patientss RBCs coated
with Ab
Add antihuman
antiglobulin
Agglutination
Indirect
Coombs test
patient serum
containing antibodies
Incubate with normal
RBCs
Add antihuman globulin
Agglutination
73
Warm antibody type
Antibodies are active at 37 C
Commonly mediated by IgG rarely IgA
Idiopathic or autoimmune
1/4
th
cases have underlying cause e.g.
SLE, drugs (penicillin, methyldopa) that
alter RBC antigens
RBCs coated with antibodies are removed
by spleen microspherocytes
Anemia, jaundice, hepatosplenomegaly,
generalized lymphadenopathy
0
74
Cold antibody type
Usually mediated by IgM type
Antibodies bind to RBCs at a lower
temperature and activate classical
complement pathway
Associated with
Infectious mononucleosis, CMV
Mycoplasma
Lymphomas (CLL)
HIV infection
75
IgM binds to RBCs & fixes complement
at low temperatures in peripheral circulation
IgM released at body temperature
from RBCs (during recirculation)
RBCs remain coated with C3b
Affected RBCs removed by
macrophages in spleen and liver
76
Hemolytic anemia secondary
to mechanical trauma
RBC destruction by physical trauma in
blood vessels (Intravascular hemolysis)
Prosthetic heart valves
Partial obstruction of blood vessels
microangiopathic hemolytic anemia
(MAHA), DIC, TTP, HUS
SLE
PBF- fragmented RBCs (schistocytes)
Anemias due to impaired
hemoglobin synthesis
78
Iron deficiency anemia
Most prevalent deficiency world wide
Anemia is due to decreased body iron
content, which leads to impaired heme
synthesis
79
Etiology
Chronic blood loss is the most
common cause. Common site of
bleeding in
Males & post menopausal females- GIT
Females in reproductive age group- FGT
Malabsorption (duodenum)
Increased demand
Dietary deficiency
80
Clinically
Non specific signs and symptoms
Koilonychia spoon shaped nails
Pica craving for salt, rocks, dirt
Plummer Vinson syndrome
Esophageal web
dysphagia
Iron deficiency anemia
81
Investigations
CBC
Hb - low
MCV - low
MCHC - low
RDW - increased
Free erythrocyte protoporphyrin (FEP)
and Zn protoporphyrin levels are
elevated
82
PBF
Microcytic hypochromic blood picture few
tear drop cells
Platelets can be increased
Bone marrow
Micronormoblasts
Decreased bone marrow iron
Soluble transferrin receptors elevated
83
Iron studies:
Serum iron levels: decreased
Serum ferritin levels: decreased
Total Iron Binding capacity
(TIBC): increased
Decreased ferritin levels stimulate
transferrin synthesis in the liver hence
TIBC increases (inversely related)
Percentage saturation: decreased
84
Anemia of chronic disease
(ACD)
Common causes include:
Chronic infections - osteomyelitis,
bacterial endocarditis, lung abscess
Immune disorders- RA, Crohn disease,
SLE
Neoplasms- Hodgkin lymphoma,
carcinoma breast
85
Chronic infections,
Chronic
inflammations
malignancies
Erythroblasts
Macrophages
Anemia
Hepicidin
Ferroportin
_
86
Investigations
PBF : Normocytic normochromic (early),
some times microcytic hypochromic
Serum Fe: decreased
Serum ferritin: increased
TIBC: decreased
Bone marrow iron: increased
87
Sideroblastic anemia
Excessive iron in the mitochondria of
erythroblasts due to defective heme
synthesis
Hereditary
Acquired
Myelodysplastic syndromes
Drugs: Isoniazid, alcohol
Lead poisoning
Pyridoxine deficiency
88
Normoblasts with iron laden
mitochondria in perinuclear position
are known as ring sideroblasts
Small amount of iron may also be seen
in a RBC (siderocyte)
Siderocytes contain iron in the form of
Pappenheimer bodies
PBF shows microcytic hypochromic
picture along with presence of normal
cells as well (dimorphic picture)
89
Low High High Low %
saturation
Low Low Low High TIBC
High High High Low Serum
ferritin
Low High High Low Serum iron
Anemia
of
Chronic
disorder
Sidero-
blastic
anemia
Thalassemia
minor
Iron
deficiency
anemia
Differential diagnosis of microcytic hypochromic
anemia
90
Normal Normal HbA2 & HbF Normal Hb
electro-
phoresis
High High Normal/
High
Absent BM Fe
Normal Normal Normal High RDW
Low Low High Low RBC
count
Anemia of
Chronic
disorder
Sidero-
blastic
anemia
Thalassemia
minor
Iron
deficiency
anemia
91
Megaloblastic anemia
Caused by folate or B12 deficiency
Characterized by presence of giant
erythroid precursors which give rise to
abnormal large RBCs (Macrocytes)
92
Pathogenesis
93
Impaired DNA synthesis
Nuclear cytoplasmic asynchrony
(nuclear maturation lags behind)
Megaloblasts
Hypersegmented
neutrophils
Abnormal
megakaryocytes
Intramedullary death (ineffective hematopoiesis
and increased bone marrow iron)
94
Causes of folate deficiency
Alcoholism
Dietary deficiency
Pregnancy / lactation
Drugs which block dihydrofolate
reductase e.g. methotrexate,
trimethoprim
Malabsorption
Increased cell turnover e.g. malignacies
95
Causes of B12 deficiency
Pernicious anemia
Vegans
Patients with ileal disease e.g. Crohns
disease
Infestation with fish tape worm
(Diphyllobothrium latum)
Chronic pancreatitis
Bacterial overgrowth e.g. autonomic
neuropathy, diverticular disease
96
Clinically
Pallor, easy fatigability, dyspnea
Sore tongue, cheilosis
Sallow complexion due to mild jaundice
Numbness, tingling sensation,
unsteadiness of gait because of
involvement of posterior columns
With severe disease lateral
corticospinal tract may also be
involved
B12
deficiency
only
97
Diagnosis
CBC
pancytopenia
MCV usually more than110 femtolitres
Reticulocyte count decreased
PBF
macrocytes, macro-ovalocytes
Hypersegmented neutrophils (earliest
change)
98
Bone marrow findings
Hypercellular marrow
Megaloblasts- large cells with delicate,
fine and reticular or sieve like chromatin
Features of dyserythropoiesis
Giant myelocytes and metamyelocytes
Megakaryocytes have abnormally large,
bizarre nuclei
99
Biochemical tests:
Increased LDH levels
Red cell folate levels decreased
Serum B12 levels decreased
Increased homocystiene levels are
seen in both B12 and folate deficiency
Increased methlymalonic acid levels In
B12 deficiency only
Serum antibodies to IF (pernicous
anemia)
Schilling test
100
Schilling test
Give parenteral B12 (to replenish stores)
Give radioactive labelled B12 orally
Measure urinary B12 levels
> 10%
(Normal)
< 5 %
Again give labeled B12
and intrinsic factor
Malabsorption
> 10%
Pernicious
anemia
< 5 %
101
Aplastic anemia
Disorder of pluripotent stem cells
Bone marrow failure and cytopenias
Anemia
Leucopenia
Thrombocytopenia
102
Etiology
Idiopathic Chemicals
Drugs
(chloramphenicol)
Toxins
Viral infections
Irradiation
Hereditary
(Fanconi Anemia)
103
Drugs, infections,
Unidentified stimulus
Altered stem cells
Activate T cells
IFN-y & TNF
Decreased
growth
potential
Marrow aplasia
Suppress stem cells
104
Clinically
Pallor, weakness
Bleeding diathesis
Increased frequency of infections
No splenomegaly
PBF:
Normocytic normochromic RBCs with
markedly decreased reticulocyte count
Leucopenia with relative lymphocytosis
Thrombocytopenia
105
Bone marrow aspiration
is usually a dry tap
Bone marrow biopsy
reveals:
Hypocellular marrow
Increased fat spaces
Prominence of
lymphoid cells
106
Pure red cell aplasia (PRCA)
Selective suppression of red blood cells
Two main types:
Primary
Secondary
Parvovirus B19
Autoimmune disorders
Thymic tumors (Thymoma)
107
Myelophthisic anemia
Associated with infiltrative diseases of the
bone marrow which cause marrow fibrosis
and destroy the marrow architecture
Immature erythroid and myeloid precursors
appear in blood (Leucoerythroblastic
picture) due to extramedullary
hematopoesis
108
Common causes include:
Bonemarrow metastasis
Granulomatous disorders e.g.
tuberculosis, sarcoidosis
Myelofibrosis (will be discussed later)
Myelophthisic anemia
Polycythemia
110
Polycythemia
Relative
Dehydration
Absolute
Autonomous proliferation
of stem cells
(Polycythemia rubra vera)
EPO
Lung diseases, high
altitude, paraneoplastic
syndrome
RBC precursors normal
EPO
Primary Secondary
111
Polycythemia vera
Neoplasm of myeloid stem cells
associated with erythrocytes,
leucocytosis and thrombocytosis
Classified as one of the
myeloproliferative disorders
Levels of erythropoietin are low
Most symptoms are related to increased
red cell mass, hematocrit and increased
blood viscosity
112
Clinically
Intense pruritus, facial plethora
Headache, dizziness
Hypertension, peptic ulcerations
Bleeding and thrombotic events because
of abnormal blood flow (hyperviscosity)
Splenomegaly, hepatomegaly
Possible gout due to increased purines
from RBC breakdown
113
Investigations
Increased red cell mass (radioactive
studies)
CBC:
Increased Hb, increased red cell count
WBC count: increased
Thromobocytosis
Bone marrow is hypercellular showing
hyperplasia of all three cell lines
(erythroid, myeloid, megakaryocytes)
About 15-20% patients develop
myelofibrosis
White Blood Cells
B-cell markers: 19, 20, 21, 22
T-cell markerse: 1- 8
115
Disorders of white blood cells
Leukopenia
Neutropenia / Agranulocytosis
Leucocytosis
Non- neoplastic / reactive including
leukemoid reaction
Neoplastic / leukemia
Non Neoplastic
Disorders of WBCs
117
Neutropenia
Markedly reduced WBC count
Usually < 1000 /L
Increased susceptibility to infections
especially (gram negative bacteria,fungal
and viral)
Etiology:
Inadequate granulopoeisis: aplastic anemia,
chemotherapy, drugs
Increased destruction: overwhelming
infections, immune mediated destruction,
splenomegaly
118
Clinically
Malaise, fever, chills
Ulcerating lesions of gingiva, floor of
mouth, pharynx (agranulocytic angina)
invasive bacterial and fungal infections
in lungs, urinary tract and other organs
are common
Remove the offending drug
Treated by G-CSF
119
Neutrophilic leucocytosis
Usually associated with shift to left
(more than 10% band cells and
presence of other immature myeloid
cells)
Neutrophilia due infective causes have
special morphological features
Increased azurophilic granules aka toxic
granulations
Cytoplasmic Dohle bodies may be seen
120
Causes of neutrophilia
Bacterial infections, abcesses
Sterile inflammations e. g. myocardial
infarction, gout, RA
Stress
Myeloproliferative disorders
Drugs, steroids, epinephrine, lithium
121
Eosinophilia
Allergic disorders (asthma, hay fever)
Skin diseases
Parasitic infestations
Drug reactions
Malignancies Hodgkin lymphoma
Collagen vascular diseases, vasculitis
122
Basophilia
Basophilic leucocytosis is rarely
reactive, and almost always indicates
myeloproliferative disease
(especially Chronic myeloid leukemia
- CML).
123
Lymphocytosis
Infections: viral (Infectious
mononucleosis, CMV,HIV etc),
whooping cough
Neoplasms: chronic lymphocytic
anemia
Endocrine: Graves disease
Drugs: phenytoin
Atypical lymphocytes in viral infections
124
Monocytosis
Chronic infection
Tuberculosis, malaria, rickettsiosis,
bacterial endocarditis
Collagen vascular disease
Inflammatory bowel disease
Myeloproliferative disorders
Malignancy
125
Leukemoid reaction
Extreme elevation in WBC count in
exaggerated response to infections,
inflammations, malignancies and allergies
Neutrophilic type of leukemoid reaction is
most common
WBC count varies from 30,000 to more
than 50,000/ L
Confused with leukemias
Leukocyte alkaline phosphate (LAP)
score is increased
126
Infectious Mononucleosis
Acute self limiting viral infection (EBV)
of B cells
Common in adolescents, young adults
Transmitted by saliva
127
Clinically
Fever, sore throat, palatal petechiae
Tender posterior cervical
lymphadenopathy
Generalized lymphadenopathy (multiple
discrete lymph nodes)
Sudden onset of rash after ampicillin
Hepatosplenomegaly
Recovery within 4-6 weeks
Rarely complicated by splenic rupture
128
EBV
infects oropharyngeal cells
Lymphoid tissue (tonsils, adenoids)
Infects B cells (CD21 receptors)
B cells proliferate
IgM, IgG
Heterophile antibodies
(monospot test)
Activates T cells, NK cells
Atypical lymphocytes/
Downey cells
129
Diagnosis
Leucocytosis with lymphocytosis
(usually > 60%) with presence of
atypical lymphocytes (Downey cells)
Monospot test detects IgM antibodies
against sheep or bovine RBCs
Assays for antibodies specific to EBV
antigens are also used
Neoplastic Disorders
of WBCs
131
Neoplasms of white blood
cells
Myeloid cells Lymphoid cells Histiocytes
Leukemias
Myeloproliferative
disorders
Myelodysplastic
syndromes
Leukemias
Lymphomas
Plasma cell
neoplasms
Histiocytosis
132
WHO classification of lymphoid
neoplasms
Precursor B cell neoplasms
Acute lymphoblastic leukemia / lymphoma
Peripheral B cell neoplasms
Precursor T cell neoplasms
Acute lymphoblastic leukemia / lymphoma
Peripheral T cell/ NK cell neoplasms
Hodgkin lymphoma
Acute Leukemia
134
Acute Leukemias
Acute lymphoblastic leukemia (ALL)
Acute myeloid leukemia (AML)
135
Acute lymhoblastic leukemia
(ALL)
Precursor B cell leukemia / lymphomas
Precursor T cell leukemia / lymphomas
136
ALL
Malignancy of lymphoblasts ( B or T)
Common in children and young adults
Involvement of bone marrow, peripheral
blood and lymph nodes
About 80% are B cell in origin and usually
present as acute childhood leukemias
T cell ALLs usually present as
lymphomas in adolescent males
137
Block in differentiation
of blasts
Accumulation of
immature Cells
(blasts)
Displacement of normal
hematopoietic elements
Anemia Thrombocytopenia Leucopenia
(Pancytopenia)
138
Clinically
Sudden onset
Anemia, fever, fatigue, frequent infections,
bleeding diathesis
Bone pains, sternal tenderness
Generalized lymphadenopathy, mild
hepatosplenomegaly
Headache, vomiting, nerve palsies with
CNS involvement, testicular infiltration
In T cell ALL, dyspnea, stridor, dysphagia
because of thymic involvement
139
ALL
The T cell type usually present later
(age of onset 15-20 years)
Mediastinal involvement common
Can present with dyspnea, pericardial
involvement
140
Diagnosis
Leucocytosis with increased number of
blasts
Some times leucopenia (aleukemic
leukemia)
Bone marrow
Hypercellular
Flooded with blasts
By definition lymphoblasts should be more
than 25%
141
LYMPHOBLASTS
Chromatin clumped,
and immature
1-2 nucleoli
Scanty cytoplasm
with no granules
PAS positive
MYELOBLASTS
Fine homogenous
chromatin
2-5 nucleoli
Cytoplasm more as
compared to
lymphoblasts
Cytoplasmic granules
and Auer rods can be
seen
Special stains- MPO,
non specific esterase
142
Immunophenotyping is done by flow
cytometry
B cells CD 10,19, CD 20
T cells CD2, CD3
Tdt positive in > 90% cases
(Tdt enzyme involved in pre-B cells
lymphocyte gene recombination)
Cytogenetic studies
143
Prognosis
Good prognosis
Age 2-10
t(12;21)
Hyperdiploidy
(> 50 chromosomes)
WBC count <
100,000
Poor prognosis
Male
Age < 2; adults
WBC count >
100,000
144
Acute myeloid leukemia
(AML)
Block in differentiation of myeloblasts
Common in adults (median age 50)
Classification
French American British (FAB)
World Health Organization (WHO)
145
Myeloid stem cell
granulocytes
monocytes
Platelets
Minimal / no
differentiation
Undifferentiated
AML-M0
Myeloblastic
Immature
AML-M1
Myeloblastic
Mature-M2
Erythrocytes
Acute
megakaryocytic
Leukemia-M7
Acute
promyelocytic
Leukemia-M3
Acute myelo-
monocytic
Leukemia-M4
Mature into
acute monocytic
leukemia M5
Acute erythro-
leukemia- M6
146
WHO classification
AML with recurrent chromosomal
rearrangements
t(15:17) (FAB M3)
t(8:21) (FAB M2)
inv(16) (FAB M4 with eosinophilia)
t(11:23)
AML with multilineage dysplasia
AML- therapy related
AML-not other wise specified
Includes rest of the subclasses of FAB
classification
147
AML
Median age of presentation is 50 years
More common in males
Risk factors include
Chemical exposure e.g. benzene
Radiation or chemotherapy (therapy
related AML)
Smoking
Myelodysplastic syndrome (MDS)
Myeloproliferative disorders (MPD)
148
Clinically
Fever, shortness of breath
Easy bruising or bleeding, petechiae
Loss of weight, loss of appetite
DIC in M3 type (due to myeloperoxidase
release)
In M4 & M5 infiltration of the gums and
skin can occur
Uncommonly masses of myeloblasts in
skin without involvement of the bone
marrow, which are known as granulocytic
sarcoma or chloromas
149
Investigations
1) CBC, PBF:
Anemia, leucocytosis, thrombocytopenia
Presence of myeloblasts
Auer rods seen in the cytoplasm
Auer rods are composed of azurophilic
granules, rich in peroxidase
clumps of Auer rods are seen in M3 type
150
2) Bone marrow aspiration/Biopsy:
Increased number of blasts
WHO criteria is 20% blasts in the
peripheral blood or bone marrow
M7 type is associated with bone marrow
fibrosis
3) Special stains:
Myeloperoxidase (MPO)
Sudan black
Non specific esterase for monoblasts
PAS for erythroblasts
151
4) Flow cytometry: CD 13, CD33 positive
5) Cytogenetic studies reveal presence
of chromosomal abnormalities
152
Prognosis
t(15:17)
t(8:21)
inv(16)
Therapy related
AML
AML with multi-
lineage dysplasia
T(11:23)
GOOD
BAD
Chronic Leukemias
154
Chronic Leukemias
Chronic lymphocytic leukemia / Small
lymphocytic lymphoma (CLL / SLL)
Chronic myeloid leukemia (CML)
155
CLL/SLL
Most common leukemia of older age group
Arise from mature B cells
Often asymptomatic or present with non
specific symptoms
Increased susceptibility to infections
Lymphadenopathy
Hepatosplenomegaly
156
Abnormal B cells
Decreased production of
Immunoglobulins
Hypogammaglobulinemia
Increased susceptibility
to infections
Autoantibody against
RBCs
Hemolytic anemia
(spherocytosis)
157
Diagnosis
Leucocytosis with lymphocytosis
Mature looking lymphocytes but
delicate, easily break during slide
preparation aka smudge cells/ basket
cells
Bone marrow: sheets of lymphoid cells
Lymph nodes: effacement of the nodal
architecture by small lymphocytes
In SLL no involvement of bone marrow
and peripheral blood
158
Immunophenotyping
CD19, 20 positive
Surface immunoglobulin positive
CD5 positive (T-cell marker on B-cells)
Prognosis usually good
Small percentage transform to
aggressive non Hodgkin lymphomas
Not strongly associated with
chromosomal translocations
159
CML
Is a myeloproliferative disorder arising from
myeloid stem cells characterized by
increased proliferation of the granulocytic cell line
without the loss of their capacity to differentiate
presents in adults 25-60 years
Characterized by distinctive translocation
9:22 (ABL gene on ch.9 & BCR on ch.22)
ABL-BCR fusion gene has tyrosinase activity
160
Clinically
Slow onset
Non-specific symptoms
Dragging sensation in left
hypochondrium because of massive
splenomegaly
161
Diagnosis
Peripheral blood:
Leucocytosis with presence of
neutrophils, myelocytes,
metamyelocytes and few blasts (< 10%)
Eosinophilia, basophilia
Leucocyte alkaline phosphatase
(LAP) score is decreased
(differentiate from leukemoid reaction)
162
Bone marrow:
Hypercellular with myeloid hyperplasia
Megakaryocytes increased in number,
small dysplastic forms
Karyotyping : reveals the Philadelphia
chromosome t (9:22) in 90 % cases
FISH (Fluorescence in situ hybridization)
and RT-PCR (reverse transcriptase
polymerase chain reaction) also be
used
163
Course
Without treatment, after 3-4 years most
patients enter accelerated phase and
blastic crisis (usually AML)
Blastic crisis can be myeloid type or
lymphoid type
Target therapy (Imatinib- an inhibitor
of tyrosine kinase) induces remission
in 90%
Bone marrow transplanation is curative
164
Hairy cell leukemia
Indolent tumor of B cells
Characterized by presence of cells with
fine cytoplasmic hair like processes
(hairy cells)
Common in old, hairy males
These B cells are positive for CD11c
and CD103
The cells mainly infiltrate bone
marrow and spleen
165
Bone marrow infiltration causes
pancytopenia (leads to dry tap)
Massive splenomegaly
Hairy cells are seen in peripheral blood
The cells are positive for tartarate
resistant acid phosphatase (TRAP) stain
Responds very well to chemotherapy
166
Chronic Myeloproliferative
Disorders
1) Chronic myelogenous leukemia
2) Polycythemia vera
3) Myelofibrosis
4) Esssential thrombocythemia
2,3,4 involve Jak-2 mutation
167
Myelofibrosis
Primary
Aka agnogenic
myeloid metaplasia
Secondary
Granulomatous
disorders e.g. TB,
sarcoidosis
Metastasis
Myeloproliferative
disorders
Irradiation
168
Primary Myelofibrosis
Is a clonal myeloid stem cell disorders
predominantly affecting the
megakaryocytes
Inappropiraite release of growth factors
(TGF- & PDGF) by megakaryocytes
Circulating stem cells home in
seconadry hematopietic organs which
is known as extramedullary
hematopoiesis or myeloid metaplasia
169
Clinically
Uncommon in persons younger than 60
years
Weakness, anemia, fever, night sweats
Hepatomegaly, massive splenomegaly
170
Investigations
In initial phases all three parameters
are increased (RBCs, WBCs & platelets)
Later on cytopenias
PBF: leucoerythroblastic picture with
presence of tear drop cells, nucleated
RBCs and immature granulocytes
BM aspiration: Dry tap
Bone marrow biopsy: increased
fibrosis ad collagenisation
171
Essential Thrombocytosis
Disorder of multipotent stem cells
mainly affecting the megakaryocyte
lineage
Characterized by marked
thrombocytosis > 600,000 per mm
3
Common in elderly age group (> 60)
Indolent course but evolve to bone
marrow fibrosis or AML
172
Clinically
Asymptomatic
Neurological symptoms: head ache,
dizziness
Thrombosis: digital pain, gangrene
Bleeding: skin, GIT, mucus membranes
173
Peripheral Blood
Thrombocytosis
Giant large platelets
Mild leukocytosis
Bone marrow
Mild to moderate hypercellularity
Megakaryocytic hyperplasia with giant
megakaryocytes
Lymph node
disorders
175
Lymphadenopathy
Non-neoplastic
Neoplastic
Inflammatory Non-inflammatory
Infective Non-infective
Acute
Chronic
Primary
Lymphomas
Non Hodgkin Hodgkin
Secondary/
Metastatic
Non Neoplastic
Disorders of the
Lymph Nodes
177
Acute non-specific
lymphadenitis
Usually involves a group of lymph nodes
draining a focus of infection
Tender, overlying skin red
If abscess formation fluctuant swelling
Histopathology:
Secondary follicles with large germinal
centres some times necrosis in the centre
of follicles
178
Chronic non-specific
lymphadenitis
Three main histological types
Follicular
Paracortical
Sinus histiocytosis
179
Follicular hyperplasia
Activation of B lymphocytes
Secondary follicles (follicles with pale
germinal centers)
Prominent phagocytic activity- tingible body
macrophages
Increased rate of mitosis
180
Paracortical Hyperplasia
Seen with viral infections
Hyperplasia of parafollicular T cells
Prominence of postcapillary venules
181
Sinus Histiocytosis
Lymph nodes draining malignancies
Distension and prominence of sinusoids
Hypertrophy of lining endothelial cells and
infiltration by macrophages
Neoplastic disorders
of the lymph nodes
183
Lymphomas
Hodgkin lymphoma (HD)
Non Hodgkin lymphoma (NHL)
184
Hodgkin lymphoma
A lymphoid neoplasm of B cell origin
Characterized by presence of distinctive
Reed Sternberg cells
RS cells are usually admixed with
reactive cells
185
Differentiated from NHL:
Almost always nodal in origin
Usually starts in single group of lymph
nodes & then spreads to contiguous
nodes
Neoplastic cells (Reed Sternberg cells)
constitute a minor component
Spill into peripheral blood is very
uncommon
Involvement of mesenteric nodes &
Waldeyers ring is uncommon
Presence of reactive inflammatory cells
186
Classification
Lymphocyte predominance
Nodular sclerosis
Mixed cellularity
Lymphocyte rich
Lymphocyte depletion
uncommon
187
Etiopathogenesis
Enigmatic
EBV genome in 70% cases of mixed
cellularity type & small % of nodular
sclerosis patients
RS cells secrete various cytokines
which are responsible for the non
neoplastic reactive background
188
Reed-Sternberg cells
Large binucleate cells or cells with
multilobated nucleolus and a large
prominent inclusion like nucleolus
Binucleate cells (the classic RS cells) have
mirror image nucleoli with acidophilic
nucleolus (owl eye appearance)
Distinct nuclear membrane
Abundant cytoplasm
Most type are CD15 and CD30 positive
189
Nodular sclerosis type
Most common type
Equally frequent in males and females
Characterized by the presence of
Collagen bands (divide node into nodules)
Lacunar type RS cells
Cervical & mediastinal
lymphadenopathy
Associated with good prognosis
190
Mixed cellularity
Association with EBV infection
More common in HIV positive patients
RS cells present against a reactive
background composed of neutrophils,
eosinophils, macrophages, lymphocytes
and plasma cells
No collagen bands
Mediastinal involvement uncommon
Lymphocyte Predominant
Primarily less than 35 years
Mostly lymphocytes with occasional
histiocytes (popcorn cells)
Good prognosis due to lymphocytes
attacking tumor cells
191
ANN ARBOR STAGING (for both HD and
NHL)
Multiple or disseminated foci of involvement of one
or more extra lymphatic organs with or without
lymphatic involvement
IV
Involvement of lymph node regions on both sides
of the diaphragm (III) which may include spleen or
extra lymphatic site or both
III
Involvement of two or more lymph node regions on
the same side of diaphragm or involvement of
limited contiguous extra lymphatic organs
II
Involvement of single lymph node region (I) or
single extra lymphatic organ
I
DISTRIBUTION OF DISEASE STAGE
Each stage is subdivided depending upon the absence (A) or
presence (B) systemic symptoms
192
Course
In Stage I and II 5 year survival rate is
about 100%
Long term survivors of radiation
therapy are at risk of developing
malignancies .e.g. carcinoma lung,
melanoma, carcinoma breast
193
Non Hodgkin lymphomas
(NHL)
Can be
B cell type (more common)
T cell type
194
Non Hodgkin lymphomas
CLL/ small lymphocytic lymphoma
Follicular lymphoma
Diffuse large cell lymphoma
Burkitts lymphoma
Plasma cell neoplasm
Hairy cell leukemia
Mantle cell lymphoma
Marginal zone lymphoma
195
Follicular lymphoma
40% of Non Hodgkin lymphoma
Arise from follicular centre cells
CD 19, 20 postive, CD 10 positive, bcl2
positive
Equal incidence in males and females
t (14:18) - fusion of bcl and Ig production
preventing apoptosis
196
Most common presenting symptoms is
painless lymphadenopathy
Waxing and waning lymphadenopathy
Very indolent course
Poor response to chemotherapy due
to low mitotic activity (no cell death)
40% progress to aggressive type of
lymphomas
197
Morphology
Vague nodular architecture
The tumor cells resemble germinal
centre cells or centrocytes
Look like small lymphocytes with
cleaved nuclei (buttock cell)
Admixed with few centroblasts like
cells
198
Diffuse large B cell lymphoma
Comprise about 50% adult Non
Hodgkin Lymphomas
Median age is 60 years
Rapidly enlarging nodal or extranodal
lesions (GI, skin, brain testes)
199
Diffuse effacement of the nodal
architecture by large lymphoid cells
3-4 times the size of small
lymphocytes, prominent nucleoli,
moderate amount of cytoplasm
Abundant mitotic activity
Respond to chemotherapy
200
Burkitts lymphoma
Common in Children / young adults
Endemic or African type (100% EBV)
Sporadic
HIV associated (25% EBV)
Maxilla, mandible are the common sites
of involvement in endemic type
In sporadic type abdominal involvement
is more common (ileum, ovaries)
201
Biopsy of the affcetd tissue reveals
sheets of intermediate sized lymphoid
cells with high mitotic activity
These cells have 2-5 prominent nucleoli,
intensely basophilic cytoplasm
Lipid filled vacuoles stain with oil red O
High mitotic rate leads to starry sky
appearance
8:14 translocation is characteristic
finding (c-myc)
Responds well to chemotherapy
Plasma cell neoplasms
203
Multiple Myeloma
Plasma cell neoplasm characterized by
involvement of skeleton at multiple sites
Clonal proliferation of plasma cells
(driven by IL-6) that secretes
monoclonal immunoglobulins in the
blood which are referred as M
component
In 60% cases M component (M spike) is
IgG, 20-25% have IgA and in 15-20%
cases either kappa or lambda chains
204
Clinically
CRAB mnemonic
Calcium elevated
Renal failure (amyloidosis)
Anemia (weakness, lethargy)
Bone lesions in vertebra, ribs, skull,
pelvis and femur
Pathological fractures
Recurrent infections
205
Bone marrow:
increased number of plasma cells (sheets)
Plasma cells may have Russell bodies
Increased number of plasma cells are also
seen in other lymphoid organs
Kideny:
Proteinaceous casts in tubules (usually
made of Bence Jones proteins)
surrounded by giant cells
Interstitial infiltrate of plasma cells
206
Diagnosis
CBC
PBF reveals rouleax formation
Bone marrow: plasma cells > 30 %
Urine examination: Bence Jones
proteins, proteinuria
Serum protein electrophoresis reveals
M spike (Myeloma spike)
Radiological studies: lytic lesions,
pathological fractures
Bleeding Disorders
208
Bleeding disorders
Three basic causes:
1. Defect in vessel wall
2. Deficiency or defect of platelets
3. Deficiency or defect of coagulation
factors
209
General investigations
1) Platelet counts: normal = 150-
400,000/ml
2) Bleeding time: prolongation indicates a
platelet defect (reduced number/
function)
3) Prothrombin time (PT): test of
extrinsic and common coagulation
pathways (factors 7, 3, 10, 5, 2,1)
4) Partial thromboplastin time (PTT): test
of intrinsic and common pathways
(factors 12, 11, 9, 8, 10, 5, 2,1)
5) Thrombin time: test of fibrinogen
210
Bleeding Due to Vessel Wall
Abnormalities
1) Endothelial Abnormalities
Infections meningococcemia,
measles, Rickettsiosis (direct
endothelial damage)
Drug reactions hypersensitivity
vasculitis (immune damage)
Henoch-Schonlein purpura (immune
damage)
Scurvy Vitamin C deficiency
Platelet Disorders
212
Bleeding due to Platelet
Disorders
Functionally abnormal platelets (normal
platelet count, prolonged bleeding
time)
Thrombocytopenia
213
Platelet function disorders
Congenital
Acquired
Drugs: aspirin and non-steroidal anti-
inflammatory drugs (N-SAIDS), heparin
Uremia (chronic renal failure)
214
Thrombocytopenia
Platelet count less than 100, 000 / L
Petechiae, purpura
Hemorrhage in internal organs
Spontaneous bleeding if platelet count
< 20, 000 / L
215
Causes
1) Decreased production: Aplastic
anemia, hematologic malignancies,
drugs, viral infections, megaloblastic
anemias
2) Shortened survival: immunological,
DIC
3) Sequestration
4) Dilutional
216
Immune mediated
thrombocytopenic purpura (ITP)
Platelet destruction by auto antibodies
Can be acute or chronic
Can be
Idiopathic
Secondary:
AIDS, SLE, viral infections
217
Pathogenesis
Autoantibodies against GP IIb-IIIa or Ib-
IX (usually IgG)
Coat the platelets
Removed by spleen
218
Clinically
Acute ITP common in children (resolves)
Preceded by a viral illness
After 1-2 weeks sudden onset of
petechiae and purpuras
Chronic ITP more common in adult
females (rarely resolves)
Insidious onset of symptoms
219
Pin point hemorrhages, bleeding gums
Increased menstrual flow
Melena, hematuria, nose bleeds
Rarely intracerebral hemorrhage
220
Investigations
CBC- low platelet count, giant platelets
Bleeding time prolonged
Prothrombin time, partial thromboplatsin
time essentially normal
Bone marrow: Compensatory INCREASE
in number of megakaryocytes, young
immature forms
Antiplatelet antibodies present
221
Course
Acute ITP is usually self limiting
In markedly reduced platelet count,
steroids are given
Chronic ITP
Steroids are usually required
Frequent relapse
Refractory cases require splenectomy
222
Thrombotic thrombocytopenic
purpura (TTP)
Pentad of
1) Thrombocytopenia
2) Neurological symptoms
3) Microangiopathic hemolytic anemia
(MAHA)
4) Fever
5) Renal impairment
223
Deposition of hyaline
Microthrombi in microcirculation
(heart, kidney, brain)
Fragmentation of erythrocytes
(schistocytes)
Impaired degradation of vWF multimers
Deficiency of an enzyme ADAMTS 13
Platelet micro aggregate formation
Hemolytic Uremic Syndrome
(HUS)
Clinically similar to TTP
Occurs in children following infection
by E. coli O157:H7 (verotoxin)
More likely to progress to renal failure
Clotting Factor Disorders
No petechiae, purpura (plaltelets are
normal)
Risk of rebleeding due to weak
hemostatic plug (missing fibrin)
225
Clotting Factor Disorders
Hereditary or Acquired
Hereditary:
typically involve a single clotting factor
defect eg. Factor VIII, vWF,
Acquired:
typically multiple factor abnormalities. Eg.
Vitamin K deficiency, severe liver disease
(PT affected), DIC (disseminated
intravascular coagulation).
Disseminated intravascular
coagulation (DIC)
Look for fibrin degradion products
Increased PT/PTT time, platelet
consumption
Causes: Obstetric procedures, infections,
neoplasms, tissue injury
226
Hereditary Clotting Factor
Deficiencies
Factor VIII deficiency (Hemophilia A)
deficiency of Von Willebrand factor
(von Willebrand disease)
227
Hemophilia A
X linked recessive
30% cases new mutations
Reduction in amount or activity of
factor VIII
Associated with spontaneous bleeding
into joints, muscles and internal organs
(following exceedingly minimal trauma)
228
Disease/bleeding severity can be
Mild Depends upon level of F VIII
Moderate
Severe
Spontaneous bleeding or prolonged
bleeding after minor procedures
Arthritis is common complication
Sometimes intracerebral hemorrhage
Increased PTT (Platelet count, BT and
PT are normal)
Treated with factor VIII transfusions
229
von Willebrand Disease
One of the most common inherited
disorders of bleeding (1% frequency)
Autosomal dominant is most common
mode of inheritance
Rarely autosomal recessive
Reduced quantity or qualitative defect
Type 1&3 reduced vWF quantity
Type 2 impared vWF quality
230
Mild bleeding diathesis
Easy bruising, epistaxis, menorrhagia
Excessive bleeding from wounds
Normal platelet count
Prolonged bleeding time
Prolonged PTT (because of secondary
decrease in factor VIII levels)
Normal PT
Ristocetin co-factor assay; active vWF
levels are reduced
You might also like
- Practical Hematology Manual #1Document48 pagesPractical Hematology Manual #1walhaliNo ratings yet
- Clinical Assessment and Examination in Orthopedics, 2nd Edition PDFDocument196 pagesClinical Assessment and Examination in Orthopedics, 2nd Edition PDFS KALYAN100% (5)
- Lab Investigations of AnemiaDocument109 pagesLab Investigations of AnemiaMadhura ShekatkarNo ratings yet
- Hematology Book For PDF 1304281501 Phpapp02 PDFDocument260 pagesHematology Book For PDF 1304281501 Phpapp02 PDFAndrea Kocsis100% (1)
- Manual Platelet CountDocument14 pagesManual Platelet CountMiyo SobremisanaNo ratings yet
- CardiopathophysiologyDocument63 pagesCardiopathophysiologyapplesncoreNo ratings yet
- CardiopathophysiologyDocument63 pagesCardiopathophysiologyapplesncoreNo ratings yet
- Spinal Cord Injury Study GuideDocument8 pagesSpinal Cord Injury Study GuideAlice Gifford100% (3)
- Hematological Investigation or Quantitative Evaluation of The Hematopoietic SystemDocument21 pagesHematological Investigation or Quantitative Evaluation of The Hematopoietic SystemMAMA LALANo ratings yet
- Hem311 Week 13 Lab - Reticulocyte CountDocument32 pagesHem311 Week 13 Lab - Reticulocyte CountSheine EspinoNo ratings yet
- Histology & Cell BiologyDocument33 pagesHistology & Cell BiologyMohSen100% (1)
- Morphology OF Red Blood CellsDocument36 pagesMorphology OF Red Blood CellsFrancis ValdezNo ratings yet
- Molecular Diagnosis in HaematologyDocument23 pagesMolecular Diagnosis in HaematologyUmar'Farouq Oni100% (1)
- Prep, Dressing, Draping The PatientDocument60 pagesPrep, Dressing, Draping The PatientTamil Villardo100% (1)
- Coag Made EasyDocument16 pagesCoag Made EasyBrian RobertsNo ratings yet
- ImmunopathologyDocument21 pagesImmunopathologyapplesncoreNo ratings yet
- White Blood Cell Disorders: Neoplastic Diseases of The BloodDocument81 pagesWhite Blood Cell Disorders: Neoplastic Diseases of The BloodMiguel Cuevas Dolot100% (1)
- Blood Smear Examination 1معدل Document74 pagesBlood Smear Examination 1معدل Kenesa100% (1)
- Validation Cell AnalyzersDocument45 pagesValidation Cell AnalyzerscandiddreamsNo ratings yet
- HPLC PPT - KarishmaDocument76 pagesHPLC PPT - KarishmaDivya GauravNo ratings yet
- Drug StudyDocument6 pagesDrug StudyDahnel Magumpara100% (1)
- Platelet CountsDocument35 pagesPlatelet Countsshikhar623No ratings yet
- Citrate Activates Accoa Carboxylase (Fa Synthesis) and F-1,6 BP (Gluconeogenesis)Document6 pagesCitrate Activates Accoa Carboxylase (Fa Synthesis) and F-1,6 BP (Gluconeogenesis)Nahome YebassewNo ratings yet
- NAFLD - NASH and Present & Future Management OptionsDocument78 pagesNAFLD - NASH and Present & Future Management OptionsSantosh AnandNo ratings yet
- RBCs Abnormal MorphologyDocument33 pagesRBCs Abnormal MorphologyLailitifa Windy SNo ratings yet
- 1 Introduction To AnemiaDocument60 pages1 Introduction To AnemiaKhisha RangasNo ratings yet
- Urinalysis and Body Fluids2020Document47 pagesUrinalysis and Body Fluids2020MONFOLA100% (1)
- WBC BasicsDocument70 pagesWBC BasicsZoe ZillaNo ratings yet
- S0850alug 1670953860959-SEU HDocument56 pagesS0850alug 1670953860959-SEU HAziz KhwajaNo ratings yet
- Hematology BMLS 103Document88 pagesHematology BMLS 103harpreetNo ratings yet
- Identification of Normal and Abnormal Forms of RedDocument32 pagesIdentification of Normal and Abnormal Forms of RedNada hasanNo ratings yet
- CC1 - Topic 1Document11 pagesCC1 - Topic 1Marie MontemarNo ratings yet
- Modified Beggs / Orthodontic Courses by Indian Dental AcademyDocument202 pagesModified Beggs / Orthodontic Courses by Indian Dental Academyindian dental academyNo ratings yet
- RBCDocument66 pagesRBCFarah mansourNo ratings yet
- Pathophysiology of PainDocument6 pagesPathophysiology of PainJorgeGalleguillosCavadaNo ratings yet
- Tune Up FitnessDocument6 pagesTune Up FitnessMarsela Giovanni100% (2)
- 2 Medicine HematologyDocument78 pages2 Medicine HematologyAmitNo ratings yet
- Red Blood Cell AbnormalitiesDocument9 pagesRed Blood Cell AbnormalitiesIez FatihahNo ratings yet
- NCP HypothermiaDocument2 pagesNCP HypothermiaJohn Paolo Ocampo100% (3)
- The Perceived Self Ecological and Interpersonal Sources of Self Knowledge Emory Symposia in Cognition PDFDocument331 pagesThe Perceived Self Ecological and Interpersonal Sources of Self Knowledge Emory Symposia in Cognition PDFtsiky100% (1)
- Classification of Neutrophilic GranulocytesDocument1 pageClassification of Neutrophilic GranulocytesripangaNo ratings yet
- Hemocytometer ProtocolDocument1 pageHemocytometer ProtocolBiolab ProtocolsNo ratings yet
- Blood Grouping ReagentsDocument7 pagesBlood Grouping ReagentsDominic EmerencianaNo ratings yet
- Megaloblastic Anemia Testing AlgorithmDocument1 pageMegaloblastic Anemia Testing AlgorithmkatNo ratings yet
- 1-Deep Breathing and Cough Exercises - DosdosDocument6 pages1-Deep Breathing and Cough Exercises - DosdosBianca Mikaela DosdosNo ratings yet
- Anemia BMLTDocument134 pagesAnemia BMLTRajkishor YadavNo ratings yet
- CML, CLLDocument118 pagesCML, CLLMunesh SherawatNo ratings yet
- CBCDocument12 pagesCBCDaNa Al-jomah100% (1)
- Automation in HaematologyDocument67 pagesAutomation in Haematologyk11a1r18No ratings yet
- Anemia and Its Classification by DR Bashir Ahmed Dar A Sopore Kashmir 1228039135310976 9Document30 pagesAnemia and Its Classification by DR Bashir Ahmed Dar A Sopore Kashmir 1228039135310976 9hercolaniumNo ratings yet
- CBC Reviewer Anaphy LabDocument9 pagesCBC Reviewer Anaphy LabARVINE JUSTINE CORPUZNo ratings yet
- Sickle Cell TestDocument14 pagesSickle Cell TestkayNo ratings yet
- Basic Haematology Exercise 1 (MKEB2403)Document10 pagesBasic Haematology Exercise 1 (MKEB2403)kiedd_04100% (8)
- The Peripheral Blood FilmDocument5 pagesThe Peripheral Blood FilmanggaririnNo ratings yet
- Clearance and GFR: Major DR Arabinda Mohan Bhattarai Lecturer (Biochemistry), NAIHSDocument25 pagesClearance and GFR: Major DR Arabinda Mohan Bhattarai Lecturer (Biochemistry), NAIHSChandan SahNo ratings yet
- Chapter Two Anemiarev - ATDocument153 pagesChapter Two Anemiarev - ATAemro TadeleNo ratings yet
- Red Cell and White Cell Counting, BloodDocument89 pagesRed Cell and White Cell Counting, BloodJovel GangcuangcoNo ratings yet
- CYTOCHEMISTRY by Brian M. Denney, RMT: PhosphatasesDocument5 pagesCYTOCHEMISTRY by Brian M. Denney, RMT: PhosphatasesHarvy Halasan0% (1)
- At HemoglobinDocument2 pagesAt HemoglobinzulfiNo ratings yet
- HAEMOPOIESISDocument6 pagesHAEMOPOIESISDiyana ZahariNo ratings yet
- Reticulocyte Count StainDocument4 pagesReticulocyte Count StainGopikonda Leela RaniNo ratings yet
- CytochemistryDocument55 pagesCytochemistrySaad Zafar Awan100% (1)
- Blood Cells MorphologyDocument20 pagesBlood Cells Morphologymoonfire2009No ratings yet
- Haematology - Blood Films.Document6 pagesHaematology - Blood Films.kkkssbb100% (1)
- Cell Inclusions: John SantangeloDocument45 pagesCell Inclusions: John Santangelosaint5470No ratings yet
- RBC AnomalyDocument38 pagesRBC AnomalyTorillo KimNo ratings yet
- Basic Tech in Clinical ChimistryDocument84 pagesBasic Tech in Clinical ChimistryAhmed AboamerNo ratings yet
- RBC Micro-Macro MeasurementsDocument11 pagesRBC Micro-Macro MeasurementsDingdongLopezNo ratings yet
- 4 - HemoglobinopathiesDocument19 pages4 - HemoglobinopathiesHamzehNo ratings yet
- White Blood Cell DisordersDocument18 pagesWhite Blood Cell DisordersLucyellowOttemoesoeNo ratings yet
- Bismillah..a.yuli FlowcytometriDocument40 pagesBismillah..a.yuli FlowcytometriYuli RohmaNo ratings yet
- The Diagnostic Use of ADVIA 2120i Siemens and An "APL Criteria" CanDocument9 pagesThe Diagnostic Use of ADVIA 2120i Siemens and An "APL Criteria" CananggaririnNo ratings yet
- Blood ReportDocument39 pagesBlood Reportputri Mentari100% (1)
- Clinical Manifestations and Diagnosis of The Thalassemias - UpToDateDocument52 pagesClinical Manifestations and Diagnosis of The Thalassemias - UpToDatesushi37No ratings yet
- Renal Pathology: Kidney and The Urinary Collecting SystemDocument37 pagesRenal Pathology: Kidney and The Urinary Collecting Systemapplesncore100% (1)
- Cardiovascular + Clinical ScenariosDocument36 pagesCardiovascular + Clinical ScenariosapplesncoreNo ratings yet
- Gastrointestinal Tract (Partial Edit)Document47 pagesGastrointestinal Tract (Partial Edit)applesncoreNo ratings yet
- Pediatric PathologyDocument27 pagesPediatric PathologyapplesncoreNo ratings yet
- Pathology of The Lung Objectives: Define and Use in Proper Context The Following TermsDocument45 pagesPathology of The Lung Objectives: Define and Use in Proper Context The Following TermsapplesncoreNo ratings yet
- Pathology of Infections Objectives: ©bharti B-Pathologyof infections-SGUSOM 2Document25 pagesPathology of Infections Objectives: ©bharti B-Pathologyof infections-SGUSOM 2applesncoreNo ratings yet
- Fluid and Hemodynamic DisordersDocument19 pagesFluid and Hemodynamic DisordersapplesncoreNo ratings yet
- Gluteraldehyde, Peracetic Acid Alcohol, Iodophors QAC (Quarternary Ammonium Compounds)Document3 pagesGluteraldehyde, Peracetic Acid Alcohol, Iodophors QAC (Quarternary Ammonium Compounds)applesncoreNo ratings yet
- Neoplasia Path NotesDocument13 pagesNeoplasia Path NotesapplesncoreNo ratings yet
- Shift Work: A Risk Factor For Central Serous ChorioretinopathyDocument7 pagesShift Work: A Risk Factor For Central Serous ChorioretinopathysampleNo ratings yet
- IB Biology Option A: Human Nutrition and HealthDocument4 pagesIB Biology Option A: Human Nutrition and HealthDániel ZentayNo ratings yet
- CarbohydratesDocument6 pagesCarbohydratesNarasimha MurthyNo ratings yet
- Sclera AnatomyDocument21 pagesSclera AnatomyMiriam Mwangi100% (1)
- Alborz B7: Pooyandegan Rah SaadatDocument203 pagesAlborz B7: Pooyandegan Rah SaadatjuanmaraviNo ratings yet
- Analzying Sensory Somatic Responces - System Reinforcemnt Worksheet1Document2 pagesAnalzying Sensory Somatic Responces - System Reinforcemnt Worksheet1Creative DestinyNo ratings yet
- Osta Lecture 4 Notes Online ENGLISH Base of Skull and BrainDocument36 pagesOsta Lecture 4 Notes Online ENGLISH Base of Skull and BrainslyfoxkittyNo ratings yet
- Acid - Base BalanceDocument13 pagesAcid - Base Balanceadam yassineNo ratings yet
- Acute Physiology and Chronic Health Evaluation (APACHE II)Document15 pagesAcute Physiology and Chronic Health Evaluation (APACHE II)Syamsul PutraNo ratings yet
- Omron M6Document26 pagesOmron M6paninaro2011No ratings yet
- Bio1301 Lecture Note - Plant Section PDFDocument32 pagesBio1301 Lecture Note - Plant Section PDFAjomNo ratings yet
- Biopharmaceutics and Pharmacokinetics: S. Lakshmana Prabu, T.N.K. Suriyaprakash, K. Ruckmani and R. ThirumuruganDocument20 pagesBiopharmaceutics and Pharmacokinetics: S. Lakshmana Prabu, T.N.K. Suriyaprakash, K. Ruckmani and R. ThirumuruganAkshay PNo ratings yet
- Textbook: Molecular Biology of The Cell 6th Edition. by Alberts Et AlDocument5 pagesTextbook: Molecular Biology of The Cell 6th Edition. by Alberts Et Al김성한No ratings yet
- Proliferasi in Vitro Dan Aklimatisasi Pisang Kepok Unti Sayang (ABB) Dengan Penambahan Bahan OrganikDocument10 pagesProliferasi in Vitro Dan Aklimatisasi Pisang Kepok Unti Sayang (ABB) Dengan Penambahan Bahan Organiksarah khoerun nisaNo ratings yet
- Juvenile HormoneDocument17 pagesJuvenile HormonejugesmangangNo ratings yet
- Chapter 25 Biochemistry Chapter ObjectivesDocument10 pagesChapter 25 Biochemistry Chapter ObjectivesLyza KateNo ratings yet
- Meningitis Bacteriana (Revisión Bibliográfica) Bacterial Meningitis (Bibliography Revision) Dra. Ana Teresa Alvarado GuevaraDocument14 pagesMeningitis Bacteriana (Revisión Bibliográfica) Bacterial Meningitis (Bibliography Revision) Dra. Ana Teresa Alvarado GuevaraMartínCárdenasOyarseNo ratings yet
- Abdominal Compartment SyndromeDocument29 pagesAbdominal Compartment Syndromesgod34100% (1)