Professional Documents
Culture Documents
CO2 Removal Process Design Report
Uploaded by
Muhammad TauseefOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CO2 Removal Process Design Report
Uploaded by
Muhammad TauseefCopyright:
Available Formats
PROJECT
REPORT
June 2
2014
Design and simulation of CO2absorption
and stripping section for removal of co2.
1
ACKNOWLEDGEMENT
All praises to Almighty ALLAH who gave us light in darkness and gave us
understanding and ability to complete our report and all respects are for his
Prophet MUHAMMAD (PBUH, on whom be ALLAH,S blessings and
salutations)
I would like to thank PROF. DR. MAHMOOD SALEEM for granting me the
chance to pursue this Assignment in an environment that facilitated my
learning.
I take immense pleasure in thanking our worthy teacher for valuable help
regarding this assignment.
Abid Hussain Roll No. PG-M10-20
ICET University of the Punjab
new campus Lahore.
2
PLANT DESIGN ASSIGNMENT REPORTE ON
DESIGNING OF SYNTHESIS GAS PURIFICATION (CO2
removal) SECTION USING ACTIVATED-MDEA
3
4
PLANT DESIGN MID TERM ASSIGNMENT
REPORTE ON
DESIGNING OF SYNTHESIS GAS
PURIFICATION (CO2 removal) SECTION USING
ACTIVATED-MDEA
BSc. Engineering (7
th
Semester)
Submitted By: Abid Hussain
Roll No. PG-M10-20
Supervised by: Prof. Dr. Mahmood Saleem
Institute of Chemical Engineering and Technology
Faculty of Engineering & Technology
University of the Punjab
New Campus Lahore
5
ABSTRACT:
Ammonia is an important and useful for the production of urea. Since then it has been widely
used in the production of other chemicals, products and fertilizers specially. The synthesis
gas stream leaving the low temperature shift converter contains approximately 18.4 mole%
Carbon dioxide on a dry gas basis. It is essential to remove all CO2 from the synthesis gas
before entering the ammonia synthesis loop. Here we will use activated MDAE as an
absorbent for CO2 removal. Solution in our process.
Hence in the first chapter of this report I have given the introduction and contextual of CO2
gas. Removal process using aMDEA as solvent. The second chapter includes the detailed
process description of purification section, equipment working , feed and product
composition and conditions. The 3
rd
chapter contained detail material and energy balance
calculation using chemcad for all the process equipment to design Syn. Gas purification
section of ammonia plant.
I had 6 week training at FAUJI FERTILIZER COMPANY ,SADIQ ABAD, RAHIM YAR
KHAN . So the design data I used in my calculation is courtesy of FAUJI FERTILIZER
COMPANY. I design the process in following steps.1
st
, draw its flow sheet , list down all
required equipment and then I performed material and energy balance of the equipment and
of the whole process using CHEMCAD.
I hope that the reader will find the information contained to be useful.
6
Contents
Chapter 1 ................................................................................................................................................. 9
1.1 INTRODUCTION ................................................................................................................................. 9
1.2Synthesis Gas purification process:- .................................................................................................. 9
1.3Background of purification processes:- ............................................................................................. 9
1.4 ......................................................................................................................................................... 10
CHAPTER 2 ............................................................................................................................................ 11
PROCESS DESCRIPTION ..................................................................................................................... 11
2.1Absorption Column:- ................................................................................................................ 11
2.2Absorbent Regeneration (CO2stripping Section:- ................................................................... 12
2.2) Low pressure flashing column:- ............................................................................................. 12
2.3) Stripper :- .............................................................................................................................. 13
CHAPTER 3 ............................................................................................................................................ 14
MATERIAL AND ENERGY BALANCE ................................................................................................... 14
CHAPTER NO.4 ...................................................................................................................................... 17
4.1 Absorber Design: ......................................................................................................................... 17
Select between Plate & Packed column: ...................................................................................... 17
Factors affecting the absorption Column : ................................................................................... 18
Foaming ......................................................................................................................................... 18
Entrainment .................................................................................................................................. 18
Weeping/Dumping: ....................................................................................................................... 19
Flooding ......................................................................................................................................... 19
State of trays & Packing: ............................................................................................................... 19
Column Diameter: ......................................................................................................................... 19
Standard Design steps:.................................................................................................................. 19
1.Calculation of theoretical number of stages:
35
.............................................................................. 21
Calculation of Diameter of Column:
37
............................................................................................... 22
Calculation of Pressure drop: ............................................................................................................ 25
Down comer Design: ......................................................................................................................... 26
Entrainment Calculation: .................................................................................................................. 27
Calculation of Height of Column: ...................................................................................................... 28
4.2 Stripper Design: ............................................................................................................................... 28
7
Stripper ............................................................................................................................................. 28
Stripping Phenomenon: ................................................................................................................ 28
Stripping Agents: ........................................................................................................................... 28
Types of Stripper: .............................................................................................................................. 28
............................................................................................................. Error! Bookmark not defined.
Standard Design Steps: ................................................................................................................. 29
Calculation of Weeping Point: ...................................................................................................... 32
Calculation of Pressure drop: ........................................................................................................ 34
Calculation of Height of Column: .................................................................................................. 36
4.5 MDEA Surge Drum: ......................................................................................................................... 37
Design Specifications .................................................................................................................... 37
CHAPTER NO.5 ...................................................................................................................................... 38
Dynamic Simmulations. .................................................................................................................... 38
CHAPTER NO.6 ...................................................................................................................................... 39
6.1 Plant Cost Estimation: ............................................................................................................... 39
6.2 Capital Investment: ................................................................................................................... 40
6.2.1 Direct costs: ................................................................................................................... 40
6.2.2 Indirect costs: .................................................................................................................... 40
6.3 Types of Cost Estimation: ...................................................................................................... 40
6.4 Methods of Estimating Capital Investment: ......................................................................... 41
6.5 Percentage of Delivered Equipment Cost: ................................................................................ 41
Cost of Absorber: .......................................................................................................................... 41
7.6 Direct Cost:
50
.................................................................................................................................. 44
7.7 In-Direct cost: .................................................................................................................................. 44
OPERATIONAL PROBLEMS. ................................................................................................................... 45
7.1 Problems occurring during operation: .................................................................................. 45
7.2 Foaming:................................................................................................................................ 45
Causes of Foaming: ....................................................................................................................... 45
Prevention of Foaming: ................................................................................................................. 46
7.3 Corrosion: .................................................................................................................................. 46
Mechanism of Corrosion: .............................................................................................................. 47
Metods of Minimizing Corrosive Attacks: ..................................................................................... 47
7.4 Chemical Losses
54
.................................................................................................................. 47
7.5 Losses due to Volatility: ........................................................................................................ 48
8
7.6 Entrainment: ......................................................................................................................... 48
References: ........................................................................................................................................... 48
9
Chapter 1
1.1 INTRODUCTION
Absorption of CO2 from process gas because carbon dioxide gas present in
synthesis gas is poison for ammonia synthesis catalyst. In this section synthesis gas is
proceed to remove CO2 and CO, producing a high purity H2and N2. Bulk removal of carbon
dioxide is talented by the use of an improved benefield low heat process which uses the four
stage flash of the semi lean solution. to minimize external heat requirements. Final removal
of remaining CO2 and CO. is accomplished by catalytically converting the CO2 to methane
and water in the mathenator using hydrogen. The MDAE low heat process circulates an
aqueous sol. containing a nominal 37% MDAE and 3 % piprazine . This solution chemically
combines with CO 2 on the process gas but not significantly with the other voters. Additives
are injected into the solvent to enhance the CO2absorption rate, inhibit corrosion and to
control foaming.
1.2Synthesis Gas purification process:-
There is long history of different processes used for gas cleansing, here is given their
name just , while MDAE method will be discussed in detail.
1) MEA (mono ethanol amine ) process
2) Benfield process (also called Hot process)
3) Activated MDEA process
1.3Background of purification processes:-
In the early days of ammonia manufacture, monoethanolamine (MEA) was frequently used
for CO2 removal from the synthesis gas. Somewhat later, hot potassium carbonate (the
Benfield, or Hot Pot process) was used, often in a split flow configuration described as a two
stage Benfield Low Heat process for energy conservation. In the last 20 years, a very
substantial fraction of these plants have been retrofitted using BASFs a-MDEA process.
Activated MDEA as solvent:-
NMethyldiethanolamine (MDEA) is a tertiary amine whose amino group is incapable of
reacting with CO2. However, it is alkaline and so is an excellent sink for protons produce by
CO2hydrolysis. Because it is nonreactive, aqueous MDEA by itself absorbs CO2 far too
slowly to be an effective solvent for giving ammonia synthesis gas. But when spiked with a
relatively small attentiveness of piperazine, a diamine that reacts extraordinarily fast with
10
CO2, the resulting blend is an excellent solvent for treating syngas and removing CO2 in the
production of LNG.
In this paper, we first present the results of a measurable study of the piperazine promotion of
MDEA, specificsly the effects of piperazine to MDEA ratio, total amine strength, and the
treating temperature on presentation of a typical ammonia syngas CO2 removal system. In a
recent patent, Waner et al. (2009) proposed using the alkali metal salts of a number of tertiary
amino acids, appropriately promoted with reactive amines such as MEA. Some of the
potentially more intereting results of our study include the utility of operating at higher
temperatures with lower rather than higher total amine concentrations, and the existence of
operating boundaries that can lead to unstable operation when approached too closely.
Following a discussion of amino acids and their mode of operation, we critically analyze the
possibility of using the potasium salt of the tertiary aminoacid dimethylglycine, promoted
with piperazine as a syngas treating solvent. The results show that it is possible to treat
syngas quite effectively using such a solvent but with much lower concentration of the
piperazine promoter. Furtermore, results suggest that the crossexchanger commonly used as
a heat integration tool in treating plants can be completely eliminated.
1.4
aMDEA Composition:-
MDEA=37%
Piprazine=3%
and remaining is water
Advantages of using a-MDEA:-
There are many advantages of using -MDEA solution some of which most important are
More CO
2
absorption.
No time for regeneration.
Low energy requirement for regeneration.
In CO
2
removal section Amerel is used as antifoaming agent for a-MDEA solution.
11
CHAPTER 2
PROCESS DESCRIPTION
After the synthsis gas has been prepared it is purified off carbon dioxide and carbon
monoxide to yield a high purity nitrogen hydrogen synthesis gas. The CO2 removal system
consists of an Absober and a CO2 Stripper with the carbonate solution circulating in a closed
loop between the two.
2.1Absorption Column:-
Process gas leaving the top of CO
2
Absorber Feed Gas Separator is now called as raw
synthesis gas which enters the CO
2
absorber at the bottom. The internal packing at the
bottom part, BED3 and BED2 , is replaced from IMTP#50 to structured packing. The lean -
MDEA solution at 50
0
C from the lean rich exchanger and lean cooler enters the absorber
from the middle inlet.
While the two stream flow conter-current through the absorber, the lean solution gradually
absorbs the CO
2
present in the feed gas ,and leaves the absorber bottom as rich solution
stream at 48
0
C to LP flash column. The treated gas at 50
0
C exits the absorber from the top
through CO
2
absorber top knokout drum. Mist carry-over to the downstream equipment is
minimized by packing in the previous lean absorption section, which is now performing as
demister.
12
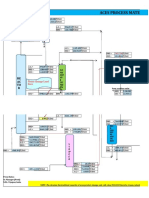
Process Flow Sheet:-
E-3
E-9
E-11 E-12 E-13 E-14 E-10
E-15
E-17
E-18
Sour gas
Rich MDAE
P-4
Regenerated MDAE
P-6
P-7
P-8
P-9
E-19
P-10
P-12
Pure syn. gass
E-20
P-14
P-15
Absorption column
Flashing column
stripper
reboiler
heater
Plate and frame heat
exchanger
Rich solution pump
FIG: 2.1 Purification section flowsheet.
2.2Absorbent Regeneration (CO2stripping Section:-
Stripping of CO
2
from a-MDEA solution, to regenerate solvent for recycling is carried out in
this section.
Regeneration takes place in following two steps.
Flashing in Low pressure flashing column and
Stripping in (Stripper)
2.2) Low pressure flashing column:-
The rich soltion at 85
0
C is flushed in the newly installed LP-Flash column at 0.9kg/cm
2
.Liquid from
LP flash column is pumped through to lean-rich exchanger where it is heated before going to the
stripper. In stipper after separation of CO
2
from the -MDEA solution, CO
2
product vapor is recycled
to the bottom part of LP flash column. CO
2
product vapor from LP flash and CO
2
vapors from stripper
is cooled to 38
0
C by direct contact cooling with quench or reflux water in a packed bed above the
striping secton of the LP flash column, quench water is circulated by the flash column Quinch Pump
13
to the LP flash quench cooler . In this exchanger the quench water is heat is rejected to the cooling
water, water condnsed from the CO2 product vapour during cooling is removed from the cooling
circuit to fulfil the water make up requirements. After being cooled the 99% CO
2
product passes
through the demister pad, exists the column and is exported for use in urea plant.
The main advantages of using LP Flash column are:
Chemically unbound CO
2
molecules removal.
No heat requirement like stripper.
2.3) Stripper :-
In LP flash column only chemical unbound molecules of CO
2
are removed, chemically bound
molecules are removed by using CO
2
stripper.
The a-MDEA solution containing chemically bound CO
2
molecules exiting from bottom of
LP flash column is sent to middle section of stripper being pumped by rich solution
pump.Steam for the purpose of striping is produced in steam generator and provision is made
to control steam supply from SH and SL heaters
Regenerated solution leaves and goes to Solution tank by gravity flow is used as
holding tank to provide residence time . The lean solution at 119
0
C from 117-F is then cooled
down sequentially in lean-rich exchanger and lean cooler to 50% with concentration, prior to
recycle into the absorber. Acid off gas and 114-C leaves striper from the top at 104
0
C is
directed to the bottom part of LP flash column.
CO2 gas separated from striper at 50C is pass through heat exchanger and cooled to 38C to
remove any entained water. Hence we obtained 99% CO2 which is transported to urea
section.
While absorber overhead gas, purified Syn. Gas , containing approx. 1000ppm of CO2 is
disengaged of any entrained liquid and preheated to about 316C , in methanator effluent
exchanger and finally sent to Methanator for further purification.
14
CHAPTER 3
MATERIAL AND ENERGY BALANCE
FlowDiagram.
CO2
aMDEA
15
aMDEA at inlet of absorber Sour gas inlet
Sweet gas outlet: stripper inlet
16
CO
2
outlet from stripper: Regenerated aMDEA
17
CHAPTER NO.4
4.1 Absorber Design:
Absorber
The removal of one or more seleced components from a mixture of gases by absorption into
a appropriate solvent.
Select between Plate & Packed column:
Vapor liquid mass transfer operation may be carried either in plate or packed column. These
two kinds of operation are quitelly different. The relative advantages of plate over packed
column are as follows:
1. Plate column are designed to deal wide range of liquid flow rates without flooding.
2. If a system contains solid contents; it will be handled in plate column, because solid will
accumulate in the voids, coating the packing materials and making it ineffective.
3. Dispersion difficulties are handld in plate column when flow rate of liquid are low as
compared to gases.
4. For large column heights, weight of the packed column is more than plate column.
5. If periodic cleaning is needed, man holes will be provided for cleaning. In packed
columns packing must be removed prier cleaning.
6. For non-foaming processes the plate column is preffered.
7. Design information for the plate column is more readily available and more reliable than
that for packed column.
8. Inter stage cooling can be provided to remove heat of reaction or solution in plate
column.
9. When temperature change is involved, packing may be damaged.
Choice of Plate Type:
There are three main types of plate, sieve plate, bubble cap and value plate. We have
selected sieve plate because:
18
1. They are lighter in weiht and less expensive. It is easier and cheaper to install.
2. Pressure drop is low as compared to valve and bubble cap plates.
3. Peak efficiency is generaliy high.
4. Maintenance cost is redced due to the ease of cleaning.
5. In case of capacity ratng, sieve plate has high rank as compared to valve and bubble
plates.
Sieve plate:
Sieve plate is simplest type of cross-flow plate. Vapour passes up through perforations in
the plate; and the liquid is retained on the plate by vapour flow. The perforations are usually
small holes, but larger holes and slots are used. The arrangement, number and size of the
holes are design parameters.
Because of their efficincy, wide operating range, ease of maintenance and cost factors, sieve
and valve trays have replaced the once highly thought of bubble cap trays in many
applications.
Factors affecting the absorption Column :
Vapor Flow Conditions:
1. Foaming
2. Entrainment
3. Weeping/dumping
4. Flooding
Foaming:
Foaming refers to the expansion of liquid due to passage of vapor or gas. Although it
provides high interfacial liquid-vapor contact, extreame foaming often leads to liquid
buildup on trays. In some cases, foming may be so bad that the foam mixes with liquid on
the tray above.
Whether foaming will occur depends primarily on physical properties of the liquid mixtures,
but is occasionally due to tray desins and condition. Whatever the cause, separation
efficiency is always reduced.
Entrainment:
Entrainment refers to the liquid carried by vapour up to the tray above and is again caused
by high vapor flow rates. It is negative because tray efficiency is reduced: lower volatile
material is carried to a plate holdng liquid of higher volatility. It could also contaminate high
purity distillate. Excessive entrainent can lead flooding.
19
Weeping/Dumping:
This phenomenon is caused by low vapor flow. The pressure exerted by the vapor is
insufficient to hold up the liquid on the tray. Therefore, liquid starts to leak through
perforations. Excessive weeping will lead to dumping. That is the liquid on all trays will crash
(dump) through to the base of the column (via a domino effect) and the column will have to
be re-started. Weeping is indicated by a sharp pressure drop in the column and reduced
separation efficiency.
Flooding:
Flooding is brought about by excessive vapour flow, causing liquid to be entrained in the
vapor up the column. The increased pressure from excessive vapor also backs up the liquid
in the down comer, causing an increase in liquid holdup on the plate above. Depending on
the degree of flooding, the extreme capacity of the column may be severely reduced.
Flooding is detecting by sharp increases in column differential pressure and significant
decrease in separation efficiency.
State of trays & Packing:
Remember that the actual number of trays required for a particular separation duty is
determined by the efficiency of the plate. Thus, any factors that cause a decrease in tray
efficiency will also change the perfomance of the column. Tray efficiencies are effected by
fouling, wear and tear and corrosion and the rates at which these occur depends upon the
properties of the liquids being proessed. Thus appropriate materials should be specified for
tray construction.
Column Diameter:
Vapor flow velocity is dependent on column diameter. Weeping determines the minimum
vapor flow required while flooding determines the maximum vapor flow allowed, hence
column capacity. Thus, if the column diameter is not sized properly, the column will not
perform well
Standard Design steps:
1) Calculation of theoretical number of stages.
2) Calculation of actual number of stages.
3) Calculation of diameter of column.
4) Calculation of weeping point.
5) Calculation of pressure drop.
6) Downcomer design.
7) Entrainment calculations.
8) Calculation of height of column.
20
ABSORBER DESIGN
21
1.Calculation of theoretical number of stages:
35
The main componnt which we want to be absorbed in MDEA is H
2
S.so, we take it as a
reference.
H
2
S:
In = 3.73
Out = .0109
Moles of H
2
S absorbed = 3.719
E
ai
=
= 0.997 or 99.7%
Minimum
for H
2
S.
(
)
min
= K
i
E
ai
L =
lean oil entring absorber.
V
n+1
=
rich gas entering absorber.
Value of K depends on T & P.
So, average tower conditions for k
i
:
T = 110 F
P =
= 433psia.
36
K
i
= 1.6
So, (
)
min
= 1.6 x 0.997
= 1.5952.
Operating (
)
= 1.25 (1.5952)
= 1.994.
Operating absorption factor
A
io
= (
22
=
= 1.246.
Theoretical stages at operating conditions.
E
ai
= A
io
N+1
A
io
/ A
io
N+1
1
0.997 = (1.246)
N+1
1.246 / (1.246)
N+1
1
(N+1) log 1.246 = log (
)
(N+ 1)(0.0995) = 1.919
N = 19.09.
It means 19 theoretical trays are needed.
1. Calculation of Actual Number of stages:
We take 70% efficiency.
So,
Actual number of stages =
= 27 stages.
Calculation of Diameter of Column:
37
Flooding velocity is given by
U
f
= K
1
Where,
U
f
= Flooding vapor velocity in m/s , base on net column cross-sectional area.
K
1
= Constant obtained from figure 11.27 vol.6 Coulson & Richardson .
F
LV
=
Where,
L
w
=Liquid mass Flow rate ,
V
w
= Vapour mass Flow rate ,
In this Case,
23
L
w
= 148.045
V
w
= 17.18
P
v
= 21.47
[
]
P
L
= 1001.48
F
LV
=
= 1.26
We use Plate Spacng 700mm.
38
K
1
= .034
Then, U
F
= 0.034
= 0.23
We take actual velocity as 85% of flooding velocity
So,
v
= 0.85 x 0.23
= 0.20
Maxium volumetric vapor flow rate =
=0 .80
Net area required = A
n
=
= 4 m
2
We take dwncomer area as 12% of total area
Column cross sectional area = A
c
=
= 4.55 m
2
Down comr area = A
d
= 4.55 -4 = .55m
2
Active area , bubbling area = A
a
= A
c
2 A
d
= 4.55 2(0.55)
24
= 3.45 m
2
Total hole aea as 10% of active area , so
Hole area = A
H
= 0.10 x 3.45 = 0.345 m
2
Column diameter = D
c
=
= 2.40 m.
2. Calculation of Weeping Point:
For the calcuation of weeping point, hole diameter must be selected so that at lowest
operation rate, the vapor flow velocity is still above weeping point.
Maximum liquid flowrate = 148.045
Minimum liquid rate , at 70% turn down = 0.70 x 148.045
= 103.631
x 100 =
x 100 = 12%
39
= 0.77
l
w
= 0.77 x 2.40 = 1.85m
we know
h
ow
= 750 *
+
2/3
L
w
= weir length, m
h
ow
= height over weir , mm liquid
L
w
= liquid flow rate
Minimum h
ow
= 750 [
]
2/3
= 109mm.
We take , h
w
= 50mm
25
h
w
+ h
ow
= 109 + 50 = 159mm.
40
K
2
= 31.2
U
h(min)
= [
]
U
h
= minimum vapor velocity through holes, m/s
D
h
= hole diameter, mm
U
h
=
]
U
h
= 0.77m/s
Actual mimum vapor velocity =
= 1.62 m/s.
So, minium operating rate will be well above weeping point.
Calculation of Pressure drop:
= 9.81 x 10
-3
h
t
P
L
= total pressure drop , P
a
(N/m
2
)
h
t
= total pressure drop , mm liquid
Total presure drop is giver by
h
t
= h
d
+ (h
w
+ h
ow
) + h
r
h
t
= total plate presure drop
h
d
= dry plate pressure drop
h
r
= resdual head
h
w
= height of weir
h
ow
= weir crest, mm liquid
h
d
= 51 [
]
2
C
o
= Ofice coefficient
26
U
h
= Vapor velocity through holes , m/s
U
h
=
= 2.32 m/s.
We tak carbon steel plate, so
plate thickness = 5mm
hole diameter = 5mm
so,
41
C
o
= 0.84
h
d
= 51 [
]
2
[
]
= 8.34 mm
h
r
=
= 12.8mm
h
t
= 12.48 + 8.34 + 50 + 109
= 179m liquid
= 9.81 x 10
-3
x 179 x 1001.48
= 1757 Pa
= 0.26 Psia (per plate)
Down comer Design:
The downomer area and the plate spacing must be such that the level of the liquid and froth
in the downomer is well below the top of outlet weir on the plate above. If the liquid rises
above the outlet weir the column will flood.
h
b
= (h
w
+ h
ow
) + h
t
+ h
dc
Where,
h
b
= downcomer bckup, measured from plate surface, mm
h
dc
= head loss in doncomer, mm
27
h
dc
= 166 [
]
where,
h
dc
= head loss in downomer, mm
L
wd
= liquid flowrate in dwncomer, kg/s
A
m
= Either downcomer area or clearance area under the downcomer A
op
which is smaller.
A
op
= h
op
L
w
Where, h
op
= height of botom edge of apron above plate
L
w
= length of weir
h
op
= h
w
10
= 50 10 = 40mm
So, A
op
= 0.040 x 1.85m
= 0.074m
h
dc
= 166 [
]
2
= 6.62mm
So, backup in downcomer = h
b
= (50 +109) +6.62 + 179
= 34..62mm = 0.33462m
Then , backup in downcoer < (plate spacing + weir height)
0.33462 < (0.700 + 0.50)
0.33462 < 0.375
So, plate efficiency is acceptable.
Entrainment Calculation:
For checking entrainment , we calculate
U
v
=
U
v
=
= 0.2 m/s
% flooding =
28
=
= 86%
We already know F
LV
F
LV
= 1.26
42
It is well below 0.1, so there is no chance of entrinment and process is satisfactory.
Calculation of Height of Column:
No. of plates = 27
Tray spacing = 0.70m
Tray thickness = 0.005m
Total thickness of trays = 0.135m
Top clearance = 1m
Bottom clearance = 1m
Total height = 20m
4.2 Stripper Design:
Stripper:
It is a counter current multi-stage separation column, with liquid feed at top and vapor
feed at the bottom stage.
Stripping Phenomenon:
Strippng is a mass transfer operation that involves the transfer of a solute (as H
2
S & CO
2
in
our case) from the liquid phase to the gas phase.
Stripping Agents:
Air
Stream
Inert gas
Hydocarbon gases
Reboled vapors (as in our case)
Types of Stripper:
i. Refluxed Stripper:
It is emloyed if simple stripping is not sufficient to achieve the desired separation and
contacting trays are needed above the feed tray.
29
ii. Reboiled Stripper:
If the botom product from a stripper is thermally stable, it may be Reboiled at the bottom of
the column.
iii. Open steam/Air stripper:
Direct stearm may also be used. Sometimes air or inert gases may also be used
(Combination of above can be made based on systems requirement)
Principle of separation: difference in volatilities
Created or added phase: vapor
Separating agent: stripping vapor
Standard Design Steps:
Calculation of
1) Theortical number of stages.
2) Actual number of stages.
3) Diamter of column.
4) Weeping point.
5) Pressure drop.
6) Downomer design.
7) Entrainment calculations.
8) Height of column
1. Calculation of theoretical number of stages:
43
The main component which we want to be stripped from MDEA is H
2
S. So, we take it as a
refence.
Let us supose that 100% of H
2
S is not stripped and very minute quantities remains in the
lean MDEA coming back from Stripper.
30
Fraction of H
2
S stripped = E
si
= 0.998
Minimum V/L for H
2
S = (
)
min
=
Value of K depends on T & P.
So, average tower conditions for value of k
i
:
T = 230 F
P = 26psia.
36
Ki = 35
So, (
)
min
=
= 0.0285
Operating (
)
= 1.25 (0.0285)
= 0.0356.
Operating striping factor
S
i
= (
. K
i
= 0.0356 x 35
= 1.246.
Theoretical stags at operating conditions.
E
Si
= S
i
N+1
S
i
/ S
i
N+1
1
0.998 = (1.246)
N+1
1.246 / (1.246)
N+1
1
(N+1) log 1.246 = log (
)
(N+ 1)(0.0955) = 2.0969
N = 20.78
It means 21 theoretcal trays are needed.
2. Calculation of Actual Number of stages:
We take 70% efficiency.
So,
31
Actual number of stages =
= 30 stages.
3. Calculation of diameter of column:
37
Flooding velocity is given by
U
f
= K
1
Where,
U
f
= Floodig vapor velocity in m/s , base on net column cross-sectional area.
K
1
= Constant obtaind from figure 11.27 vol.6 Coulson & Richardson .
F
LV
=
Whre,
L
w
=Liquid mass Flow rate ,
V
w
= Vapur mass Flow rate ,
In this case
In this Case,
L
w
= 315
V
w
= 20.38
P
v
= 1.96
[
]
P
L
= 935.24
F
LV
=
= 0.71
We use Pate Spacing 800mm.
38
K
1
= .05
Then, U
F
= .054
32
= 1.8
We take actul velocity as 85% of flooding velocity
So, U
v
= 0.85 x 1.18
= 1.00
Maximum volmetric vapor flow rate =
=10.4
Net area required = A
n
=
= 0.4 m
2
We take downcomer areaas 12% of total area
Column cross sectional are = A
c
=
= 11.82 m
2
Down comer area = A
d
= 11.2 10.4 = 1.42m
2
Active area , bubbling area = A
a
= A
c
2 A
d
= 11.82 2(1.42)
= 8.98 m
2
Total hole area as 10% of activ area , so
Hole area = A
H
= 0.10 x 8.98 = 0.898 m
2
Column diameter = D
c
=
= 3.88 m.
Calculation of Weeping Point:
For the calculation of weepig point, hole diameter must be selected so that at lowest
operation rate, the vapor flow velcity is still above weeping point.
Maximum liquid flowrate = 315
Minimum liquid rate , at 70% turn down = 0.70 x 315
33
= 220.5
x 100 =
x 100 = 12%
39
= 0.77
l
w
= 0.77 x 3.88 = 2.99m
we know
h
ow
= 750 *
+
2/3
L
w
= weir length, m
h
ow
= height over weir , mm liquid
L
w
= liquid flow rate
Minimum h
ow
= 750 [
]
2/3
= 138mm.
We take , h
w
= 50mm
h
w
+ h
ow
= 138 + 50 = 188mm.
40
K
2
= 31.2
U
h(min)
= [
]
U
h
= minimum vapor velocity through holes, m/s
D
h
= hole diameter, mm
U
h
=
]
U
h
= 6.17m/s
Actual minimum vapor velocity =
= 8.11 m/s.
So, minimum operating rate will be well above weeping point.
34
Calculation of Pressure drop:
= 9.81 x 10
-3
h
t
P
L
= total pressure drop , P
a
(N/m
2
)
h
t
= total pressure drop , mm liquid
Total pressure drop is giver by
h
t
= h
d
+ (h
w
+ h
ow
) + h
r
h
t
= total plate pressure drop
h
d
= dry plate pressure drop
h
r
= residual head
h
w
= height of weir
h
ow
= weir crest, mm liquid
h
d
= 51 [
]
2
C
o
= Orifice coefficient
U
h
= Vapor velocity through holes , m/s
U
h
=
= 11.6 m/s.
We take carbon steel plate, so
plate thickness = 5mm
hole diameter = 5mm
so,
41
C
o
= 0.84
h
d
= 51 [
]
2
[
]
= 20 mm
h
r
=
= 13mm
35
h
t
= 188 + 13 + 20
= 221mm liquid
= 9.81 x 10
-3
x 221 x 935.24
= 2027.6 Pa
= 0.29 Psia (per plate)
4. Downcomer Design:
The downcomer area and the plate spacing must be such that the level of the liquid and
froth in the downcomer is well below the top of outlet weir on the plate above. If the liquid
rises above the outlet weir the column will flood.
h
b
= (h
w
+ h
ow
) + h
t
+ h
dc
Where,
h
b
= downcomer backup, measured from plate surface, mm
h
dc
= head loss in downcomer, mm
h
dc
= 166 [
]
2
where,
h
dc
= head loss in downcomer, mm
L
wd
= liquid flowrate in downcomer, kg/s
A
m
= Either downcomer area or clearance area under the downcomer A
op
which is smaller.
A
op
= h
op
L
w
Where, h
op
= height of bottom edge of apron above plate
L
w
= length of weir
h
op
= h
w
10
= 50 10 = 40mm
So, A
op
= 0.040 x 2.99m
= 0.120m
h
dc
= 166 [
]
2
36
= 13.07mm
So, backup in downcomer = h
b
= (50 +138) +13.07 + 221
= 422.07mm = 0.422m
Then , backup in downcomer < (plate spacing + weir height)
0.422 < (0.800 + 0.50)
0.422 < 0.425
So, plate efficiency is acceptable.
5. Entrainment Calculation:
For checking entrainment , we calculate
U
v
=
U
v
=
= 1 m/s
% flooding =
= 85%
We already know F
LV
F
LV
= 0.71
42
It is well below 0.1, so there is no chance of entrainment and process is satisfactory.
Calculation of Height of Column:
No. of plates = 30
Tray spacing = 0.800m
Tray thickness = 0.005m
Total thickness of trays = 0.15m
Top clearance = 1m
Bottom clearance = 1m
Total height = 25m
37
4.5 MDEA Surge Drum:
Flow rate of MDEA = 2349334.04 Ib/hr
Density of MDEA at 160
o
F = 64.6 Ib/ft
3
Basis 24 hours
Vol. of MDEA for 24 hr = 2349334.04 x 24/64.6 = 872817.6 ft
3
Total vol. of vessel = vol. of MDEA + 10% allowance
= 872817.6 x 1.10 = 960099.36 ft
3
Let us suppose that 0.3% of MDEA solution is slipped in the surge tank
=960099.36 *0.03=2858.29 ft
3
=81m
3
V = d
2
h/4
Let, h/d = 3 or h = 3d
[1]
V = 3 d
3
/4
From here, d = 3.25m so, h=9.75 m
Design Specifications
Time of operation = 24hr
Dia. Of vessel = 3.25m
Height of Vessel = 9.75m
Recommended material of instruction is carbon steel.
38
CHAPTER NO.5
Dynamic Simmulations.
39
CHAPTER NO.6
6.1 Plant Cost Estimation:
As the process design is completed it becomes possible to make accurate cost estimation
because detailed specification can thus be obtained from various manufactures. However
no design project should proceed to the final stages before costs are considers and the cost
estimation should be made throughout all the early stages of the design when complete
specifications are not available. Evaluation of costs in the preliminary design is said pre
design cost estimation. Such estimation should be capable of providing a basis for company
management to decide whether or not further capital should be invested in the project.
An evaluation of costs in the preliminary design phase is sometimes called as guess
estimation and often rule of thumb are used. A plant design obviously must present a
process that is capable of operating under conditions which will yield a profit.
A capital investment is required to any industrial process, and determination of necessary
investment is an important part of plant design project. The total investment for any
40
process consists of physical equipment and facilitates in the plant plus the working capital
for money which must be available to pay salaries. Keep raw materials and products on
hand and handle other special items requiring a direct cash layout.
6.2 Capital Investment:
Before industrial plant can be put into operation, large amount of money must be supplied
to purchase and install the necessary machinery and equipment, land services facilitates
must be obtained and plant must be erected, complete with all pipe control services. In
addition it is necessary to have money available for payment of expenses involved in plant
operation.
The capital needed to supply the necessary manufacturing and plant facilities is called fixed
capital. Fixed cost capital investment while necessary for the operation of the plant termed
as Working Capital. The sum of fixed capital investment and the working capital is known as
total capital investment.
Fixed capital investment classified into two subdivisions: namely
Direct costs
Indirect costs
6.2.1 Direct costs:
The direct cost items are incurred in the construction of planet in addition to the cost of
equipment:
Purchase equipment
Purchase equipment installation
Instrumentation
Piping
Electrical Equipment and materials
Building (including services)
Service facilities
Taxes
6.2.2 Indirect costs:
These include:
Design and engineering
Contractors expanses
Contractors fee
Contingency
6.3 Types of Cost Estimation:
Various methods are employed for estimating capital investment are as follows:
41
Preliminary estimate
Definitive estimate
Detailed estimate
In choosing the method for cost estimation following factors are considered:
Amount of detailed information available
Accuracy desired
Time spent on estimation
6.4 Methods of Estimating Capital Investment:
Seven methods of estimating capital investment are outlined below:
Detailed item estimate
It cost estimate
Percentage of delivered equipment cost
Lang factor for approximation of capital investment
Power factor applied to plant capacity ratio
Investment cost per capacity
Turnover ratio
6.5 Percentage of Delivered Equipment Cost:
This method for estimating the fixed or total capital investment requires determination of
the delivered equipment cost. The other items included in the total direct plan cost are then
estimated as Percentage of Delivered Equipment Cost.
The percentage used in making an estimation of this type should be determined on the basis
of type of process involved, design complexity required, material of construction, location of
the plant, past experiences, and other items depend on the particular unit under
consideration.
Purchased equipment cost for common plant equipment = C
e
= E
C
e
= a + b (S)
n
Where a & b are cost constants
S = size parameter
n = exponent for that type of equipment
Cost of Absorber:
We know that
42
C= a+b(S)
n
Diameter of Absorber = 2.4 m
sizing factor (S) = 2.4
a = 110, b = 380, n= 1.8
46
so,
C = 110+380(2.4)
= $1947 ( Cost/Tray)
Total cost of one absorber = 1947 x 27
= $52569
As we have used two absorber, so
Cost of both Absorbers = $52569 x 2
= $105138
This cost is for year 2007, so by applying inflation rate of 3.3% per year, we can find cost in
2013.
C = 105138(1.033)
6
= $127750.21
1. Cost of Exchanger:
We know
C= a+b(S)
n
Sizing parameter of exchanger (S) = 584 m
2
a = 1350, b = 180, n = 0.95
47
so,
C = 1350+180(584)
0.95
= $73313
This cost is for year 2007, so by applying inflation rate of 3.3% per year, we can find cost in
2013.
43
C = 73313(1.033)
6
= $89080.
2. Cost of Inlet Gas Separator:
Diameter = D = 2.06 m
Length = L = 6.34 m
From Graph
48
C = 22000
Pressure Factor = 1.4 (at 30 psia)
So,
C = 22000 x 1.4 = $30800
This cost is for year 2004, so by applying inflation rate of 3.3% per year, we can find cost in
2013.
C = 30800(1.033)
9
= $41253.
3. Cost of Lean Solvent pump:
Capacity of Pump = 3919 GPM
From Graph
C = $25000
This cost is for year 1988, so by applying inflation rate of 3.3% per year, we can find cost in
2013.
C = 25000(1.033) = $49436.
4. Cost of MDEA Surge Tank:
Capacity of tank = 81 m
3
We know that
C = C= a+b(S)
n
a = 5000, b = 1400, n = 0.7 (cone roof)
49
so, C = 5000+1400(81)
0.7
= $35343
44
This cost is for year 2004, so by applying inflation rate of 3.3% per year, we can find cost in
2013.
C = 35343(1.033)
9
= $47337.
E = $354856
7.6 Direct Cost:
Component
% of E Cost ($)
Purchased Equipment Installation 0.25E 88714
Instrumentation installation 0.07E 24840
Piping 0.08E 28388
Electrical 0.05E 17723
Building 0.05E 17723
Yard improvement 0.02E 7097
Service facilities 0.15E 53228
Land 0.01E 3549
Total direct cost = D = 241262
7.7 In-Direct cost:
Engineering & Supervision = 0.33E = $117102 (1)
Construction Expenses = 0.41E = $145490 (2)
Total in-direct Cost = $262592
Total Cost = direct Cost + indirect cost
= $503855
Contactors Fee = X = 0.05 (D+I) = $12063
Contingency = Y = 0.10 (D+I) = $24126
Fixed Capital Investment = (D+I+X+Y) = $540043
Working Capital Investment = 0.15(D+I+X+Y) = $81006
45
OPERATIONAL PROBLEMS.
7.1 Problems occurring during operation:
One of the reasons that alkanolamine processes have become the predominant choice for
both refinery gas giving and natural gas purification is their comparative freedom from
operating difficulties. However, several factors can result in undue expense and cause
difficulties in the operation of alkanolamine units. Chief among these, from an economic
standpoint are corrosion and amine loss. Other operating problems, which occasionally limit
the capacity of plant for gas purification, include foaming and plugging of equipment. In
many cases, operation can be significantly improved by daily monitoring of key plant
operating variables and by proper control and design of treating plant.
7.2 Foaming:
Foaming of alkanolamine solution is perhaps the most common operating problem in amine
treating units. It is most frequently encountered in contractor, but may also occur in the
stripping column.
Causes of Foaming:
Specific cause of foaming includes the following:
Water soluble surfactants in the feed gas (e.g. well treating mixes, pipeline corrosion
inhibitors) which lowers the amine metaphors surface tension. Excessive antifoam can
also cause foaming.
Liquid hydrocarbons e.g. entrained compressor lubricating oils in the feed gas or
hydrocarbons condensation within the amine absorber.
Particulate contaminants (e.g. mill scale, FeS correction products, rust contained in the
feed gas or produced within amine treating units. Solids such as FeS do not cause
foaming but concentrate at liquid/gas interface and stabilize the foam by increasing the
surface viscosity retarding film drainage.
Oxygen adulteration of feed gas or amine unit (usually at the amine sump or amine
storage tank) and reation of amine heat stable salts. Dissolved iron can catalyze the
reaction of amine with oxygen to foam carboxylic acid.
Feed gas adulteraction such as carboylic acid, which react with amine to form heat
stable salts.
Contamination of amine unit with gases and oils during a turnaround.
Amines filter elements that have been washed with surfactants or contaminated with
oils during manufactue.
Contaminants in the amine plant makeup water such as boiler feed water treating
chemicals and corrosion inhibitors.
46
Prevention of Foaming:
Foaming can be reduced or controlled by proper care of the amine solution. The
following techniqes reduce the amine solution contamination and minimize foaming:
A properly desined feed gas inlet separator and filter should be provided. A feed gas
coalscer should be considered for feed gas stream contaminated with compressor
lubricating oils and other finally dispersed aerosols. A properly size slug catcher should
be provided if slgs can accumulate in the feed gas line.
A feed gas water wash should be considered when the feed gas streams is severely
contaminated wth carboxylic acid or water soluble, surface active pollutes. A feed gas
water wash can also remove aerols and ultra fine chemicals.
Onsite of offsie amine solution recovering to remove heat stable salts and amine
degradation poducts. No more than 10% of the amine should be tied up as stable salts.
Caustic additin to neutralize heat stable salts to mitigate corrosion and thereby reduce
iron sulfite formation.
A properly sized rich amine flash drum remove entrained and dissolved hydrocarbons.
Liquid skimming facilitate in the absorber sump, the rich amine flash drum, the
regenerator sump and the amine regenerator overhead accumulator.
New plants and old plants that have undergone a major turnaround or often
contaminated with oils, greases welding fluxes and corrosion inhibitors. A hot caustic
wash (2-5 wt% caustic soda) followed by a hot condensate wash can remove these
impurities and help to prevent foaming.
Addition of antifoam is carried out.
7.3 Corrosion:
By far the most serious operating problem encountred with amine gas purification
process is corrosion as would be expected this problem has been given widest attention.
Generally, it occurs in regenerator heat exchanger and pumps. The extent and type of
corrosion has been observed to depend upon such factors as the amine used, the
presence of impurities in the solution leading with acid gas, the temperature and
pressure, predominant in various part of the plant, the velocity with which the solution
flows and others. However, it appears that the principal corroding agents are the add
gases. The rate of corrosion growths with increase acid gas concentration n solution.
Corrosion due to hydrogen sulfide and carbon dioxide is frequently observed a filter
shell and the hot end heat exchanger tubes. To minimize corrosion by hydrogen sulfide
and carbon dioxide, the acid gases must be shell in a relatively corrosive form until
regeneration of amine solution is stripping still
Overloading the amine solution will increasethe casual for corrosion due to pressure
discount or high temperature in the heat exchanger. This danger can be remedied
bymaintaining adequate pressure on the amine solution and by operating the unit at as
low and acid gas alkanolamine ratio as possible. This ration should not exceed 0.05
47
moles of acid gas per mole of alkanolamne and should be event less of condition
licences
Mechanism of Corrosion:
It is known that free or aggressive carbon dioxide causes severe corrosion particularly at
elevated temperature and in the presence of water
It is believed that the metallic iron with carbondi acid which results in the formation of
stable iron bicarbonate. Further heating of solution any cause the release of carbon dioxide
and the precipitate of the iron as the relatively insolule carbonate.
Hydrogen sulfide attacks steel as an acid with the subsequent formation of insoluble ferrous
sulfite. This compound forms a coating on the metal surface which does not adhere tightly
and therefore affords little protection from further corrosion. There is no satisfactory
correlation available for carbon dioxid hydrogen sulfide mixture, which relates the corrosive
attacks to be probable with any givenratio of hydrogen sulfide to sulfur dioxide.
However, certain generalized obseration has been made. It appears that in plant handling
predominantly carbon dioxide, ver small extent of hydrogen sulfide may actually reduce
corrosion. On the other hand, eac of the acid gases growths the corrosive attacks of the
other
Methods of Minimizing Corrosive Attacks:
Corosion can be reduced by various methods, including certain protection in the operation
ad process design of purification plants. Use of more expensive corrosion resistant material
nd continues or periodic removal of corrosion promoting agent from the solution.
Acombination of several of these measures usually leads to most satisfactory and
e\conomical to reduce corrosion attacks;
The temperature of the solution in the reboiler and the temperature of the steam
usedin the reboiler should be kept as low as proble.
Use of high temperature heat carrying media, uch as oil, should be avoided to maintain
the lowest possible skin temperature of metal
Pressure regenerator with its supplemenry high temperatures results in severe corrosion
of reboiler tubes; it is, therefore, good ractice to maintain the lowest possible pressure
on the stripping column and reboiler
To prevent oxygen from entering te system, it is prudent to maintain a blanket of inlet
gas over all serving of the solutin, which could be exposed to atmosphere and to ensure
the pressure the suction side ofall pumps.
Continuous removal of suspeded solids (by nitration) and the decomposition product (by
distillation of a side stream) enerally helps to reduce corrosion.
7.4 Chemical Losses
The loss of a solvent can bea serious operating difficulty in alkanolamine gas purification
plants. Corrosion can be icurred by entrainment of the solution in the gas stream
48
vaporization or chemical dgradation of the amine. Loss of the solvent by entrainment or
vaporization is undesirable \not only because of the cost of chemicals but also because of
the contamination of the ipelines by liquid deposited on the walls. In addition when
alkanolamine solution are ued to purify the gas to be used in catalytic process, entrainment
by vaporization of solvet esult in a serious poisoning of the catalyst.
7.5 Losses due to Volatility:
Glycol volatility losses are usually significant in ethylene glycol, di-ethylene glycol but very
less in tri-ethylne and higher glycols, which have very high boiling points. Hence usually a
very small amunt of glycol is lost by evaporation into gas stream in absorbers and also in
regeneators.
Prevention from Volatility Losses:
Volatility losses can be prevented by following methods:
A cold water flux is convyed at the top plate of regenerating column.
Normally absorbers are operted at lower temperatures (80-110
0
F recommended) to
avoid losses.
7.6 Entrainment:
In many cases most of the glycol loss occurs as carry over of solution with the product gas.
Entrainment losses are fashined either by inefficient mist withdrawal or by foaming and
subsequent carry over solution. Entrainment losses from glycol absorber vary significantly
contingent on the mechanical design of both the upper solution of absorber and mist
elimiation devices.
Prevention from Entrainment Losses:
Entrainment can be mi diminishd by the following techniques:
Using efficient mist eliminaton equipment.
Application of the foam inhibitor.
References:
1. ARTHUR KOHL AND RICHARD NELSON, Gas Purification, Edition 5th.
2. Basic Principles and Calculations in Chemical Engineering.7th Edition.
3. Coulson & Richardson's Chemical Engineering - Volume 6.
4. GAS PROCESSORS SUPPLIERS ASSCIATION, Engineering Data Book
49
You might also like
- Absorber Design FinalDocument27 pagesAbsorber Design FinalTamara NwaserNo ratings yet
- Stamicarbon Urea Process Data PDFDocument1 pageStamicarbon Urea Process Data PDFtreyzzztylerNo ratings yet
- Mass Transfer and Absorbers: International Series of Monographs in Chemical EngineeringFrom EverandMass Transfer and Absorbers: International Series of Monographs in Chemical EngineeringRating: 4.5 out of 5 stars4.5/5 (3)
- Simulation of Methanol Production From Biomass Gasification in Interconnected Fluidized BedsDocument9 pagesSimulation of Methanol Production From Biomass Gasification in Interconnected Fluidized BedsKelly TorresNo ratings yet
- Fixed-Bed Reactor Design and Diagnostics: Gas-Phase ReactionsFrom EverandFixed-Bed Reactor Design and Diagnostics: Gas-Phase ReactionsRating: 4 out of 5 stars4/5 (5)
- Final Year Design Project Thesis Report Session 2018Document153 pagesFinal Year Design Project Thesis Report Session 2018RiholoNo ratings yet
- 2008 Morikawa TEC IFA ACES21 Advanced Urea Production Technology - 2Document15 pages2008 Morikawa TEC IFA ACES21 Advanced Urea Production Technology - 2MubasharNo ratings yet
- REACTOR DESIGN FOR AMMONIA OXIDATIONDocument5 pagesREACTOR DESIGN FOR AMMONIA OXIDATIONabdul rehmanNo ratings yet
- Aces Process Material Balance: RE AC TO RDocument4 pagesAces Process Material Balance: RE AC TO Rwaheed ahmadNo ratings yet
- The Reaction of CO2 With Ethanolamines PDFDocument4 pagesThe Reaction of CO2 With Ethanolamines PDFekmagisNo ratings yet
- Graduation-Project - Sulfuric AcidDocument195 pagesGraduation-Project - Sulfuric AcidMuntazer QasimNo ratings yet
- Amine Unit Start UpDocument11 pagesAmine Unit Start UpthinkpadNo ratings yet
- Reactor DesignDocument9 pagesReactor DesignKin Wai CheahNo ratings yet
- Natural Gas As Feedstock For Fertilizer: A Thesis Submitted in Partial Fulfillment of The Requirements For The Degree ofDocument65 pagesNatural Gas As Feedstock For Fertilizer: A Thesis Submitted in Partial Fulfillment of The Requirements For The Degree oframachandran_chemNo ratings yet
- Ammonia MEB Final PDFDocument30 pagesAmmonia MEB Final PDFMANU BTech MCA Third YearNo ratings yet
- HCl Gas Absorption ProcessDocument2 pagesHCl Gas Absorption Processsundhar100% (2)
- Internship ReportDocument39 pagesInternship ReportNaumanTahir100% (1)
- Ammonia Energy 2520 BalanceDocument7 pagesAmmonia Energy 2520 Balanceapi-3714811No ratings yet
- Material BalanceDocument6 pagesMaterial BalanceMehran Rasheed GorayaNo ratings yet
- Calculating Multiplication Factors for Converting Oleum to Sulphuric AcidDocument12 pagesCalculating Multiplication Factors for Converting Oleum to Sulphuric AcidYalamati Satyanarayana100% (1)
- 2 Thermodynamic Property Methods in Aspen PlusDocument10 pages2 Thermodynamic Property Methods in Aspen PlusNorman_Mpofu21100% (1)
- CD4061 No Es PDFDocument25 pagesCD4061 No Es PDFFernando AmoresNo ratings yet
- Material and Energy BalanceDocument9 pagesMaterial and Energy BalanceSana100% (1)
- Final ReportDocument46 pagesFinal ReportVarun Gupta100% (1)
- Integrated Low Pressure Methanol ProcessDocument20 pagesIntegrated Low Pressure Methanol ProcessKhalidMadaniNo ratings yet
- Bubble Column ReactorDocument21 pagesBubble Column ReactorMuhammad Hamzah SyahrirNo ratings yet
- Material and Energy BalanceDocument28 pagesMaterial and Energy Balancemuhammad arslan100% (1)
- AICHE Coal Gasification ReportDocument23 pagesAICHE Coal Gasification ReportMuzzy VoraNo ratings yet
- Urea Plant Material Balance (ACES Process)Document7 pagesUrea Plant Material Balance (ACES Process)muks19950% (2)
- The Use of MDEA and Mixtures of Amines For Bulk CO2 RemovalDocument9 pagesThe Use of MDEA and Mixtures of Amines For Bulk CO2 RemovalTrùm Dầu Mỏ BkNo ratings yet
- Design of Gas Absorber For The Exhaust Gases of Ammonia PlantDocument11 pagesDesign of Gas Absorber For The Exhaust Gases of Ammonia PlantVan LimNo ratings yet
- (MEA) CO2 Capture PFDsDocument95 pages(MEA) CO2 Capture PFDsfNo ratings yet
- Amine Absorber Equipment SpecificationDocument7 pagesAmine Absorber Equipment SpecificationGracylla RoseNo ratings yet
- Absorption Stripping StagesDocument61 pagesAbsorption Stripping StagesAzmi Musa100% (1)
- Proposal For UreaDocument24 pagesProposal For UreaUmar ZamanNo ratings yet
- Nirbhay Urea Final PDFDocument99 pagesNirbhay Urea Final PDFHimanshu vikram100% (1)
- SWEETENING (Chemical Absorption) PresentationDocument54 pagesSWEETENING (Chemical Absorption) Presentationarsalan amirpour75% (4)
- Absoeber Striper Final ReportDocument27 pagesAbsoeber Striper Final ReportMuneebNo ratings yet
- Design of packed absorber column for multi-component gas scrubbingDocument104 pagesDesign of packed absorber column for multi-component gas scrubbingNana kwadwoNo ratings yet
- Amine Process Simulation Model DevelopmentDocument9 pagesAmine Process Simulation Model DevelopmentacetilenaNo ratings yet
- Plant Design For Methanol Distillation Unit: February 2021Document51 pagesPlant Design For Methanol Distillation Unit: February 2021maged1998100% (1)
- MATERIAL BALANCE TITLEDocument46 pagesMATERIAL BALANCE TITLEG Vamsee KrishnaNo ratings yet
- Economic Aspects of Setting Up Purge Gas Recovery Unit (PGRU) With Ammonia Production ProcessDocument7 pagesEconomic Aspects of Setting Up Purge Gas Recovery Unit (PGRU) With Ammonia Production ProcessWilly ChandraNo ratings yet
- Urea - Kirk Othmer PDFDocument15 pagesUrea - Kirk Othmer PDFusman_hafeez86No ratings yet
- Urea PDFDocument11 pagesUrea PDFSteve WanNo ratings yet
- Brian JR Geoffroy CHNG 3012 Part II PDFDocument103 pagesBrian JR Geoffroy CHNG 3012 Part II PDFjanelle ramdahinNo ratings yet
- Experiment 4a – Pressure Drop in Packed ColumnsDocument21 pagesExperiment 4a – Pressure Drop in Packed ColumnsMohamad Samer KansouNo ratings yet
- Urea Plant Material Balance ACES ProcessDocument5 pagesUrea Plant Material Balance ACES ProcessSTEFAN TOTHNo ratings yet
- Methane Syngas Methanol MicroprocessingDocument14 pagesMethane Syngas Methanol MicroprocessingAtieyNoryhati-dzNo ratings yet
- Design of AbsorberDocument9 pagesDesign of Absorberhaseeb tahir50% (2)
- Packed Bed SO3Document112 pagesPacked Bed SO3Michelle MendozaNo ratings yet
- CHE 425 Engineering Economics and Design Principles NotesDocument93 pagesCHE 425 Engineering Economics and Design Principles NotesAudrey Patrick Kalla50% (2)
- Manufacture of UreaDocument86 pagesManufacture of UreamohamedNo ratings yet
- Cre Una PDFDocument164 pagesCre Una PDFChetana PatilNo ratings yet
- Material Balance of Ammonium Sulphate ProductionDocument5 pagesMaterial Balance of Ammonium Sulphate ProductionShahbaz AlamNo ratings yet
- Allyl Chloride Production PDFDocument4 pagesAllyl Chloride Production PDFmarisolNo ratings yet
- Mass ConverterDocument18 pagesMass ConverterDinesh CR7No ratings yet
- Process Flow Diagram For Ammonia SynthesisDocument6 pagesProcess Flow Diagram For Ammonia SynthesisHanan Ahmed Ibrahim100% (1)
- Sound Level 42 Cabin and All Areas of Ammonia-IIDocument2 pagesSound Level 42 Cabin and All Areas of Ammonia-IIMuhammad TauseefNo ratings yet
- 09 HyC10Document10 pages09 HyC10Muhammad TauseefNo ratings yet
- 10 OctaneBenzeneManagmDocument16 pages10 OctaneBenzeneManagmMuhammad TauseefNo ratings yet
- 09 HyC10Document10 pages09 HyC10Muhammad TauseefNo ratings yet
- 01 StrategicBusinessDocument2 pages01 StrategicBusinessMuhammad TauseefNo ratings yet
- Investigatory Report WewDocument7 pagesInvestigatory Report WewStephen GaleonNo ratings yet
- Schematic For Potable Water SystemDocument17 pagesSchematic For Potable Water Systemmkchy12No ratings yet
- MCQ CH 2 ElectrochemistryDocument2 pagesMCQ CH 2 ElectrochemistryGaurav SonarNo ratings yet
- Stainless Steel Molecular Etching PDFDocument6 pagesStainless Steel Molecular Etching PDFp.designNo ratings yet
- MInirhizotron ThecniquesDocument16 pagesMInirhizotron ThecniquesHector Estrada MedinaNo ratings yet
- 91988v00 Modeling Industrial Chemical Processes With Matlab and Simulink 04 PPDocument4 pages91988v00 Modeling Industrial Chemical Processes With Matlab and Simulink 04 PPEdenson Flores TrujilloNo ratings yet
- Structure and Bonding (Chapter 3) Exam Questions: 141 Minutes 141 MarksDocument34 pagesStructure and Bonding (Chapter 3) Exam Questions: 141 Minutes 141 Marksrejymol100% (1)
- Applications of Polymer Gels in Tissue Engineering, Drug Delivery and MoreDocument18 pagesApplications of Polymer Gels in Tissue Engineering, Drug Delivery and MorePathik ShahNo ratings yet
- Cylinder Design Standards WSNZDocument5 pagesCylinder Design Standards WSNZjamilNo ratings yet
- Catálogo SKODADocument14 pagesCatálogo SKODAruben colqueNo ratings yet
- Aggregate Impact Value TestDocument6 pagesAggregate Impact Value Testnadz_fynazNo ratings yet
- Introduction To Medical TechnologyDocument22 pagesIntroduction To Medical TechnologyKaycee Gretz LorescaNo ratings yet
- Unit 3 Exam-SolutionsDocument8 pagesUnit 3 Exam-SolutionsbrunosipodNo ratings yet
- MTDKDocument9 pagesMTDKraviteja1840No ratings yet
- Measurement of Leaf Water Potential: by The Dye MethodDocument5 pagesMeasurement of Leaf Water Potential: by The Dye MethoderuditeramanaNo ratings yet
- Questionnaire On Positive Isolation StandardDocument11 pagesQuestionnaire On Positive Isolation StandardBata JenaNo ratings yet
- Lab Report: ME2151-2 MetallographyDocument4 pagesLab Report: ME2151-2 Metallographyandy100% (1)
- Bradford Method Protein Assay GuideDocument3 pagesBradford Method Protein Assay GuideDoreliaNo ratings yet
- Light in August EssayDocument7 pagesLight in August Essayppggihnbf100% (2)
- Purifying Biodiesel: Comparing Ion Exchange and AdsorptionDocument77 pagesPurifying Biodiesel: Comparing Ion Exchange and AdsorptionFabianMurielRuizNo ratings yet
- Milipore CleanlinessDocument76 pagesMilipore Cleanlinesswendypost73No ratings yet
- Injections and Implanted Drug ProductsDocument3 pagesInjections and Implanted Drug ProductsHAROLD TANNo ratings yet
- RHA ResumeDocument2 pagesRHA ResumeJames McFarlaneNo ratings yet
- Plusco428 Wireline Products 28 Vis Honey Oil With Inhibitor For Pressure ApplicationDocument7 pagesPlusco428 Wireline Products 28 Vis Honey Oil With Inhibitor For Pressure ApplicationebeNo ratings yet
- External Diffusion Effects On Heterogeneous Reactions: A. Sarath BabuDocument59 pagesExternal Diffusion Effects On Heterogeneous Reactions: A. Sarath BabuboiroyNo ratings yet
- Carbohydrate As Biology Answers OCR AQA EdexcelDocument4 pagesCarbohydrate As Biology Answers OCR AQA EdexcelbekoNo ratings yet
- Experiment - 5 Raymond Classifier: Name: Aman Agrawal Roll No:18CH30003Document6 pagesExperiment - 5 Raymond Classifier: Name: Aman Agrawal Roll No:18CH30003akshay agrawalNo ratings yet
- Army Public School Bhopal: TOPIC:-" "Document20 pagesArmy Public School Bhopal: TOPIC:-" "Gourav Pathariya100% (1)
- Pressure Force and Area RAGDocument3 pagesPressure Force and Area RAGruuki0% (2)
- Chemistry Aqa A Level AlkenesDocument21 pagesChemistry Aqa A Level AlkenesAttec OinotnaNo ratings yet