Professional Documents
Culture Documents
Cardiac Toxic Responses
Uploaded by
Daismar ArenasOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cardiac Toxic Responses
Uploaded by
Daismar ArenasCopyright:
Available Formats
CARDIAC TOXIC RESPONSES
The ultimate functional effect of cardiac toxic manifestations is decreased cardiac output and
peripheral tissue hypoperfusion, resulting from alterations in biochemical pathways, energy
metabolism, cellular structural and function, electrophysiology, and contractility of heart. These
morphological and functional alterations induced by toxic exposure are referred as toxicologic
cardiomyopathy.
Drugs or xenobiotics can directly cause both heart failure and heart hypertrophy. Under severe
acute toxic insults, myocardial death becomes the predominant response leading to cardiac
dilation and heart failure. In most cases, myocardial survival mechanisms can be activated so
that myocardial apoptosis is inhibited. The survived cardiomyocytes often become hypertrophy
through activation of calcium-mediated fetal gene expression and other hypertrophic program. If
toxic insult continues, the counter-regulatory mechanism against heart hypertrophy such as
activation of cytokine-medicated pathways eventually lead to myocardial cell death through
apoptosis or necrosis, dilated cardiomyopathy, and heart failure.
In the study of cardiac toxicology, the manifestations of cardiac toxicity in human patients and
animal models are critical parameters serving as indices of cardiac toxicity. These manifestations
are expressed in the forms of cardiac arrhythmia, hypertrophy, and heart failure. These abnormal
changes reflect myocardial functional alterations resulting from both acute and chronic cardiac
toxicity.
CARDIAC ARRHYTHMIA
Cardiac rhythms under physiological conditions are set by pacemaker cells that are normally
capable of developing spontaneous depolarization and responsible for generating the cardiac
rhythm, the so-called automatic rhythm. A cardiac rhythm that deviates from the normal
automatic rhythm is called cardiac arrhythmia, often manifested in the form of tachycardia (fast
heart rate). There are several classes of tachycardia, including sinus tachycardia, atrial
tachycardia, ventricular tachycardia, and torsade de pointes (TfP) (a life-threatening
tachycardia). In addition, subclasses such as atrial fibrillation, atrial flutter, and accelerated
idioventricular rhythm provide further description of manifestations of arrhythmia.
CARDIAC HYPERTROPHY
There are two forms of cardiac hypertrophy: concentrentic hypertrophy, which is often observed
during pressure overload and is characterized by new contractile-protein units assembled in
parallel resulting in a relative increase in the width of individual cardiac myocytes. By contrast,
eccentric hypertrophy is characterized by the assembly of new contractile-protein units in series
resulting in a relatively greater increase in the length than in the width of individual myocytes,
occurring in human patients and animal models with dilated cardiomyopathy. TOXICOLOGIC
CARDIOMYOPATHY IS OFTEN MANIFESTED IN THE FORM OF ECCENTRIC
HYPERTROPHY.
The development of cardiac hypertrophy can be divided into three stages:
Developing hypertrophy during which period the cardiac workload exceeds cardiac
output
Compensatory hypertrophy in which the workload/mass ratio is normalized and normal
cardiac output is maintained
Decompensatory hypertrophy in which ventricular dilation develops and cardiac output
progressively declines, and overt heart failure occurs.
HEART FAILURE
A traditional definition of heart failure is the inability of heart to maintain cardiac output
sufficient to meet the metabolic and oxygen demands of peripheral tissues. This definition has
been modified to include changes in systolic and diastolic function that reflect specific
alterations in ventricular function and abnormalities in a variety of subcellular processes. Forms
of heart failure include:
1. Low output vs High output
a. Low output condition where in metabolic demand of the body is within normal
limits but the heart in unable to meet them.
b. High output condition where in the metabolic demand of the body are high and the
heart is unable to meet them (i.e Hyperthyroidism, anemia)
2. Left sided vs Right sided
a. Left sided The heart cannot adequately pump blood from the left ventricle, therefore
resulting to accumulation of blood in that area. Its manifestations include, nocturnal
dyspnea, dry wheezing cough, exertional fatigue and weakness, and nocturia.
b. Right sided The heart is unable to pump blood from the right ventricle, therefore
resulting blood accumulation in that area. It includes tightness and swelling of the
skin, nausea and vomiting, anorexia, bloating and abdominal pain.
ACUTE CARDIAC TOXICITY
Acute cardiac toxicity is referred to as cardiac response to a single exposure to a high dose of
cardiac toxic chemicals. It is often manifested by cardiac arrhythmia and myocardial apoptosis. It
is difficult to measure acute cardiac toxicity. Taking for instance, a single high dose of arsenic
can lead to cardiac arrhythmia and sudden death, which is easy to measure, however, a single
oral dose of monensin (20mg/kg) leads to diminished cardiac function progressing to heart
failure requires a long-term observation. Exposure can directly lead to heart failure, which is
different from an often-observed hypertrophy response, which may or may not progress into
heart failure.
CHRONIC CARDIAC TOXICITY
It is the cardiac response to long-term exposure to chemicals, which often manifested by cardiac
hypertrophy and the transition to heart failure. About 25% of human patients with
cardiomyopathy are recognized as having idiopathic cardiomyopathy. Recognition of chronic
cardiac toxicity in the pathogenesis of cardiomyopathy is clinical relevance, and this knowledge
can be used to prevent and treat patients with toxicologic cardiomyopathy.
BIOMARKERS FOR CARDIAC TOXICITY
Myocardial injury can be divided into two major classes: structural and nonstructural injuries.
The structural damage of the heart includes cell death and the associated histopathological
changes such as myocardial infarction. Functional deficits often accompany the structural injury.
Nonstructural damage represents functional deficits without apparent structural alterations.
Myocardial adaptation to intrinsic and extrinsic stress leading to myocardial structural changes
such as hypertrophy should be in the category of structural damage because the progression of
hypertrophy leads to heart failure in which cell death is a major determinant factor. Myocardial
structural changes and functional alterations can be indirectly measured by echocardiography
and electrocardiogram in combination with stress testing. The data generated from these
measurements can be considered in a broad sense as biomarkers. However, in clinical practice
and experimental approach, biomarkers are referred to asindexes of myocardial injury measured
from blood samples. The fundamental principle of the biomarkers is that molecules that are
released from the myocardium under various injury conditions are readily detectable from blood
samples.
Availability of Biomarkers
Currently validated biomarkers that are included in clinical diagnostic testing guidelines are all
related to myocardial structural injury. Developing biomarkers for nonstructural injury is most
challenging and demands implantation of more advanced technologies such as functional
genomics and proteomics. In addition, currently available biomarkers have limitations, although
they are useful.
Creatine Kinase There are three major CK isoenzymes identified; CK-MM is the principal form
in skeletal muscle, CK-MB presents in myocardium in which CK-MM is also found, and CK-BB
is the predominant form in brain and kidney. Elevation of serum CKMB is considered a
reasonably specific marker of acute myocardial infarction.
Myoglobin Myoglobin is found in all muscle types and its value as a biomarker of myocardial
injury is based on the fact that serum concentrations of myoglobin increase rapidly following
myocardial tissue injury, with peak values observed 14 hours after acute myocardial infarction.
Elevation of serum myoglobin is likely reflective of the extent of myocardial damage.
B-Type Natriuretic Peptide BNP is a cardiac neurohormone secreted by the ventricular
myocardium in response to volume and pressure overload, and the release of BNP appears to be
directly correlated with the degree of ventricular wall tension. BNP is now accepted as a
biomarker for congestive heart failure and is included in the European guideline for the diagnosis
of chronic heart failure.
C - reactive protein The acute phase reactant CRP is a marker of systemic and vascular
inflammation, which appears to predict future cardiac events in asymptomic individuals. In
particular, inflammation has been shown to play a pivotal role in the inception, progression, and
destabilization of atheromas. A predictive value of CRP for the prognosis of coronary heart
disease is thus proposed. The measurement of CRP appears to provide additional prognostic
information when cTnT is measured at the same time.
Cardiac Troponins Cardiac troponin T (cTnT) and I (cTnI) are constituents of the myofilaments
and expressed exclusively in cardiomyocytes. It is thus of absolute myocardial tissue specificity.
In healthy persons, serum cTnT or cTnI are rarely detectable. Therefore, any measurable
concentrations of serum cTnT or cTnI reflect irreversible myocardial injury such as myocardial
infarction. The clinical experience has arrived at a recommendation that cTn measurement
becomes the gold standard for diagnosis of acute myocardial infarction.
CARDIAC TOXIC CHEMICALS
Many substances can cause cardiac toxic responses directly or indirectly. However, only
chemicals that primarily act on the heart or whose cardiac toxicity is the primary concern should
be categorized as cardiac toxic chemicals. Clinically, the most recognized toxicologic
cardiomyopathy is found in alcoholic heart muscle disease, which is often referred to as
alcoholic cardiomyopathy (ACM). In this section, ACM will be discussed as a prototype of
toxicologic cardiomyopathy. However, cardiomyopathy induced by therapeutic drugs is also well
recognized, but it is often referred to as cardiomyopathy induced by specific chemicals, such as
anthracycline cardiomyopathy. In this context, the chemicals that cause cardiac toxicity can be
classified in multiple ways, enumerating some, it includes: (1) pharmaceutical chemicals, (2)
natural products, and (3) environmental and industrial chemicals.
ALCOHOL AND ALCOHOLIC CARDIOMYOPATHY
ACM has been recognized for a long time and the prevalence of ACM in selected populations
ranges from 23% to 40%. Alcohol is believed to be the causal chemical in up to 40% of all
patients with nonischemic, dilated cardiomyopathy. ACM is characterized by an increase in
myocardial mass, dilation of the ventricles, wall thinning, ventricular dysfunction, and heart
failure. The pathogenesis of ACM is not completely understood. However, it is clear that the
duration of heavy alcohol use in patients is a critical factor. The pathogenesis of heart failure
begins after an index event such as alcohol-induced cardiac muscle injury that produces an initial
decline in pumping capacity of the heart. Following this initial decline, a variety of
compensatory mechanisms are activated,including the adrenergic nervous system, the renin
angiotensin system, and the cytokine system. Some of these compensatory changes have been
detected in alcoholic. However, with time, the sustained activation of these systems can lead to
secondary end-organ damage within the ventricle by activating and accelerating the left ventricle
remodeling and subsequent cardiac decompensation, resulting in the transition from
asymptomatic to symptomatic heart failure. It was proposed that the metabolite acetaldehyde is
responsible for some of the cardiac injury associated with ethanol consumption. The metabolic
enzyme responsible for the conversion of ethanol to acetaldehyde is alcohol dehydrogenase,
which is absent in cardiac myocytes. Studies have indicated that the impaired liver function of
alcoholics may be sufficient to generate quantities of acetaldehyde that can reach the heart. The
direct effects of acetaldehyde on the myocardium include inhibition of protein synthesis,
inhibition of Ca2+ sequestration by the SR, alterations in mitochondrial respiration, and
disturbances in the association of actin and myosin.
PHARMACEUTICAL AGENTS
Antiarrhythmic Agents Antiarrhythmic drugs have historically been classified based upon a
primary mechanism of action: Na+ channel blockers (class I), -adrenergic blockers (class II),
drugs that prolong action potential duration, especially K+ channel blockers (class III), and Ca2+
channel blockers (class IV). However, this classification is artificial because most of the drugs
have multiple mechanisms of action.
Class I antiarrhythmic agents are primarily Na+ channel blockers, including disopyramide,
encainide, flecainide, lidocaine, mexiletine, moricizine, phenytoin, procainamide, propafenone,
quinidine, and tocainide. Blockade of cardiac Na+ channels results in reduction of conduction
velocity, prolonged QRS duration, decreased automaticity, and inhibition of triggered activity
from delayed afterdepolarizations or early after depolarizations. The primary concern of Na+
channel blocker toxicity is that proarrhythmic effects are seen at a much higher incidence in
those patients with a previous history of myocardial infarction or with acute myocardial
ischemia. The proarrhythmic effects of these drugs would also be more prevalent in patients with
other cardiac complications.
Class II antiarrhythmic drugs are -adrenergic receptorblocking drugs, including acebutolol,
esmolol, propranolol, and sotalol. The catecholamines increase contractility, heart rate, and
conduction through activation of -adrenergic receptors in the heart. These effects can be
explained by increased adenylyl cyclase activity, increased cyclic AMP, activation of protein
kinase A, and phosphorylation and activation of L-type Ca2+ channels, thereby increasing
intracellular Ca2+, and particularly the amplitude of the Ca2+ transient. Therefore, antagonists of
-adrenergic receptors in the heart lead to effects that are opposite that of catecholamines, and
are useful for the treatment of supraventricular tachycardia. The main adverse cardiovascular
effect of -adrenergic receptor antagonists is hypotension. These drugs may also exacerbate AV
conduction deficits (e.g., heart block) and promote arrhythmias during bradycardia.
Class III antiarrhythmic drugs are primarily K+ channel blockers. These drugs include
amiodarone, bretylium, dofetilide, ibutilide, quinidine, and sotalol. Blockade of K+ channels
increases action potential duration and increases refractoriness. Prolonged action potential
duration contributes to the development of early after depolarizations and promotion of
tachycardia, especially polymorphic ventricular tachycardia (torsades de pointes). The most
noticeable adverse effect of these drugs is QT prolongation and torsadogenesis. Most of the
drugs in this class also affect other ion channels and/or receptors. Amiodarone and quinidine also
block Na+ channels, whereas sotalol inhibits -adrenergic receptors in the heart. Amiodarone
prolongs action potential duration and effective refractory period of Purkinje fibers and
ventricular myocytes, and the most common adverse cardiovascular effect of amiodarone is
bradycardia. Amiodarone may also have cardiotoxic effects by stimulating excessive Ca2+
uptake, especially in the presence of Procaine.
Class IV antiarrhythmic drugs are Ca2+ channel blockers and include diltiazem and verapamil.
The dihydropyridine Ca2+ channel blockers are not used to treat arrhythmias because they have
a greater selectivity for vascular cells; however, these drugs may also alter cardiac ion
homeostasis when plasma concentrations of the drugs are elevated. The dihydropyridines interact
with Ca2+ channels in the inactivated state of the channel, and because vascular smooth muscle
resting potentials are lower than cardiac cells, the time spent in the inactivated state is relatively
longer in vascular smooth muscle, thus providing some preference of dihydropyridines for the
vasculature. Bepridil, verapamil, and diltiazem exert negative inotropic and chronotropic effects.
These drugs also exert a negative chronotropic effect, thus they may produce bradycardia. In
contrast, the dihydropyridine Ca2+ channel blockers typically induce a reflex tachycardia
subsequent to peripheral vascular dilation and baroreceptor reflex leading to increased
sympathetic outflow from the medulla.
Cardiac glycosides (digoxin and digitoxin) are inotropic drugs used for the treatment of
congestive heart failure. Ouabain is a cardiac glycoside commonly used in the laboratory for
electrophysiological experiments in cardiac myocytes. The mechanism of inotropic action of
cardiac glycosides involves inhibition of Na+, K+-ATPase, elevation of intracellular Na+,
activation of Na+/Ca2+ exchange, and increased availability of intracellular Ca2+ for
contraction. Consequently, cardiotoxicity may result from Ca2+ overload, potentially including
reduction in resting membrane potential (less negative), delayed afterdepolarizations, and
premature ventricular contraction or ectopic beats. The principal adverse cardiac effects of
cardiac glycosides include slowed AV conduction with potential block, ectopic beats, and
bradycardia. During overdose, when the resting membrane potential is significantly altered and
ectopic beats are prevalent, ventricular tachycardia may develop and can progress to ventricular
fibrillation.
Sympathomimetics Catecholamines represent a chemical class of neurotransmitters synthesized
in the adrenal medulla (epinephrine and norepinephrine) and in the sympathetic nervous system
(norepinephrine). These neurotransmitters exert a wide variety of cardiovascular effects. Because
of their ability to activate- and -adrenergic receptors, especially in the cardiovascular system, a
number of synthetic catecholamines have been developed for the treatment of cardiovascular
disorders and other conditions such as asthma and nasal congestion. Inotropic and chronotropic
catecholamines used to treat bradycardia, cardiac decompensation following surgery, or to
increase blood pressure (e.g., hypotensive shock) include epinephrine, isoproterenol, and
dobutamine, and these drugs typically display nonselective activation of adrenergic receptors.
More selective 2-adrenergic receptor agonists used for bronchodilatory effects in asthma
include albuterol, bitolterol, fenoterol, formoterol, metaproterenol, pirbuterol, procaterol,
salmeterol, and terbutaline. High oral doses of albuterol or terbutaline or inhalation doses (i.e.,
enhanced delivery to the stomach instead of the lungs with subsequent systemic absorption) of
these drugs may lead to nonselective activation of 1-adrenergic receptors in the heart with
subsequent tachycardia. Sympathomimetic drugs that is more selective for - adrenergic
receptors include the nasal decongestants ephedrine, phenylephrine, phenylpropanolamine, and
pseudoephedrine. As with the asthma drugs, at high doses these nasal decongestants can produce
tachycardia, and a number of deaths have been reported.. Of particular interest is the high
concentration of ephedra alkaloids that may be present in some herbal remedies or
neutraceuticals, especially in products containing ma huang. Tachycardia may occur from the
consumption of large amounts ofephedra alkaloids, which may predispose the myocardium to
ventricular arrhythmias.
Antracyclines and other anti-neoplastic agents Cardiotoxicity is recognized as a serious side
effect of chemotherapy for malignant cancers, especially with well-known antitumor agents such
as doxorubicin, daunorubicin, 5-fluorouracil, and cyclophosphamide.
Anthracyclines (doxorubicin or Adriamycin and daunorubicin) are widely used antineoplastic
drugs for the treatment of breast cancer, leukemias, and a variety of other solid tumors.
Unfortunately, the clinical usefulness of these drugs is limited because of acute and chronic
cardiotoxic effects. The acute effects mimic anaphylactictype responses, such as tachycardia and
various arrhythmias. These effects are usually manageable and most likely are due to the potent
release of histamine from mast cells sometimes observed in acute dosing. In addition, large acute
doses can also cause left ventricular failure. The greatest limiting factor of the anthracyclines is
associated with long-term exposure, which usually results in the development of
cardiomyopathies and, in severe cases, congestive heart failure.
5-Fluorouracil Clinical evidence of 5-fluorouracil cardiotoxicity ranges from mild precordial
pain and ECGabnormalities (ST segment elevation, high peaked T waves, T-wave inversions,
and sinus tachycardia) to severe hypotension, atrial fibrillation, and abnormalities of ventricular
wall motion. The mechanism of cardiotoxicity of fluorouracil is unknown, but it may relate to
impurities present in commercial products of the drug, one of which is metabolized to
fluoroacetate, a compound that might participate in fluorouracil induced cardiotoxicity.
Cyclophosphamide High doses of cyclophosphamide given to cancer or transplant patients may
lead to severe hemorrhagic cardiac necrosis. The mechanism of the cardiotoxicity of this drug is
not clear, but there is suggestive evidence that the toxic metabolite of cyclophosphamide, 4-
hydroperoxycyclophosphamide, may alter the ion homeostasis in cardiac myocytes, resulting in
increased Na+ and Ca2+ content and reduced K+ levels
Centrally acting drugs
Some of central nervous system (CNS)-acting drugs have considerable effects on the
cardiovascular system, including tricyclic antidepressants (TCAs), general anesthetics, some of
the opioids, and antipsychotic drugs.
TCAs including amitriptyline, desipramine, doxepin, imipramine, and protriptyline have
significant cardiotoxic effects, particularly in cases of overdose. The effects of TCAs on the heart
include ST segment elevation, QT prolongation, supraventricular and ventricular arrhythmias
(including torsades de pointes), and sudden cardiac death. In addition, as a result of peripheral -
adrenergic blockade, TCAs cause postural hypotensionthe most prevalent cardiovascular
effect. Furthermore, the risk of TCA induced cardiotoxicity is significantly enhanced in children
and by concomitant administration of other drugs that alter ion movement or homeostasis in the
heart (e.g., the Na+ channel-blocking class I antiarrhythmic agents), or use in patients with
cardiovascular disease.
Antipsychotic drugs include the phenothiazines (acetophenazine, chlorpromazine, fluphenazine,
mesoridazine, perphenazine, thioridazine, and trifluoperazine), chlorprothixene, thiothixene, and
other heterocyclic compounds (clozapine, haloperidol, loxapine, molindone, pimozide, and
risperidone). As with TCAs, the most prominent adverse cardiovascular effect of antipsychotic
drugs is orthostatic hypotension. However, the phenothiazines (e.g., chlorpromazine and
thioridazine) may exert direct effects on the myocardium, including negative inotropic actions
and quinidine-like effects.
General anesthetics as exemplified by enflurane, desflurane,halothane, isoflurane,
methoxyflurane, and sevoflurane have adverse cardiac effects, including reduced cardiac output
by 2050%, depression of contractility, and production of arrhythmias (generally benign in
healthy myocardium but more serious in cardiac disease. These anesthetics may sensitize the
heart to the arrhythmogenic effects of endogenous epinephrine or to -receptor agonists.
Halothane has been found to block the L-type Ca2+ channel by interacting with dihydropyridine-
binding sites, to disrupt Ca2+ homeostasis associated with the SR, and to modify the
responsiveness of the contractile proteins to activation by Ca2+ (Bosnjak, 1991).
Propofol is an intravenously administered general anesthetic that also decreases cardiac output
and blood pressure.
Local anesthetics
In general, local anesthetics have few undesirable cardiac effects. However, when high systemic
concentrations of cocaine and procainamide are attained, these chemicals may have prominent
adverse effects on the heart.
Cocaine acts as a local anesthetic agent by blocking conduction in nerve fibers through
reversibly inhibiting Na+ channels and stopping the transient rise in Na+ conductance. In the
heart, cocaine decreases the rate of depolarization and the amplitude of the action potential,
slows conduction speed, and increases the effective refractory period. The other major
pharmacologic action of cocaine is its ability to inhibit the reuptake of norepinephrine and
dopamine into sympathetic nerve terminals (sympathomimetic effect). Cocaine also, indirectly
through its actions on catecholamine reuptake, stimulates - and -adrenergic receptors, leading
to increased cyclic AMP and inositol triphosphate levels. These second messengers will, in turn,
provoke a rise in cytosolic Ca2+, which causes sustained action potential generation and
extrasystoles. The net effect of these pharmacologic actions is to elicit and maintain ventricular
fibrillation. In addition, cocaine causes cardiac myocyte death and myocardial infarction, but the
mechanism of action remains to be elucidated.
Other local anesthetic drugs include benzocaine, bupivacaine, etidocaine, lidocaine,
mepivacaine, pramoxine, prilocaine, procaine, procainamide, proparacaine, ro-pivacaine, and
tetracaine. Lidocaine and procainamide are also used as antiarrhythmic drugs. Extremely high
doses of these drugs cause decreases in electrical excitability, conduction rate, and force of
contraction likely through inhibition of cardiac Na+ channels.
Antihistamines
The most severe adverse effect of the second generation histamine H1 receptor antagonists
(antihistamines) is their association with life-threatening ventricular arrhythmias and sudden
cardiac death. Terfenadine and astemizole cause altered repolarization, notched inverted T
waves, prominent TU waves, prolonged QT interval, first- and second-degree AV block,
ventricular tachycardia or fibrillation, and torsades de pointes. These antihistamines produce
cardiac arrhythmias by blocking the delayed rectifier K+ channel and prolonging action potential
duration in cardiac myocytes. The prolonged action potential duration promotes early
afterdepolarizations and predisposes the myocardium to ventricular arrhythmias. However,
terfenadine also inhibits L-type Ca2+ channels in rat ventricular myocytes at concentrations near
or below that required to inhibit delayed rectifier K+ current. Therefore, both inhibition of Ca2+
and inhibition of K+ current likely contribute to the cardiotoxic actions of terfenadine.
Steroids and related hormones
Estrogens, progestins, androgens, and adrenocortical steroids are major steroid hormones
produced by mammals including humans. Myocardial tissue contains steroid receptors; therefore
the heart serves as a target organ for steroid effects. It also has been shown that cardiac tissue can
synthesize steroid hormones, although the capacity for synthesis may be much lower than more
classic steroid synthesizing tissues. There are two major mechanisms of action of the hormones:
the first is to alter gene expression and the second is to change signaling transduction pathways.
Estrogens are synthesized in ovaries, testes, and adrenal glands, and estrogen is an active
metabolite of testosterone. The older versions of estrogenic oral contraceptives that contained
high amounts of estrogens were associated with increased risk of coronary thrombosis and
myocardial infarction; however, lower doses of estrogens have been found by numerous
investigators to impart protective effects on the cardiovascular system, including antiapoptotic
effects, and beneficial effects on lipid metabolism such as decreased low-density lipoproteins
(LDL cholesterol) and increased high-density lipoproteins (HDL cholesterol). Estrogens alter
cardiac fibroblast proliferation, but they can either increase or decrease proliferation of these
cells.
Progestins are also synthesized in the ovaries, testes, and adrenal glands. Naturally occurring and
synthetic progestins include desogestrel, hydroxyprogesterone, medroxyprogesterone,
norethindrone, norethynodrel, norgestimate, norgestrel, and progesterone. As part of hormone
replacement therapy, progestins serve an opposing role to estrogens. Unfortunately, estrogen
treatment opposed with progestins may negate the cardiovascular benefits of estrogens on lipid
metabolism. Very little is known about the direct effects of progestins on the heart. Although
progestins could exert deleterious effects on the heart, more studies are required to investigate
mechanisms.
Glucocorticoids and mineralocorticoids are primarily synthesized in the adrenal glands.
Naturally occurring glucocorticoids include corticosterone, cortisone, and hydrocortisone
(cortisol), and the mineralocorticoid is aldosterone. A large number of synthetic glucocorticoids
are used for treatment of various autoimmune and inflammatory diseases. Both aldosterone and
glucocorticoids appear to stimulate cardiac fibrosis by regulating cardiac collagen expression
independently of hemodynamic alteration. Furthermore, aldosterone and glucocorticoids induce
hypertrophic growth and alter expression of Na+, K+-ATPase, Na+/H+ antiporter, and chloride/
bicarbonate exchanger of cardiac myocytes in vitro. Clinically relevant cardiac hypertrophy has
been observed in premature infants undergoing dexamethasone treatment. The mechanisms
responsible for the direct effects of these chemicals remain poorly understood.
INDUSTRIAL AGENTS
Solvents
Industrial solvents can exert adverse effects on the heart directly or indirectly; both are related to
their inherent lipohilicity. Solvents may affect cardiac physiological functions such as
contraction and energy production by directly dispersing into plasma membranes. However, the
effects of solvents on the heart would be more related to their actions on the neuro-hormonal
regulation of cardiac function. Solvents may disrupt sympathetic and parasympathetic control of
the heart as well as cause release of circulating hormones such as catecholamines, vasopressin,
and serotonin, which in turn affects cardiac function.
Halogenated Alkanes
The highly lipophilic nature of halogenated alkanes allows them to cross the bloodbrain barrier
readily. This action, coupled with their CNS-depressant activity, makes these compounds ideally
suited for anesthetics (halothane, methoxyfluorane, and enflurane). Halogenated hydrocarbons
depress heart rate, contractility, and conduction. In addition, some of these agents sensitize the
heart to the arrhythmogenic effects of-adrenergic receptor agonists such as endogenous
epinephrine. Fluorocarbons (freons) have been reported to have this sensitizing effect on the
myocardium. Chronic exposure to halogenated hydrocarbons may cause myocardial degenerative
response.
ANIMAL AND PLANT TOXINS
Animal toxins in the venom of snakes, spiders, scorpions, and marine organisms have profound
effects on the cardiovascular system. There are also a number of plants such as foxglove,
oleander, and monkshoodthat contain toxic constituents and have adverse effects on the
cardiovascular system.
You might also like
- Crash Cart Inventory ListDocument2 pagesCrash Cart Inventory ListSalah ElbadawyNo ratings yet
- Digitalis Toxicity Treatment and ManagementDocument39 pagesDigitalis Toxicity Treatment and ManagementAnonymous 3OoumAUytNo ratings yet
- By: Prabhat Khare: Asst. ProfessorDocument25 pagesBy: Prabhat Khare: Asst. ProfessorSUMIT KUMAR PANDITNo ratings yet
- Common Antidotes Used in Clinical PracticeDocument42 pagesCommon Antidotes Used in Clinical PracticeAUASSIRINo ratings yet
- Medicinal Chemistry-Ii: 1.anti-Infective Agents: FDocument14 pagesMedicinal Chemistry-Ii: 1.anti-Infective Agents: FAnonymous ionOPaqlkNo ratings yet
- What Are IV FluidsDocument5 pagesWhat Are IV FluidsRegine Mae Encinada100% (1)
- Cardiovascular Agents: Florianne E. Adlawan, R.NDocument31 pagesCardiovascular Agents: Florianne E. Adlawan, R.NadlawanflorianneNo ratings yet
- Introduction To Pharmacoepidemiology 2015 PDFDocument20 pagesIntroduction To Pharmacoepidemiology 2015 PDFNovria Rizki HarahapNo ratings yet
- Cataract NotesDocument4 pagesCataract NotesJeremy LauNo ratings yet
- At A Glance - Drug Distribution in The Body.Document10 pagesAt A Glance - Drug Distribution in The Body.Ismail Hossain Siragee100% (1)
- A. Eitel M. Scherrer K. Kümmerer: in Cooperation WithDocument43 pagesA. Eitel M. Scherrer K. Kümmerer: in Cooperation Withbubu_bubulinaNo ratings yet
- LECTURE 9 Positive InotropicDocument5 pagesLECTURE 9 Positive InotropicanaNo ratings yet
- Cardiovascular System: Antihypertensive DrugsDocument73 pagesCardiovascular System: Antihypertensive Drugsأمجد محمدNo ratings yet
- 1479912608-SHTM 2010omr PART 1Document41 pages1479912608-SHTM 2010omr PART 1DCHNo ratings yet
- Drug Study (Print3copiesDocument8 pagesDrug Study (Print3copiesPhylum ChordataNo ratings yet
- Cardiac BiomarkersDocument7 pagesCardiac BiomarkersAnand VeerananNo ratings yet
- What Is Crash CartDocument3 pagesWhat Is Crash CartEricson SomeraNo ratings yet
- CapDocument6 pagesCapRem AlfelorNo ratings yet
- ABG AnalysisDocument9 pagesABG AnalysisHoney PrasadNo ratings yet
- A Guide On Intravenous Drug Compatibilities Based On Their PHDocument10 pagesA Guide On Intravenous Drug Compatibilities Based On Their PHSergio M JuniorNo ratings yet
- Syringes NeedlesDocument52 pagesSyringes NeedlesRamchandra KenyNo ratings yet
- Indication Specific Action Side Effects/ Adverse Effects Nursing Consideration/ Patient TeachingDocument6 pagesIndication Specific Action Side Effects/ Adverse Effects Nursing Consideration/ Patient TeachingKrista Madranca CastroNo ratings yet
- HyperlipidemiaDocument17 pagesHyperlipidemiabent alfay7a2No ratings yet
- ToxicologyDocument31 pagesToxicologyHeart Hacker HarryNo ratings yet
- Drugs Acting On Cardio Vascular SystemDocument16 pagesDrugs Acting On Cardio Vascular SystemANUSHYA B PSGRKCWNo ratings yet
- Poisoning First AidDocument13 pagesPoisoning First AidGerra Mae Tubio CandelasaNo ratings yet
- Stres Test PDFDocument15 pagesStres Test PDFStevco Donev MakedonskiNo ratings yet
- BronchodilatorsDocument41 pagesBronchodilatorsLeong Zhee ChuanNo ratings yet
- Chlorpheniramine MaleatDocument200 pagesChlorpheniramine MaleatAchmad Fachry100% (1)
- Review Literature Journal: Institute of Nursing EducationDocument12 pagesReview Literature Journal: Institute of Nursing EducationEr Shah Rukh QadriNo ratings yet
- Paracetamol PDFDocument49 pagesParacetamol PDFEhb90210No ratings yet
- Radiation TherapyDocument2 pagesRadiation Therapyapi-446211408No ratings yet
- Angina PectorisDocument4 pagesAngina PectorisAnn CunananNo ratings yet
- PDIS - Calculation of Medication DosesDocument41 pagesPDIS - Calculation of Medication DosesMark Angelo JaurigueNo ratings yet
- Cardiac Glycosides 815Document19 pagesCardiac Glycosides 815SanskritiNo ratings yet
- Heparin LitigationDocument34 pagesHeparin LitigationjvalgalNo ratings yet
- Anthelmintics For Animal UseDocument20 pagesAnthelmintics For Animal UseSunil100% (2)
- Common Drug InteractionsDocument1 pageCommon Drug InteractionsLuxs23No ratings yet
- Pharmaceutical Care PlanDocument13 pagesPharmaceutical Care PlanMuhammad Mustafa IjazNo ratings yet
- Tonsillitis, Tonsillectomy and Adenoidectomy: - Literature ReadingDocument52 pagesTonsillitis, Tonsillectomy and Adenoidectomy: - Literature ReadingdestiNo ratings yet
- Unit 3. Information Search ProcessDocument34 pagesUnit 3. Information Search ProcessJenilyn FarnacioNo ratings yet
- Intrathecal Injection (LP) ENGDocument1 pageIntrathecal Injection (LP) ENGAlina RadescuNo ratings yet
- Renewal Application of LTO 1.2Document2 pagesRenewal Application of LTO 1.2Hazel BisaNo ratings yet
- Abbreviation Meaning: AbdominalDocument3 pagesAbbreviation Meaning: AbdominalMariel GentilesNo ratings yet
- Micro Chart #3 - Italics OnlyDocument27 pagesMicro Chart #3 - Italics Onlyapi-26938624100% (1)
- Cardiac BiomarkersDocument10 pagesCardiac BiomarkersfelipetheNo ratings yet
- ICSH Guidelines For Bone Marrow Lee Oct 2018Document16 pagesICSH Guidelines For Bone Marrow Lee Oct 2018Mia FernandezNo ratings yet
- GonadoDocument32 pagesGonadoshailendra meena50% (2)
- Aseptic Dispensing SOPsDocument19 pagesAseptic Dispensing SOPsvickanovriNo ratings yet
- Cancer MarkerDocument4 pagesCancer MarkerAnita BhattiNo ratings yet
- The Physiology of ShockDocument37 pagesThe Physiology of ShockGauxy AromboNo ratings yet
- 61 80 Otc DrugsDocument8 pages61 80 Otc DrugsIra YaoNo ratings yet
- Old Drugs For A New Use (Prescription)Document17 pagesOld Drugs For A New Use (Prescription)Karol Denisse Hernández BastidaNo ratings yet
- Cilastati Imipenem Drug InfoDocument19 pagesCilastati Imipenem Drug InfoCosmina GeorgianaNo ratings yet
- Radio PharmaceuticalsDocument48 pagesRadio PharmaceuticalsKris Joy EbonNo ratings yet
- Final Checklist Nursing Skills Checklist-1 2Document7 pagesFinal Checklist Nursing Skills Checklist-1 2api-380107556No ratings yet
- Paracetamol Poisoning - Wikipedia PDFDocument96 pagesParacetamol Poisoning - Wikipedia PDFBhanothu JyothiNo ratings yet
- Unit 5 SuppositoriesDocument47 pagesUnit 5 SuppositoriesAsif ANo ratings yet
- Diagnosis and Treatment of Digoxin Toxicity PDFDocument3 pagesDiagnosis and Treatment of Digoxin Toxicity PDFRozi AbdullahNo ratings yet
- Myocardial InfarctionDocument10 pagesMyocardial Infarctionshahdalaswad10No ratings yet
- Taketomo 2019Document328 pagesTaketomo 2019Oscar AguileraNo ratings yet
- AIIMS PG Entrance May 2004 Solved Question Paper With KeyDocument15 pagesAIIMS PG Entrance May 2004 Solved Question Paper With KeySAIFNo ratings yet
- Recalls 2 - Np3Document70 pagesRecalls 2 - Np3Kurt Nino TorresNo ratings yet
- Throat Mcqs Points Rida NaqviDocument5 pagesThroat Mcqs Points Rida NaqviÅli Raza ChaudaryNo ratings yet
- MRCP PearlsDocument682 pagesMRCP Pearlssherbiny alnaghyNo ratings yet
- DR Anuj Raj BijukchheDocument95 pagesDR Anuj Raj BijukchheMUHAMMAD JAWAD HASSANNo ratings yet
- Block 4 Renal Lecture 1 MCQDocument5 pagesBlock 4 Renal Lecture 1 MCQMahmoud ElshrkawyNo ratings yet
- 5b9f87df82236UG Syllabus (MBBS) AIIMS RaipurDocument8 pages5b9f87df82236UG Syllabus (MBBS) AIIMS RaipurBri MinNo ratings yet
- Reviwer 29Document3 pagesReviwer 29chaSephNo ratings yet
- De Paula 2017Document21 pagesDe Paula 2017TAINAH DE PAULANo ratings yet
- Chapter 20 - 22Document8 pagesChapter 20 - 22Stephanie LeeNo ratings yet
- Epidemiology of Alzheimer DiseaseDocument17 pagesEpidemiology of Alzheimer DiseaseAndi SulvanNo ratings yet
- Heart Disease Knowledge QuestionnaireDocument2 pagesHeart Disease Knowledge QuestionnaireFerdy LainsamputtyNo ratings yet
- Physiology Chapter 30 Renal Regulation of PotassiumDocument32 pagesPhysiology Chapter 30 Renal Regulation of PotassiumDaniel AdamsNo ratings yet
- Lung FunctionsDocument25 pagesLung FunctionsFatimah SNo ratings yet
- PY 5.16: Record Arterial Pulse Tracing Using Finger Plethysmography in A Volunteer or Simulated EnvironmentDocument6 pagesPY 5.16: Record Arterial Pulse Tracing Using Finger Plethysmography in A Volunteer or Simulated EnvironmentADITYA SARAFNo ratings yet
- SRP Practical Endocrinology HarrowerDocument700 pagesSRP Practical Endocrinology HarrowerGuillermo BelliNo ratings yet
- Grassi, Et Al. Lercanidipine Update. J of Pharmacology and Pharmacotherapy 2017Document11 pagesGrassi, Et Al. Lercanidipine Update. J of Pharmacology and Pharmacotherapy 2017Ruth RachmawatyNo ratings yet
- Medical-Surgical Nursing Assessment and Management of Clinical Problems 9e Chapter 66Document15 pagesMedical-Surgical Nursing Assessment and Management of Clinical Problems 9e Chapter 66sarasjunkNo ratings yet
- Pipeline Update 30JAN2024 0Document19 pagesPipeline Update 30JAN2024 0RC CharlesNo ratings yet
- Phenylephrine Hydrochloride Drug MonographDocument1 pagePhenylephrine Hydrochloride Drug MonographMuhammad ArsalanNo ratings yet
- Journal ClubDocument28 pagesJournal Clubapi-355090691No ratings yet
- The Nature of Disease Pathology For The Health Professions 2nd Edition Ebook PDFDocument62 pagesThe Nature of Disease Pathology For The Health Professions 2nd Edition Ebook PDFcarol.williams649100% (41)
- Disfunctii Vaculare, Ateroscleroza Si Calcificari VasculareDocument24 pagesDisfunctii Vaculare, Ateroscleroza Si Calcificari VasculareMikaela W. Marcu100% (1)
- DefinitionDocument2 pagesDefinitionAlmustapha Babangida DanganiNo ratings yet
- Cardiac Troponin T Is Not Increased in PatientsDocument5 pagesCardiac Troponin T Is Not Increased in PatientsAnanda Putri ImsezNo ratings yet
- Machanical Ventilator Nursing Care PalnDocument13 pagesMachanical Ventilator Nursing Care PalnAnnie Priscilla100% (1)
- Final Scientific Program EORCAPS 2013 For StudentsDocument3 pagesFinal Scientific Program EORCAPS 2013 For StudentsdrgauravkguptaNo ratings yet
- Session 10Document2 pagesSession 10Sistine Rose Labajo100% (1)
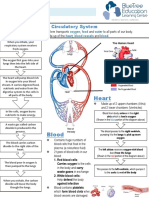
- Circulatory System Notes SLDocument1 pageCirculatory System Notes SLRathna PalaniappanNo ratings yet