Professional Documents
Culture Documents
Ringkasan Eter Epoksida
Uploaded by
Fiamfibi0 ratings0% found this document useful (0 votes)
34 views5 pageseter epoksida yg diringkas
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documenteter epoksida yg diringkas
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
34 views5 pagesRingkasan Eter Epoksida
Uploaded by
Fiamfibieter epoksida yg diringkas
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 5
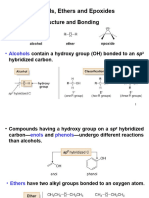
Chapter 18: Ethers
Naming of Ethers
Physical Properties
Preparation of Ethers
General Structure
R
O
R' R
S
R' H
S
R'
Ether
Thiol
Sulfide
R or R' can be alkyl, aryl or vinyl and the R groups may be attached in a ring shape
Simple ethers are named by naming the two substituent groups followed by the word ether.
O
ethyl methyl ether
O
diphenyl ether
O
tert-butyl isopropyl ether
O
cyclohexyl propyl ether
If other functional groups are present then the group is considered to be an alkoxy substituent.
O
3-propoxy-cyclohexene
O
OH
2-Methoxy-ethanol
O
1-Propoxy-but-2-yne
O O
Dimethoxy-methane
H
3
C
O
CH
3
112
Boiling points of ethers are elevated relative to similar alkanes. This
is due to a molecular dipole caused by the R-O-R bond.
Simple, symmetric ethers are prepared industrially by the dehydration of alcohols:
OH
diethyl ether synthesis:
2
H
2
SO
4
O
OH OH
2
This reaction is an easy way to make symmetric ethers, yet is impractical for most laboratory applications.
Cleavage of Ethers
Williamson Ether Synthesis: SN2 reaction with alkoxide as nucleophile on a primary carbon with a good leaving group
R O Na
X R'
R=any alkyl, phenyl or vinyl group
X=any good leaving group (Cl, Br, I or tosylate)
R
O R'
Alkoxides can be genarated with sodium hydroxide in some cases or sodium hydride (NaH).
R OH
NaH
R O Na H
2
Williamson ether synthesis reactions are usually only done with a primary electrophilc substrate since
otherwise E2 elimination reactions would compete.
Alkoxymercuration of Alkenes
Hg(CF
3
COO)
2
OH
O
HgO
2
CF
3
NaBH
4
O
Very simlar to alcohols synthesis using oxymercuration. This varierty uses the trifluoracetoxy ligand for Hg
instead of acetate. An alcohol serves as the nucleophile instead of water.
A mercurinium bridge is formed followed by nucleophilc attack by the alcohol and subsequent reduction.
The alcohol nucleophile will attack the more subsituted carbon due to greater partial positive charge at this
site.
Ethers may be cleaved to yield an alcohol and an alkyl halide. Strong conditions are usually necessary for
ether cleavage. Note: If two or more equivalents of acid are used further dehydration can occur on formed alcohols.
HI or HBr in refluxing water are need for cleavage of ether with primary or secondary substituents. These
ethers are cleaved via an S
N
2 mechansim.
With tertiary, allylic, or benzylic substituetns an S
N
1 mechanism is observed for cleavage which can be
done with strong acids such as H
2
SO
4
or CF
3
COOH.
O
H
2
SO
4
O
H
O
H
H
O
H
O
O
HI
H
I
OH
I
nucleophile attacks less substituted carbon
more stable carbocation formed
Claisen Rearrangement
O
Look for
this group!!!
Thermally catalyzed, 6 electron pericyclic
O
O
H
OH
Cyclic Ethers and Epoxides
Cyclic ethers have essentially the same reactivity of their acyclic counterparts. Epoxides are an
exception, owing their reactivity to a strained three membered ring.
O
O
O
O
tetrahydrofuran
(THF) 1,4-dioxane
ethylene oxide
(an epoxide)
Prepartion of Epoxides
Epoxides can be formed by oxidation of a doble bond with a peroxy acid (peracid). A common example
of such a reagent is meta-chloroperoxybenzoic acid (m-CPBA).
O O
H
O
Cl
m-CPBA
O
m-CPBA
Mechanism:
O
O
H
O
R
O
O
OH
R
Epoxide formation via an intramolecular Williamson ether synthesis of a halohydrin.
OH
Br
NaOH
O
Br
O
groups anti to one another
Br
2
H
2
O
Opening/Cleavage of Epoxides
Crown Ethers
Epoxides can be opened in a few different ways. Epoxides react at conditions milder then those needed to
cleave non-strained ethers. Nucleophilic attack occurs from the backside of the epoxide oxygen. It is often
possible for mixtures of products to result.
O
H Cl
O H
Cl
attack is S
N
2 like: on the less substituted carbon
O
H Cl
Acid Catalyzed: Primary and Secondary carbons
Acid Catalyzed: Tertiary carbons
O
H Cl
O H
O H
Cl
O
H
Cl
attack is SN1 like: more stable carbocation formed followed by attack
major
product
major
product
Base-Catalyzed Epoxide Openigs-occur due to ring strain
O
OH
O
HO
H
2
O
OH
HO
attack is S
N
2 like: on the less substituted carbon
Hydroxide acts as a nuclophile displacing the epoxide oxygen as a leaving group. Other nucleophiles,
such as Grignard reagents, alkoxides, hydrides, can perform this same reaction.
O
O
O
O O
O
18-crown-6
K
Host molecules for cation guests of various sizes.
Named by specifying the number of atoms and the number of oxygens
separated by the word crown.
(total atom #)-crown-(oxygen #)
Thiols and Sulfides
Thiols (Mercaptans)- Sulfur analog of alcohols, named like alcohols except use thiol instead of -ol
Sulfides- Sulfur analog of ethers, named like ethers except use sulfide instead of the word ether
SH Mercapto Group
Br
Preparation of thiols
NaSH
S
N
2
SH Note: prone to many side products
Br
SH
H
2
N NH
2
S
NH
2
NH
2
S
H
2
O
OH
Disulfide formation from thiols
SH
2
oxidation
Br
2
or I
2
S
S
reduction
Zn, H
H
2
N
SH
O
OH
cysteine
S
Sulfides
R X S
R
S
N
2
S
H
3
C I
S
Trialkyl Sulfonium Ion
S
Oxidation of Sulfides to Sulfones
H
2
O
2
S
O
m-CPBA
S
O
O
Preparation
DMSO
(dimethylsulfoxide)
You might also like
- 7 - Ethers and EpoxidesDocument37 pages7 - Ethers and Epoxidesrna6802No ratings yet
- Ethers and Epoxides: Ethers Nomenclature of EthersDocument10 pagesEthers and Epoxides: Ethers Nomenclature of EtherssarahNo ratings yet
- Ethers and Epoxides Thiols and SulfidesDocument18 pagesEthers and Epoxides Thiols and SulfidesTrescia Mae EstilloreNo ratings yet
- EthersDocument16 pagesEthersajay gudlaNo ratings yet
- Organic Chemistry Chap 11 Study GuideDocument49 pagesOrganic Chemistry Chap 11 Study GuideYarys YauNo ratings yet
- Notes CH 14 Wade 7Document14 pagesNotes CH 14 Wade 7tonyromofan100% (1)
- Alcohols and Ethers CHM457Document51 pagesAlcohols and Ethers CHM457AIMAN IMAN SHAIFUDDINNo ratings yet
- 18: Ethers and Epoxides Thiols and Sulfides: Based On Mcmurry'S Organic Chemistry, 9 EditionDocument48 pages18: Ethers and Epoxides Thiols and Sulfides: Based On Mcmurry'S Organic Chemistry, 9 Edition張湧浩No ratings yet
- Ethers, Epoxides, and Sulfides: Organic Chemistry, 7Document46 pagesEthers, Epoxides, and Sulfides: Organic Chemistry, 7Mon Jake Caoile PavicoNo ratings yet
- Description: o o o oDocument7 pagesDescription: o o o oAirome CorpuzNo ratings yet
- Useful Reactions PDFDocument8 pagesUseful Reactions PDFagusrimbombanteNo ratings yet
- 4.3 Alkyhalide PreparationDocument23 pages4.3 Alkyhalide PreparationDawit BirhanuNo ratings yet
- Alcohols 1Document13 pagesAlcohols 1Suresh VedpathakNo ratings yet
- Enols and EnolatesDocument36 pagesEnols and EnolatesManuel Curitol PiutrinNo ratings yet
- AldehydeKetonesNotessee PDFDocument7 pagesAldehydeKetonesNotessee PDFSubhabrata MabhaiNo ratings yet
- Ethers: By: DR Saima NajmDocument10 pagesEthers: By: DR Saima NajmSaima NajmNo ratings yet
- Senyawa KarbonilDocument61 pagesSenyawa KarbonilsalmaNo ratings yet
- Thiols, Ethers, and SulfidesDocument56 pagesThiols, Ethers, and Sulfidesgsy2023-9150-52879No ratings yet
- Alcohols, Ethers and PhenolsDocument45 pagesAlcohols, Ethers and Phenolsshivam08No ratings yet
- Alcohol Ether and ExpoksideDocument64 pagesAlcohol Ether and ExpoksideAhmadBadruzzamanShuib100% (1)
- AlcoholDocument8 pagesAlcoholSri DharshanNo ratings yet
- EP101 Sen LNT 008 Ketone&Aldehyde May11Document18 pagesEP101 Sen LNT 008 Ketone&Aldehyde May11Sàtz ÑÖÑïtNo ratings yet
- 18: Ethers and Epoxides Thiols and Sulfides: Based On Mcmurry'S Organic Chemistry, 7 EditionDocument30 pages18: Ethers and Epoxides Thiols and Sulfides: Based On Mcmurry'S Organic Chemistry, 7 EditionEileen EunJung KimNo ratings yet
- Alcohol, Phenol and EtherDocument21 pagesAlcohol, Phenol and EtherAditya NandaNo ratings yet
- Classification and Nomenclature of Alcohols, Phenols and EthersDocument16 pagesClassification and Nomenclature of Alcohols, Phenols and EthersTr Mazhar PunjabiNo ratings yet
- Ethers, Epoxides, and Sulfides: Organic Chemistry, 7Document34 pagesEthers, Epoxides, and Sulfides: Organic Chemistry, 7Elahine Yuliheht Orozco OcampoNo ratings yet
- Chapter 2.4 Alcohol, Ether & EpoxidesDocument52 pagesChapter 2.4 Alcohol, Ether & Epoxides0JTINGNo ratings yet
- Chapter Four PowerpointDocument109 pagesChapter Four PowerpointthanaNo ratings yet
- 19 Enolates Enamines-2Document59 pages19 Enolates Enamines-2ronNo ratings yet
- EtherDocument23 pagesEtherOng Kok LeongNo ratings yet
- Chapter 16 Lecture NotesDocument30 pagesChapter 16 Lecture NotesJuliaNo ratings yet
- Alchohols Phenols and EthersDocument5 pagesAlchohols Phenols and EthersPritika Yamini SaiNo ratings yet
- 8 Alcohols-2 and EthersDocument24 pages8 Alcohols-2 and EthersNova sounds - No copyright musicNo ratings yet
- Alcoholes, Fenoles, Aminas, Haluros de Alquilo y Arilo.Document22 pagesAlcoholes, Fenoles, Aminas, Haluros de Alquilo y Arilo.agmirandaNo ratings yet
- Unit 11 - Alcohols, Phenols and EthersDocument41 pagesUnit 11 - Alcohols, Phenols and EthersPoovaraahan RaghuveeranNo ratings yet
- Alcohol and PhenolDocument117 pagesAlcohol and Phenolsulihah12100% (2)
- Carbonyl Chemistry I: Mechanism of Acetal and Ketal FormationDocument8 pagesCarbonyl Chemistry I: Mechanism of Acetal and Ketal FormationSubhabrata MabhaiNo ratings yet
- Nomenclature - : Ol. Other Substituents Are Named and Numbered As Done in AlkanesDocument7 pagesNomenclature - : Ol. Other Substituents Are Named and Numbered As Done in AlkanesGulshan BatraNo ratings yet
- ALKANOLSDocument25 pagesALKANOLSKoki KingNo ratings yet
- AcetalyDocument8 pagesAcetalySayan Kumar KhanNo ratings yet
- Alcohols and Phenols (ROH, Functional GRP - OH.)Document24 pagesAlcohols and Phenols (ROH, Functional GRP - OH.)MadhureemaNo ratings yet
- Alkohol Eter EpoksidaDocument73 pagesAlkohol Eter EpoksidaGepsa AprilianaNo ratings yet
- CH 18Document32 pagesCH 18Dimas MitraNo ratings yet
- Part 1 Alkanes: Organic ChemistryDocument5 pagesPart 1 Alkanes: Organic ChemistryRuonan QinNo ratings yet
- Ch-11 Part-2 Alcohols, Phenols ðersDocument57 pagesCh-11 Part-2 Alcohols, Phenols ðersBhavishya VermaNo ratings yet
- Alcohols, Phenols and EpoxidesDocument134 pagesAlcohols, Phenols and EpoxidesStudent 365100% (1)
- Chap IV - Etherepox - ThiolsulfidesDocument36 pagesChap IV - Etherepox - Thiolsulfideshelia.jafari2No ratings yet
- Jack Westin MCAT Content Organic ChemistryDocument17 pagesJack Westin MCAT Content Organic ChemistryLoraNo ratings yet
- Unit 7-10 SM Theory Book 2 EM For 2022GRDocument19 pagesUnit 7-10 SM Theory Book 2 EM For 2022GRThilanka LiyanageNo ratings yet
- Physical and Chemical Properties of AlcoholsDocument24 pagesPhysical and Chemical Properties of AlcoholsmeerasahibfarhanNo ratings yet
- Reactions of AlcoholDocument20 pagesReactions of AlcoholHaslimi HassanNo ratings yet
- CHEM 109-Chepter 6Document28 pagesCHEM 109-Chepter 6naifalfarraj3No ratings yet
- Alcohols & Phenols:: GeneralizationsDocument27 pagesAlcohols & Phenols:: GeneralizationsdoudoudoudouNo ratings yet
- Organic Chemistry,: Alcohols, Ethers, EpoxidesDocument69 pagesOrganic Chemistry,: Alcohols, Ethers, EpoxidesilhamfaturachmanagusNo ratings yet
- Chap 7Document35 pagesChap 7أسامة المنتصرNo ratings yet
- Organic Chemistry,: Chapter 9 and 12 Alcohols, Ethers, Epoxides Oxidation - ReductionDocument26 pagesOrganic Chemistry,: Chapter 9 and 12 Alcohols, Ethers, Epoxides Oxidation - Reductionsyaripatul haniNo ratings yet
- Chapter 25b: Phenols and Quinones: Suggested Problems: 12, 14, 15, 16, 33, 37, 42 (Problems in Bold Face Are HIGHLYDocument4 pagesChapter 25b: Phenols and Quinones: Suggested Problems: 12, 14, 15, 16, 33, 37, 42 (Problems in Bold Face Are HIGHLYaNo ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Nutritional Benefits in Cheese: Palanivel Ganesan Youn-Ho HongDocument24 pagesNutritional Benefits in Cheese: Palanivel Ganesan Youn-Ho HongasdfNo ratings yet
- Exploring Protein StructureDocument23 pagesExploring Protein StructureTravel UnlimitedNo ratings yet
- ORGANIC NamingDocument15 pagesORGANIC Namingapi-3835692100% (2)
- Mims Indonesia - April.2017.en - SampleDocument958 pagesMims Indonesia - April.2017.en - SampleAdetia MaharaniNo ratings yet
- (Tomon) Efek Antioksidan Pada Teh Hijau Terhadap Kadar Kolesterol Darah - KatekinDocument9 pages(Tomon) Efek Antioksidan Pada Teh Hijau Terhadap Kadar Kolesterol Darah - KatekinPutri PutriNo ratings yet
- Dielectric Constants Chart: How To Use This GuideDocument10 pagesDielectric Constants Chart: How To Use This GuideDewet VirmondNo ratings yet
- Free RadicalDocument4 pagesFree RadicalRavi PreethiNo ratings yet
- Petrochemical: Petrochemicals AreDocument9 pagesPetrochemical: Petrochemicals AreprathapNo ratings yet
- Chapter 1 Fundamentals of Organic ChemistryDocument5 pagesChapter 1 Fundamentals of Organic ChemistryOchem90No ratings yet
- Prekursor 16 Mei 23 (Perubahan Kandungan Neozep Forte)Document2 pagesPrekursor 16 Mei 23 (Perubahan Kandungan Neozep Forte)binarobinNo ratings yet
- Assignment - HydrocarbonsDocument7 pagesAssignment - HydrocarbonsYash KumarNo ratings yet
- Annual Review of Pharmacology and Toxicology 2005 PDFDocument773 pagesAnnual Review of Pharmacology and Toxicology 2005 PDFditabokNo ratings yet
- Multiple choice questions (單選題) : 100%Document13 pagesMultiple choice questions (單選題) : 100%德瑞克No ratings yet
- Nomenklatura Organskih SpojevaDocument13 pagesNomenklatura Organskih Spojevaplaninka_jaksic4160No ratings yet
- Protiens and Amino AcidsDocument9 pagesProtiens and Amino AcidsMUHAMMAD SHOAIB MUNAWARNo ratings yet
- List Stok MMF 29 Agustus 2022Document8 pagesList Stok MMF 29 Agustus 2022Inna TrissNo ratings yet
- Experiment 3 Nucleic AcidsDocument5 pagesExperiment 3 Nucleic AcidsLloyd Patrick D. Gilig83% (6)
- Daftar Produk PT Harum Sentosa: NO Nama ObatDocument10 pagesDaftar Produk PT Harum Sentosa: NO Nama ObatLutfia LatifahNo ratings yet
- Castor OilDocument19 pagesCastor OilPraks SaxenaNo ratings yet
- Volume-02 Functional Group Organic Chemistry-IDocument271 pagesVolume-02 Functional Group Organic Chemistry-IVishalNo ratings yet
- Biology OxidationDocument50 pagesBiology Oxidationderhangker100% (3)
- VXL-List of Dossier in SpanishDocument3 pagesVXL-List of Dossier in SpanishSingh PushpanjaliNo ratings yet
- Macromolecules Worksheet Answer KeyDocument10 pagesMacromolecules Worksheet Answer KeyHarriet KirklandNo ratings yet
- Lecture - 3 Cell Metabolism: Dr. S. Annie Jeyachristy Lecturer, Unit of Biochemistry Faculty of MedicineDocument15 pagesLecture - 3 Cell Metabolism: Dr. S. Annie Jeyachristy Lecturer, Unit of Biochemistry Faculty of MedicinecheckmateNo ratings yet
- Mnemonic For Remembering AntiarrhythmicsDocument1 pageMnemonic For Remembering AntiarrhythmicsJenny Jose Kalathiveetil100% (1)
- Objective Life ScienceDocument913 pagesObjective Life ScienceCBSE UGC NET EXAMNo ratings yet
- Fix's BrowDocument438 pagesFix's BrowFransNo ratings yet
- Synthesis of Lactones and LactamsDocument1,093 pagesSynthesis of Lactones and LactamsnestorNo ratings yet
- DNA NotesDocument26 pagesDNA NotesMuli Maroshi100% (1)
- Biology-ModuleSixLessonThreePathwayThreeActivity RedoDocument7 pagesBiology-ModuleSixLessonThreePathwayThreeActivity Redomiranda50% (4)