Professional Documents
Culture Documents
Gojo - Triclosan Viricidal Activity
Uploaded by
kmsrajuOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Gojo - Triclosan Viricidal Activity
Uploaded by
kmsrajuCopyright:
Available Formats
VirucidalPerformanceofVariousProfessionalHandHygieneProdu VirucidalPerformanceofVariousProfessionalHandHygieneProducts cts
AgainstAvianInfluenzaAH5N1 AgainstAvianInfluenzaAH5N1
CAMBondiMPH CAMBondiMPH
1 1
,JWArbogastPhD ,JWArbogastPhD
1 1
,DRMacingaPhD ,DRMacingaPhD
1 1
,RLambkin ,RLambkin WilliamsPhD WilliamsPhD
2 2
,EMoaneBBSc ,EMoaneBBSc
2 2
1.GOJOIndustries,Inc.,Akron,Ohio,USA2.RetroscreenVirologyLtd.,London,UK
ABSTRACT ABSTRACT
BACKGROUND/OBJECTIVES: BACKGROUND/OBJECTIVES: Influenza A is a pathogen of substantial public health concern worldwide. The evidence
for human-to-human transmission of Influenza A H5N1 has identified it as a strain with pandemic potential, which would
present significant challenges to the healthcare infrastructure in terms of both infection control and patient volume. While
standard hand hygiene practices are recommended by leading public health organizations like the CDC and WHO to
prevent transmission, quantitative data on the effectiveness of hand hygiene products against these strains are not
readily available. In an effort to determine the virucidal performance of hand hygiene products on the potential pandemic
strain Avian Influenza A H5N1, we evaluated the virucidal efficacy of various professional hand hygiene products
commonly used in healthcare settings against Avian Influenza A NIBRG-14 [H5N1] using in-vitro techniques.
METHODS: METHODS: 40L of virus was added to 360 L of test article and incubated at room temperature for 15 seconds. The
reaction was terminated by 10-fold (v/v) dilution in MDCK infection media, supernatant was titrated 10-fold (v/v) across a
96 well plate, and cells were incubated for 1 hour. After incubation, cells were washed twice with PBS, fresh infection
media was added and cells were incubated for 3 days. After the final incubation period, the Hemagglutination Assay (HA)
was used to determine the presence of virus.
RESULTS: RESULTS: Four currently marketed ethanol instant hand sanitizers (3 gel, 1 foam), an ethanol surgical hand antiseptic, a
Triclosan foaming handwash, and a Chloroxylenol foaming handwash exhibited complete kill (>3log to >4log reduction of
H5N1 virus above the detectable limit) after an exposure of only 15 seconds.
CONCLUSIONS: CONCLUSIONS: These data demonstrate that the well-formulated 0.3% foaming Triclosan and 0.5% Chloroxylenol hand
washes and the five 62%-81.4% alcohol-based hand antiseptics evaluated in this study are in fact capable of rapidly
inactivating Avian Influenza A H5N1. Although Influenza A is an enveloped virus, and thus generally considered
susceptible to various antimicrobial agents, these data provide scientific support for the CDC and WHO position on hand
hygiene as the primary prevention measure for Influenza infection in healthcare.
Avian Influenza A NIBRG-14 [H5N1] was procured from the
reference collection of the National Institute for Biological Standards
and Control, London, England. Cytotoxicity assays were performed
to determine the detection limit for each test substance. 40L of
virus was added to 360 L of test article and incubated at room
temperature for 15 seconds. The reaction was terminated by 10-fold
(v/v) dilution in MDCK infection media, supernatant was titrated 10-
fold (v/v) across a 96 well plate of MDCK cells and incubated for 1
hour. After incubation, cells were washed twice with PBS, fresh
infection media was added and cells were incubated for 3 days.
After the final incubation period, the Hemagglutination Assay (HA)
was used to determine the presence of virus, where the presence of
agglutination indicated virus presence. HA results were used to
calculate the virus titer using the Krber Calculation.
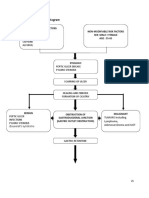
METHODS METHODS
Virucidal Assay Virucidal Assay CONCLUSIONS & DISCUSSION CONCLUSIONS & DISCUSSION
Our results show Our results show:
Alcohol-based instant hand sanitizers with active ingredient
levels 62% ethanol completely inactivate AIA H5N1 in 15 seconds
in-vitro.
Well-formulated handwashes with Triclosan (0.3%) or
Chloroxylenol (0.5%) are able to completely inactivate AIA H5N1 in
only 15 seconds in-vitro.
Avian Influenza can be effectively inactivated by hand hygiene
products that are currently being used in the healthcare setting.
Vigilant hand hygiene practices with efficacious hand hygiene
products should be a key component of Avian Influenza H5N1
infection control used in conjunction with respiratory precautions.
Until now the virucidal activity of commonly used hand hygiene
products versus H5N1 was unknown. The general opinion that
enveloped viruses are susceptible to and controllable with the
appropriate hand hygiene practices
1-2
has been confirmed in the case
of Avian Influenza A H5N1 by these results. Due to the perception of
Avian Influenza A H5N1 as a potential pandemic strain, there has
been an emphasis on airborne transmission over direct contact
transmission. As the natural transmission of AIA predominantly
occurs after direct contact, and AIA can survive on porous and non-
porous surfaces for 1-2 days and on hands for >5 minutes
5-6
, hand
hygiene has been identified by the World Health Organization as the
primary prevention measure for AIA H5N1 infection
2-4
. Therefore,
proper hand hygiene in the healthcare setting using products with
proven performance against AIA H5N1 is critical not only for
pandemic preparedness, but also as a key infection control
component for AIA H5N1 and other strains of influenza.
REFERENCES REFERENCES
1. Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control
Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of
America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm. Rep. 2002; 51(RR-
16):1-45.
2. World Health Organization. Prevention and control of influenza due to avian influenza virus A (H5N1). Version 30 January 2004.
3. Brankston G, Gitterman L, Hirji A, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect. Dis. 2007;7:257-
7.
4. Tellier R. Review of aerosol transmission of influenza A virus. Emerg. Infect. Dis. 2006;12(11):1657-62.
5. Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect.
Dis. 2006;6:130.
6. Inglesby TV, Nuzzo JB, OToole T, Henderson DA. Disease mitigation measures in the control of pandemic influenza. Biosecur.
Bioterror. 2006;4(4):376-83.
RESULTS RESULTS
Product Product
Active Active
Ingredient Ingredient
Exposure Exposure
Time Time
Log Log
10 10
Reduction Reduction
1 1
Percent Percent
Reduction Reduction
1 1
PURELL
Instant Hand Sanitizer 62% Ethanol 15 seconds >3.50 >99.96%
PURELL
Instant Hand Sanitizer with
Dermaglycerin System
62% Ethanol 15 seconds >3.79 >99.98%
GOJO
Medical Hand Sanitizer
(International Healthcare Product)
76.9% - 81.4%
Ethanol
15 seconds >4.50 >99.99%
PURELL
Instant Hand Sanitizer Foam 62% Ethanol 15 seconds >3.50 >99.96%
PURELL
Surgical Scrub 70% Ethanol 15 seconds >3.75 >99.98%
GOJO
PROVON
Foaming Handwash
with Triclosan and Moisturizers
2
0.3% Triclosan 15 seconds >4.00 >99.99%
GOJO
MICRELL
Antibacterial Foam
Handwash
0.5%
Chloroxylenol
15 seconds >4.25 >99.99%
40 40 L L H5N1 H5N1
360 360 L L Test Article Test Article
15 Second 15 Second
Incubation Incubation
Reaction Termination Reaction Termination
10 10- -fold Dilution ( fold Dilution (v/v v/v) )
MDCK Infection Media MDCK Infection Media
10 10- -fold Supernatant Titration & 1 fold Supernatant Titration & 1
Hour Incubation on MDCK Cells Hour Incubation on MDCK Cells
P
B
S
W
a
s
h
X
2
P
B
S
W
a
s
h
X
2
A
d
d
M
D
C
K
M
e
d
i
a
A
d
d
M
D
C
K
M
e
d
i
a
3 Day Incubation 3 Day Incubation
Hemagglutination Assay Hemagglutination Assay
for H5N1 Detection for H5N1 Detection
1. 1. A designation of A designation of > > denotes a complete inactivation of Avian Influenza A H5N1 denotes a complete inactivation of Avian Influenza A H5N1
2. To reduce 2. To reduce cytotoxicity cytotoxicity, the terminated reaction mixture was passed through a , the terminated reaction mixture was passed through a Sephadex Sephadex
column column
Cytotoxicity Cytotoxicity Assay Assay
4 hour 4 hour
incubation incubation
+ + tCPE tCPE
observation observation
PBS PBS
Wash x2 Wash x2
+ MDCK + MDCK
media media
added added
10 10- -fold fold
supernatant supernatant
titration + 1 titration + 1
hour hour
incubation incubation
Reaction Reaction
diluted diluted
10 10- -fold fold
in MDCK in MDCK
infection infection
media media
360 360 L L
test test
article + article +
40 40 L L
MDCK MDCK
infection infection
media media
K K rber rber Calculation Calculation
For additional information contact Cara Bondi, MPH: mccrackenc@gojo.com 2007 GOJO Industries, Inc., all rights reserved
You might also like
- Graduate Schools Comparison Worksheet - 0Document3 pagesGraduate Schools Comparison Worksheet - 0kmsrajuNo ratings yet
- Clariant Parabens PDFDocument5 pagesClariant Parabens PDFkmsrajuNo ratings yet
- Barrel Transfer Pump PDFDocument2 pagesBarrel Transfer Pump PDFkmsrajuNo ratings yet
- Tamil Varuda Rasi Palangal Kara Varudam Apr 2011 Apr2012Document29 pagesTamil Varuda Rasi Palangal Kara Varudam Apr 2011 Apr2012shanthiganeshmohanNo ratings yet
- Laundry Detergent StabilizersDocument5 pagesLaundry Detergent StabilizerskmsrajuNo ratings yet
- Galetta XLAnalystDocument16 pagesGaletta XLAnalystkmsrajuNo ratings yet
- SOFW Dec2010Document80 pagesSOFW Dec2010kmsrajuNo ratings yet
- Lubrication: 11.1 Lubrication of Rolling BearingsDocument9 pagesLubrication: 11.1 Lubrication of Rolling BearingskmsrajuNo ratings yet
- Lubrication: 11.1 Lubrication of Rolling BearingsDocument9 pagesLubrication: 11.1 Lubrication of Rolling BearingskmsrajuNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- c23 Microbiology Tortora TestbankDocument16 pagesc23 Microbiology Tortora Testbankwhitewave25No ratings yet
- ICD 10 - Chapter 1 Certain Infectious and Parasitic DiseasesDocument16 pagesICD 10 - Chapter 1 Certain Infectious and Parasitic DiseasesHuseikha VelayazulfahdNo ratings yet
- Cutaneous Microabscesses by Mycobacterium Chelonae: Case Reports From A Tertiary Care Centre, TripuraDocument4 pagesCutaneous Microabscesses by Mycobacterium Chelonae: Case Reports From A Tertiary Care Centre, TripuraIJAR JOURNALNo ratings yet
- Chronic Complications of Diabetes: Retinopathy, Nephropathy, NeuropathyDocument48 pagesChronic Complications of Diabetes: Retinopathy, Nephropathy, NeuropathyReina TarihoranNo ratings yet
- DEPRESSION ArticleDocument3 pagesDEPRESSION ArticleSamuel AladejareNo ratings yet
- Consequences of Overweight and ObesityDocument2 pagesConsequences of Overweight and Obesitykezia lohoNo ratings yet
- Tatalaksana Hiperosmolar Hiperglicemic State (SHH)Document16 pagesTatalaksana Hiperosmolar Hiperglicemic State (SHH)Vidya VidyutNo ratings yet
- Mini-OSCE Simulation: Hypertensive Disorders in PregnancyDocument16 pagesMini-OSCE Simulation: Hypertensive Disorders in PregnancyNinaNo ratings yet
- Coronavirus Panic The Infectious MYTHDocument25 pagesCoronavirus Panic The Infectious MYTHLouisa LivingstoneNo ratings yet
- Learners' Activity Sheets: Health 8Document11 pagesLearners' Activity Sheets: Health 8Leode Joy Tulang100% (1)
- Sandra Peterson Case NotesDocument3 pagesSandra Peterson Case NotesOana Maria Grigore0% (1)
- Tuberculosis: krisbantas/EPM/s1 1Document33 pagesTuberculosis: krisbantas/EPM/s1 1Yoerdy Agusmal SaputraNo ratings yet
- NCP Risk For InfectionDocument2 pagesNCP Risk For InfectionNathalie kate petallarNo ratings yet
- Schematic DiagramDocument3 pagesSchematic Diagramrn msnNo ratings yet
- 9115 TD Health Fact Sheet KenyaDocument2 pages9115 TD Health Fact Sheet KenyaSarfraz RajaNo ratings yet
- HOO - Coronavirus - Blanket - Isolation 7 1 20Document3 pagesHOO - Coronavirus - Blanket - Isolation 7 1 20mattbettNo ratings yet
- Whipworm (Trichuris Trichiura) PDF - Thushara Balasuriya, MSIDocument5 pagesWhipworm (Trichuris Trichiura) PDF - Thushara Balasuriya, MSIThushara_balasuriya833% (3)
- General InspectionDocument7 pagesGeneral InspectionAsushriNo ratings yet
- Visible Veins Eds - Google SearchDocument1 pageVisible Veins Eds - Google SearchRae MichelleNo ratings yet
- Csom (TT)Document24 pagesCsom (TT)SamirNo ratings yet
- S60 - LPL - Noida 3 N-27, Sec-18, Commercial Complex, Near. Atta Market, Noida.120-3029866/3142530 NoidaDocument2 pagesS60 - LPL - Noida 3 N-27, Sec-18, Commercial Complex, Near. Atta Market, Noida.120-3029866/3142530 NoidaGaurav singhNo ratings yet
- Strongyloides Stercoralis Hyperinfection Syndrome: A Case SeriesDocument8 pagesStrongyloides Stercoralis Hyperinfection Syndrome: A Case SerieslfjuradozNo ratings yet
- Women'S Health Talk Kozri Lo Lasante Madanm: DR Annabelle D. Marie MO Ministry of Health SeychellesDocument32 pagesWomen'S Health Talk Kozri Lo Lasante Madanm: DR Annabelle D. Marie MO Ministry of Health SeychellesAnna Diana MariaNo ratings yet
- Coccidiosis Project - Medina ShousherDocument15 pagesCoccidiosis Project - Medina ShousherMEDINA SHOUSHERNo ratings yet
- Confounding and Effect Modification ExplainedDocument49 pagesConfounding and Effect Modification Explainedtakele loveNo ratings yet
- Test Bank For Frommers Radiology For The Dental Professional 10th Edition by Stabulas Savage DownloadDocument8 pagesTest Bank For Frommers Radiology For The Dental Professional 10th Edition by Stabulas Savage Downloadchristinedaughertyrwycgtpmxj100% (24)
- Notes in PE and HEALTH (Grade 11)Document9 pagesNotes in PE and HEALTH (Grade 11)disneymovies143No ratings yet
- Surgical Management For Lung CancerDocument45 pagesSurgical Management For Lung CancerarifgteguhNo ratings yet
- Wenture AbcysisDocument3 pagesWenture AbcysisdfsdfsrfewfefweNo ratings yet
- Wuchereria BancroftiDocument6 pagesWuchereria BancroftiAinur RohmahNo ratings yet