Professional Documents
Culture Documents
Bits
Uploaded by
janakiram2010Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Bits
Uploaded by
janakiram2010Copyright:
Available Formats
1. Which of the following crystal structure characterises the ferritic stainless steel?
(b )
a) Face centred cubic b) Body centred cubic c) Simple hexagonal d) Octagonal
2. How many atoms are there per unit cell in a body centre cubic lattice system (a)
a) 2 b) 3 c) 4 d) 6
3. How many molecules per unit cell are there in a face centred cubic lattice? (b)
a) 2 b) 4 c) 6 d) 8
4. The atomic packing factor (that is volume of atoms/volume of cell) for FCC crystal structure
is (b)
a) 0.68 b) 0.74 c) 0.89 d) 0.16
5. The atomic packing factor (that is volume of atoms/volume of cell) for BCC crystal structure
is (a)
a) 0.68 b) 0.74 c) 0.83 d) 0.24
6. The atomic packing factor for which of the following crystals lattice structures are same? (a)
a) HCP & FCC b) HCP & BCC c) FCC & BCC d)BCC & BCT
7. with increase of inter atomic bond strength of substance, their (d)
a) Melting & Boiling points decreases b) Melting point increases
c) Boiling points increases d) Melting & Boiling points increases
8. which of the following is not a primary bond (c)
a) Ionic bond b) covalent bond c) Molecular bond d) Metallic bond
9. Metallic bonds in metals (b)
a) Are uni-directional i.e., do not act equally strong in all directions
b) Leads to highly coordinated close packed structures accounting for plastic properties of
Metals & their ability to form alloys
c) Have the valence electrons bound to a particular pair of atoms
d) Are same as electrovalent bonds.
10. Covalent bond is (d)
a) Also known as homo polar bond b) formed when electrons are shared between
Atoms c) found in Cl
2
& CH
4
molecules d) a secondary bond
11. Vander wall bond is (d)
a) A primary bond b) involves the transfer of electrons between the atoms
c) Involves sharing of electrons between the atoms d) is a weak bond
12. Which of the following is not an inter molecular (Vander wall) bond (d)
a) Dispersion bond b) Dipole bond c) Hydrogen bond d) Ionic bond
13. The bond between the atoms of a solid metal is called _____bond (b)
a) Ionic b) metallic c) electrovalent d) Dispersion bond
14. What type of bond is present in Diatomic molecules (e.g.O
2
, H
2
etc.) (b)
a) Vander wall bond b) covalent c) ionic d) metallic
15. What type of bond exists between Na & Cl ions when they form NaCl (c)
a) Vander wall bond b) covalent c) ionic d) metallic
16. Energy in the most stable state of an atom is (b)
a) zero b) minimum c) maximum d) in between minimum & maximum
17. Co-ordination number of (I,e. an atom possessing the number of nearest neighbours) in a
BCC structure is (b)
a) 4 b) 8 c) 10 d) 12
18. Co-ordination number in both FCC & HCP structure is (c)
a) 4 b) 8 c) 12 d) 14
19. An electro graph is used for the study of (b)
a) Composition of gases b) phase transformation in metals/alloys
c) Intensity of electron beams d) Structure of the composites
20. Number of nearest neighbours of an atom or in an octahedral hole of a close packed
structure is (a)
a) 6 b) 8 c) 10 d) 12
21. Which of the following does not crystallise in HCP structure (b)
a) Beryllium b) iron c) Mg d) Mo
22. Which of the following crystallise in BCC structure (a)
a) Cr b) Pb c) Zn d) Al
23. Atomic packing factor in case of copper crystals is a (b)
a) 1.66 b) 0.52 c) 0.78 d) 0.64
24. ______ responsible for holding together the protons and neutrons present in the nucleus of
atom. (a)
a) Continuous exchange of sub-nucleonic particles b) centrifugal forces
c) Electromagnetic force d) electrostatic force
25. Which of the following is not a characteristic of the metallic bond (a)
a) Directionality b) high conductivity c) Opacity d) Ductility
26. Co-ordination number in HCP, FCC and BCC unit cell are respectively a (a)
a) 12,8 & 12 b) 6,8 & 12 c) 8, 12 & 12 d) 12, 12 & 8
27. While in case of BCC unit cell, one central atom is surrounded by 8 identical atoms; in case of
a FCC unit cell, a central atom is in contact with ____ identical atoms (d)
a) 4 b) 6 c) 10 d) 12
28. Mixed ionic covalent bonds are normally found in (b)
a) High strength metals b) semiconductors c) thermal insulators d) high electrical
conductors
29. Lattice constant of FCC or BCC is equal to the length of the (c)
a) line having the highest linear density b) line having the lowest linear density
c) Cube edge d) body diagonal
30. Pick out the correct statement (c)
a) The primitive cell of an atom is also termed as unit cell
b) Neutrons and electrons cant be diffracted
c) The intensity of scattered wave is minimum, when the troughs and crests of X-ray beams
coincide
d) The net enthalpy change due to mixing of components is zero in real solution
31. Which of the following crystallise in HCP structure (c)
a) Au b) Sb c) Zn d) Mo
32. According to Hume Rotherys rule regarding substitutional solid solutions, the ___ for their
complete solubility (a)
a) Crystal lattice structure of 2 metals should be the same
b) Atomic diameters of the 2 metals should be different
c) Chemical affinity of the 2 metals should be high
d) Chemical composition of 2 metals should be high
33. Metals with large valence electrons ___ with a lower valence metal (a)
a) Have poor solubility b) have good solubility c) completely miscible d) completely
miscible, only when the crystal lattice structure of the 2 metals are same
34. Which of the following metals do not have FCC crystal lattice (d)
a) Cu b) Al c) Pb d) Na
35. Which one has BCC lattice of crystals (a)
a) Na b) Zn c) Ag d) Pb
36. Allotropic forms of metals have the same (d)
a) Physical properties b) crystal structure c) Chemical properties d) Mechanical
properties
37. Materials with metallic bonds in atoms are necessarily (a)
a) Ductile under stress b) hard c) gases at room temperature d) Having low electrical
conductivity
38. Solids have covalent bonds have (b)
a) High electrical conductivity b) low electrical conductivity c) low ductility d) low
hardness
39. Iron exist in more than one type of lattice structure (e.g. BCC/FCC) depending upon its (b)
a) Carbon content b) Temperature c) Melting point d) hardness
40. At room temperature iron is ____ in lattice arrangement (a)
a) BCC b) FCC c) HCP d) Octagonal
41. Metallic crystal structure is non-existent in the ____ form (b)
a) Orthorhombic b) Trapezium c) Triclinic d) tetragonal
42. Hume Rotherys rule predicts the existence of solid solubility in which ___ factor is not
included (b)
a) Crystal structure b) atomic weight c) atomic size d) electro negativity
43. Three important lattice pattern in the which most of the common metals crystallises are
FCC, BCC & HCP which of the following metals has HCP structure (d)
a) Gold b) Silver c) Platinum d) Zinc
44. Materials having ___ lattice structures are usually most ductile (a)
a) FCC b) HCP c) BCC d) Octagonal
45. Which of the following is a line defect found in metal crystals? (c)
a) Grain boundaries b) cracks c) edge dislocation d) grain size
46. The c/a ratio in ideal HCP structure is _____ (b)
a) 1.568 b) 1.633 c) 1.856 d) 2
47. _____ has the maximum density among the following (c)
a) Al b) Iron c) Copper d) Mg
48. Dislocation is ___ defect (b)
a) Point b) line c) surface d) grain boundary
49. In FCC lattice, the packing sequence of atoms is (d)
a) AB AB AB b) BC BC BC. C) AC AC AC.. d) ABC ABC..
50. In the HCP crystals the only plane with high atomic density is ____ (b)
a) (1000) b) (0001) c) (0010) d) (0100)
You might also like
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Seminar DR RN PatraDocument1 pageSeminar DR RN Patrajanakiram2010No ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Invoice No. Date Amount RsDocument2 pagesInvoice No. Date Amount Rsjanakiram2010No ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Recruitment For Asst. Prof. Phase IDocument1 pageRecruitment For Asst. Prof. Phase Ijanakiram2010No ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Course: Advanced Manufacturing Processes Module No. 4: Advanced Welding ProcessesDocument10 pagesCourse: Advanced Manufacturing Processes Module No. 4: Advanced Welding Processesjanakiram2010No ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Electric Arc Furnace SteelmakingDocument3 pagesElectric Arc Furnace Steelmakingjanakiram2010No ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- TIMCAL Brochure Fuel CellsDocument8 pagesTIMCAL Brochure Fuel Cellsjanakiram2010No ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Hashimoto 2010Document12 pagesHashimoto 2010Julian GutierrezNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Ieee STDDocument22 pagesIeee STDBalaji SpNo ratings yet
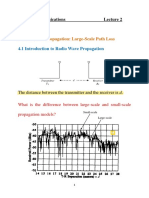
- Mobile Radio Propagation: Large-Scale Path LossDocument4 pagesMobile Radio Propagation: Large-Scale Path LossQusai HammashNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Exercise 8th GradeDocument4 pagesExercise 8th GradesheillahermantoNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Boiler Perform CalculateDocument29 pagesBoiler Perform CalculateAnsarNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- 0625 s14 QP 33 PDFDocument20 pages0625 s14 QP 33 PDFHaider AliNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Atomic Structure-EngDocument49 pagesAtomic Structure-EngUğurörengülNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Symmetry Transfer Coefficient: Factor and Confusion KineticsDocument5 pagesSymmetry Transfer Coefficient: Factor and Confusion KineticsAitor PastorNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- PICASSO SNOLAB 12a 0Document44 pagesPICASSO SNOLAB 12a 0Ivan FelisNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- PDF 4 Mechanics of DBDocument16 pagesPDF 4 Mechanics of DBRizette PaloganNo ratings yet
- Transport Phenomena - MSC - Lecture 10Document25 pagesTransport Phenomena - MSC - Lecture 10showravNo ratings yet
- DPP 3 SolutionDocument1 pageDPP 3 SolutionOmprakash DhakaNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- EXP - 8 - Determination of Venturimeter ConstantDocument6 pagesEXP - 8 - Determination of Venturimeter Constantpratyush mishraNo ratings yet
- T SC 2550160 Ks2 Year 5 Properties and Changes of Materials Revision Activity Mat Ver 4Document6 pagesT SC 2550160 Ks2 Year 5 Properties and Changes of Materials Revision Activity Mat Ver 4khushbakhtNo ratings yet
- Neet 2023 Question PaperDocument46 pagesNeet 2023 Question Paperhinot95530No ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- CM1131 - L25 - Phase Diagrams of Carbon Dioxide and Water - IVLEDocument13 pagesCM1131 - L25 - Phase Diagrams of Carbon Dioxide and Water - IVLENafeesahbmiNo ratings yet
- 2023 March EsasDocument9 pages2023 March EsasFamero Tarcena VincentNo ratings yet
- Prediction of Molar Volume For Pure Compounds Usingpeng-Robinson Equation of StateDocument4 pagesPrediction of Molar Volume For Pure Compounds Usingpeng-Robinson Equation of StateHarshil TejaniNo ratings yet
- Bridgeless PFCDocument18 pagesBridgeless PFCsolid690No ratings yet
- MSC - Nastran 2007 Implicit Nonlinear (SOL 600) User's GuideDocument532 pagesMSC - Nastran 2007 Implicit Nonlinear (SOL 600) User's GuideDonNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- 07 Mixing - Food Process Engineering TechnologyDocument25 pages07 Mixing - Food Process Engineering Technologyecky_moury6742No ratings yet
- Extra Solved Questions Class Ix Term II ChemistryDocument3 pagesExtra Solved Questions Class Ix Term II Chemistrychhabra navdeep100% (1)
- Gujarat Technological University: W.E.F. AY 2018-19Document2 pagesGujarat Technological University: W.E.F. AY 2018-19Priyank ChhatriwalaNo ratings yet
- Kinematics PracticeDocument16 pagesKinematics Practicekirki pNo ratings yet
- W. F. Chen, Plasticity For Structural Engineers, 1988-153Document1 pageW. F. Chen, Plasticity For Structural Engineers, 1988-153ahmedNo ratings yet
- Electromagnetic I EELE 3331: Electrostatic FieldsDocument84 pagesElectromagnetic I EELE 3331: Electrostatic Fieldsmae100% (1)
- Extension of The Double-Ellipsoidal Heat Source Model Tonarrow-Groove and Keyhole Weld ConfigurationsDocument13 pagesExtension of The Double-Ellipsoidal Heat Source Model Tonarrow-Groove and Keyhole Weld Configurationsal-masriNo ratings yet
- Periodic Table of ElementsDocument22 pagesPeriodic Table of ElementsZennith Orions100% (1)
- GEO REPORT StampedDocument44 pagesGEO REPORT Stampedhameed6101986No ratings yet
- Last Minute Review MCQDocument34 pagesLast Minute Review MCQIB Experts for 7on7 Pvt. Ltd.No ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)