Professional Documents
Culture Documents
1 PB
Uploaded by
pedroflu1230 ratings0% found this document useful (0 votes)
18 views8 pagesshampoo
Original Title
58451-58290-1-PB
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentshampoo
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
18 views8 pages1 PB
Uploaded by
pedroflu123shampoo
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 8
Proceedings of the 2006 WEPAN Conference, Copyright 2006, WEPAN-Women in Engineering
Programs and Advocates Network
Chemical Engineering and Cosmetics: Making the Connection
Between Chemistry and Engineering Processes in Product
Manufacturing
Rosemary Frollini, Annette M. Jacobson
Carnegie Mellon University
Abstract--This workshop will provide an activity for middle school and high school educators
and those involved in outreach projects to assist them in promoting engineering and science
careers to youth. It will also provide an opportunity to bring industrial technology into the
classroom using cosmetic and personal care products as a focus. Workshop participants will
explore the processes involved in the manufacture of soap and the role of the chemical engineer.
Each participant will manufacture their own personal care product. Using process symbols for
chemical engineering unit operations, they will design a process flow sheet that accurately
describes the steps taken to make their product. In any product, each ingredient has its own
essential function in the product formulation. Ingredients determine the properties and
effectiveness of the final product. A discussion of the ingredients in a sampling of popular
consumer products will lead to the final activity, product testing. Product testing is a necessary
and important part of product manufacturing. Specifically, we will consider product testing with
regards to ingredients with a function important to skin care, pH adjusters. Participants will
develop an awareness of the part played by the engineer and scientist in the formulation and
processing of products that they use everyday.
Chemical engineers use science and mathematics to transform materials in nature into an
amazing variety of products that we use every day. These products include cosmetics, medicine,
clothing, food and toys, just to name a few. The chemical engineer develops a PROCESS which
describes how you take the materials (chemicals) and combine them to form a PRODUCT. A
PROCESS is an orderly description of each task that must be accomplished to make the product.
You can think of following a recipe to make a cake as an example of a process. If you do not
follow the directions properly, you will not get the best looking or best tasting cake.
The proper chemistry required to make a product is essential. It is here that scientists and
engineers work together. Chemists provide the chemistry needed to make a product. The
engineer takes the chemical recipe and forms it into a series of steps (the process) defined by
equipment needed to perform each step. The chemistry defines the order of each step in the
process and the engineer uses mathematics to determine how much product can be made, the
quantity of initial ingredients needed and how fast the product can be made. This is of course
very important, since a company wants to make a product that is affordable for people, as well as
profitable.
In this paper we present an activity suitable for middle and high school students. The student
makes a cosmetic product that they can keep. Once they have experienced the steps involved in
Proceedings of the 2006 WEPAN Conference, Copyright 2006, WEPAN-Women in Engineering
Programs and Advocates Network
making the product, they are able to construct the process diagram necessary to describe the
manufacture of the product. This exercise gives the student a first hand look at the important
role of chemical engineers in making useful products for society.
The Chemical Manufacturing Process
In the production of cosmetics and personal care products, the highest standard of quality control
must be followed to assure the manufacture of safe products of uniform quality. It is the job of
the chemical engineer to design and oversee the manufacturing process.
All of the steps in the process must be determined including temperature control, mixing speed,
addition of ingredients, etc. These process requirements are essential to the quality of the
finished product.
Figure 1: Process for the Manufacture of Soap
(A Process Flow Diagram)
For example, consider a process for making a bar of soap shown in Figure 1 (The Soaps and
Detergents, 2005). Liquid and solid ingredients are combined in a large tank and mixed into a
thick suspension called a slurry. The slurry is pumped to a tower, and atomized through small
nozzles under pressure to produce small drops which fall through a current of hot air, drying
them into small granules or pellets. The pellets pass to a finishing line where they are blended
with additional ingredients (additives) such as fragrances and colorants.
Proceedings of the 2006 WEPAN Conference, Copyright 2006, WEPAN-Women in Engineering
Programs and Advocates Network
The mixture is sent through rollers for blending to a uniform texture. It is then extruded, cut into
bars and stamped into its final shape.
Each step in the process is called a unit operation. Mixing, separating, drying, cutting, heating
and cooling are examples unit operations. These can be illustrated by words and symbols.
Figure 2 shows some symbols which can be used to define (or illustrate) the unit operations of
making a cosmetic product. As shown for the soap making process, the symbols are organized
together to form the process.
Figure 2: Process Symbols for Unit Operations
A more general way of looking at a process is to develop a simple block diagram from a
description of the process. Block flow diagrams are illustrated as a set of connected blocks, or
process units. Lines with arrows connect the blocks and indicate the direction of the process
flow or stream. Raw materials always enter (as input) on the left and products leave (as
output) on the right.
Only four kinds of process units are used in block flow diagrams; mixers, reactors, separators,
and splitters. Mixers combine two or more materials (inputs). One or more chemical reactions
take place inside a reactor. An input stream is separated into two or more outputs by a
separator. The outputs from a separator have different chemical compositions from each other
and from the input. The change in chemical composition is due to physical operations, not a
chemical reaction. A splitter also separates an input into two of more outputs but now the
outputs have the same chemical composition. (Murphy, 2007)
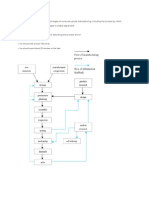
A block flow diagram for the soap manufacturing process is given in Figure 3.
Proceedings of the 2006 WEPAN Conference, Copyright 2006, WEPAN-Women in Engineering
Programs and Advocates Network
Water Additives
Solid
Ingredients
Liquid
Ingredients
Soap
Mixer Separator Mixer
Mixer Splitter
Figure 3: Block Flow Diagram for Soap Manufacturing
The first part of the activity involves the manufacture of a personal care product. BATH
FIZZIES fizz when placed in water because of a reaction between two of the ingredients,
sodium bicarbonate (baking soda) and citric acid. When in contact with water, they react to form
carbon dioxide gas the fizz just like the tiny bubbles you see when you open a bottle of soda.
Directions for Bath Fizzie Manufacture
Mix the following ingredients together in a bowl:
cup baking soda, cup citric acid, cup Epsom salt.
Measure carefully. Be careful not to get any moisture (water) into the dry ingredients or
they will begin to react.
When the dry ingredients are well mixed, add tablespoon of baby oil. Measure
carefully. When everything is well mixed, add an additional tablespoon of baby oil.
Mix again. The mixture will seem dry.
Press a small amount into both sides of a mold, being careful not to get any in the tracts
where the mold snaps together. If you do, use a toothpick to clean it out. Put the mold
together. If using a one piece mold, fill as directed.
Place a rubber band on the mold to hold it together if needed. Let the BATH FIZZIE
remain in the mold for 24-48 hours, then remove and toss into a bath tub full of water.
Be careful as even the small amount of baby oil in your BATH FIZZIE may cause the
tub to become slippery!
Bath Fizzie Flow Diagrams
After manufacture, it is now possible to prepare a block flow diagram for the manufacturing
process of the bath fizzie product. In addition, students can use unit operations symbols to draw a
Proceedings of the 2006 WEPAN Conference, Copyright 2006, WEPAN-Women in Engineering
Programs and Advocates Network
process flow diagram. This activity demonstrates how one takes a written description of
directions, and translates it into a process diagram. Once the process diagram is created the
engineer knows what kinds of equipment are needed. It is now possible to perform mathematical
calculations around each piece of equipment to determine the quantity of input materials you
need for a specific output of product.
The Chemistry of Cosmetics and Personal Care Products
Ingredient selection is an important step in the manufacturing process, because the ingredients
determine the properties and effectiveness of the final product. The next part of the activity
involves a discussion of the ingredients found in common cosmetics and personal care products
(Antczak & Antczak, 2001). After pointing out that ingredients are listed in order of the amount
present in the product (with the one present in the largest amount first), it is important to note
that the exceptions are ingredients acting as medications. Then, this active ingredient is listed
first.
Each ingredient has its own specific function. Common terms used in describing these functions
are then discussed. A few examples are antioxidant, binding agent, emollient, foaming agent,
humectant, preservative, surfactant, pH adjuster, UV absorber and viscosity adjuster. Lists of
ingredients and their functions can be found at the websites referenced (Botanicals, 2005;
USFDA, 2005). Students are then asked to identify the function of the ingredients in a few
products.
In examining the ingredients in lipstick, they may find that it contains bentonite clay to control
thickness, lanolin, a moisturizer, oxybenzone, a UV absorber and mica, a mineral which adds
sparkle. Other products that can be examined are eyeliner, foaming bath grains, toothpaste,
deodorant sticks, gel skin cleanser, and hand cream. The final product that can be examined is
the bath fizzie that was just made.
The Importance of pH
As each product is studied, it will be noted that many contain pH adjusters. So why is this an
important ingredient? pH is a chemical term used as a measure of acidity. pH ranges from 1
(very acidic) to 14 (very basic). A pH of 1-6 is acidic, 8-14 basic and a pH of 7 is considered to
be neutral.
The human skin and hair have a pH between 5 and 6, which means that they are slightly acidic.
This is due to a complex mixture of natural chemicals covering the skin that have the ability to
neutralize bases. This acidic environment helps to prevent the growth of bacteria and also
protects against substances with a high pH. It has been shown that soaps and cleansers of high
pH (basic) can be very irritating to the skin (How Does the pH, 2006; Skin Care, 2006) and
can temporarily remove this acid barrier. This is why shampoos, lotions, creams, and most soaps
have a pH adjusted to the pH of the skin, 5-6. In general, products with pHs above 8 may be
irritating to the skin.
In the final part of the activity, products including household cleaners are tested using broad
range pH paper to determine the pH and predict which ones may be irritating to the skin.
Proceedings of the 2006 WEPAN Conference, Copyright 2006, WEPAN-Women in Engineering
Programs and Advocates Network
Included for testing are shampoo, lotion, astringent, gel hand cleaner, laundry detergent and
liquid household cleaner.
Practical Information for Presenters
Acting as an effective presenter requires both organization and knowledge of the target audience.
Advanced planning is always required in order to have the needed supplies on hand and to
anticipate any problems. The activities presented here can be easily adapted to any age group.
We suggest that all of the activities be done in groups of 2-4 students. Equipment can then be
shared along with the experience! The recipe for the bath fizzies makes 1 cup of product so
the number of fizzies depends on the size of the mold used. In general, unless the group is
very small, a small mold is best to use in order to keep the amount of supplies needed
manageable. Some suggestions include plastic containers used for fruit or pudding cups, candy
molds, plastic eggs, or tea strainer balls.
The chemicals used in the Bath Fizzie activity are all available to the general public. Baking
soda, Epsom salts and baby oil can be purchased in a grocery store. (It is usually necessary to
process the Epsom salts in a coffee grinder so that it is more finely ground.) Citric acid is used
in baking, wine and cheese making, as well as in soap making and can be purchased from local
stores and web sites specializing in these activities.
Other types of oils can be used as the binder, but baby oil was chosen because of its low cost,
pleasant scent, and low probability of allergic reaction. Other oils which are often used in soap
and bath salts are apricot kernel oil, sweet almond oil, grape seed oil, olive oil, sunflower oil, and
wheat germ oil. Even though only a small amount of baby oil is used in the bath fizzie, it is still
enough to result in a slippery tub just as using bath oil does. This should always be pointed out.
Broad-range pH paper indicates pH from usually 2-12 pH units by simple color change.
It can be purchased from scientific supply houses such as Fisher Scientific and Flinn Scientific.
Any type of small container can be used for pH testing, but one with separate chambers of some
sort will keep the liquids from spreading into each other. Artist trays are very inexpensive and
easy to find at craft or art supply stores.
The above activities are easy to expand for more advanced students. For example, discuss the
chemical reaction of the bath fizzie, either in terms of the chemical names or the actual chemical
formula.
Citric Acid + Sodium Bicarbonate Sodium Citrate + Carbon Dioxide + Water
C
6
H
8
O
7
+ 3NaHCO
3
Na
3
C
6
H
5
O
7
+ 3CO
2
+ 3H
2
Proceedings of the 2006 WEPAN Conference, Copyright 2006, WEPAN-Women in Engineering
Programs and Advocates Network
Figure 4 Chemical Structure of Citric Acid Molecule
Advanced students could draw the chemical structure of the citric acid molecule (Fig. 4). They
could also discuss the substitution of the hydrogen ion on the carboxyl group of the citric acid
with the sodium ion from the bicarbonate.
Mathematics is easily included by calculating the percentage of each component in the
bath fizzie mixture. An additional task that could be done by all levels is preparing a product
label, listing the ingredients in order of their amount in the product.
To test their understanding of the chemical engineering concepts presented in the first activity,
ask the students to develop a flow chart for an activity they may be more familiar with such as
baking cookies or making ice cream.
For the ingredient activities, it is useful to generate an abbreviated list of ingredient uses,
with only those needed for the products you plan to examine. Use of long lists is often very
time-consuming and sometimes frustrating for students. Students may determine the ingredient
functions in one of their own cosmetic or personal care products by using internet search engines
to find any ingredient that may not be on the list provided.
The final part, pH testing provides an opportunity for discussions of acids and bases in the
household in general. Very young students can understand the concept of pH in terms of a simple
number line with the numbers 1 to 6 being acids, 7 being neutral, and 8 to 14 being bases.
These activities align well with the National Science Standards and are easily incorporated into
the curriculum. They have been presented to teachers and high school girls through a variety of
summer programs. Here are some representative comments from participants of the Cosmetics
Workshop. Teachers felt that the activity had real world applications and that students would pay
more attention to the ingredients in a product when making purchases because of the heightened
awareness that this activity provided. Students had comments that included liked to see how
products are made, liked hands-on and make and take activities, and enjoyed discovering
why certain ingredients are in there. They also wanted to add more ingredients like color and
fragrance to their product and wanted to spend additional time on the activity.
Besides bath fizzies, other products that are easily manufactured by the student are lip gloss,
hand lotion and liquid hand soap (Bath and Beauty, 2006; How to, 2006; Homemade,
Proceedings of the 2006 WEPAN Conference, Copyright 2006, WEPAN-Women in Engineering
Programs and Advocates Network
2006; Adventures, 2006; Sarquis, 1995; Sarquis, 1995). Presenters will find that they can
engage audiences of all ages with these exercises because they are relevant to everyday products
and will spark an interest in and an awareness of the role of the chemical engineer in the
manufacturing process.
References
Adventures in Lip Balm. Retrieved March 24, 2006 from www.notmartha.org/tomake/lipbalm.html
Antczak, S., and Antczak, G., (2001). Cosmetics Unmasked, Harper Collins Publishers, London,
Botanicals and Ingredients. Retrieved Sept. 2005 from www.beautyandthebeat.bigstep.com/generic4.html
Homemade Soap, Lip Gloss, and Face Masks. Retrieved March 24, 2006 from
www.kidzworld.com/site/p1897.htm
How does the pH of Skin Serve as a Barrier Against Bacteria?. Retrieved March 24, 2006 from
www.madsci.org/post/archives/1999-04/923688996.Me.r.html
How to Make Lip Gloss. Retrieved March 24, 2006 from
www.creativekidsathome.com/activities/activity_40.html
Murphy, R.M. (2007). Introduction to Chemical Processes, New York: McGraw Hill
Sarquis, M., Ed. (1995) Dirt Alert-The Chemistry of Cleaning, Science in Our World, Vol. 3,
Middleton, Ohio: Terrific Science Press
Sarquis, M., Ed. (1995) Fat Chance-The Chemistry of Lipids, Science in Our World, Vol. 4
Middleton, Ohio: Terrific Science Press
Skin Care About Your Skins pH. Retrieved March 24, 2006 from www.herballuxuries.com/skinspH.html
The Soaps and Detergents Association, Soaps and Detergents Manufacturing. Retrieved Sept. 2005 from
http://sdahq.org/cleaning/manufact/products/ingredients1.html
http://sdahq.org/cleaning/manufact/products/ingredients2.html
US Food and Drug Administration, Chemical Ingredients Found in Cosmetics. Retrieved Oct. 2005 from
http://vm.cfsan.fda.gov/~dms/cos-chem.html
Author Contact Information
Rosemary Frollini, rf3n@andrew.cmu.edu
Annette Jacobson, jacobson@andrew.cmu.edu
You might also like
- Life Cycle Assessment of Soap and DetergentsDocument14 pagesLife Cycle Assessment of Soap and Detergentstewegeb5100% (3)
- C 2Document22 pagesC 2sorinavramescuNo ratings yet
- Introduction To Cleaner Production Assessments With Applications in The Food Processing IndustryDocument8 pagesIntroduction To Cleaner Production Assessments With Applications in The Food Processing IndustryDua SweetoNo ratings yet
- Shampoo Industry - Report 1: Lacey Prasad - 300279457Document18 pagesShampoo Industry - Report 1: Lacey Prasad - 300279457Ginna MartinNo ratings yet
- Life Cycle Assessment of Soap and DetergentsDocument15 pagesLife Cycle Assessment of Soap and Detergentsmarion tesfaNo ratings yet
- Life Cycle Analysis of Star Soap and Detergents FactoryDocument15 pagesLife Cycle Analysis of Star Soap and Detergents Factorymarion tesfaNo ratings yet
- Chemistry Research TaskDocument4 pagesChemistry Research Taskapi-218511741No ratings yet
- A Comprehensive Framework For Surfactant Selection and Design For Emulsion Based Chemical Product Design PDFDocument12 pagesA Comprehensive Framework For Surfactant Selection and Design For Emulsion Based Chemical Product Design PDFArley NovaNo ratings yet
- Simulation System Project ReportDocument36 pagesSimulation System Project ReportAlifa SalsabilaNo ratings yet
- Product Life Cycle MappingDocument5 pagesProduct Life Cycle MappingElusor NetworkNo ratings yet
- Industrial Project N°1Document2 pagesIndustrial Project N°1kevinNo ratings yet
- Earth, Inc.: Using Nature's Rules to Build Sustainable ProfitsFrom EverandEarth, Inc.: Using Nature's Rules to Build Sustainable ProfitsRating: 3.5 out of 5 stars3.5/5 (3)
- CHM144L Industrial Chemistry Lab Detergent ExperimentDocument3 pagesCHM144L Industrial Chemistry Lab Detergent Experimentzidrick benjaminNo ratings yet
- Byekora Endever Qa Assignment.Document6 pagesByekora Endever Qa Assignment.byekora barnabusNo ratings yet
- Scrutinizing The Viscosity Measures of Two Formulations of Face Cream Light An Application of Statistical Quality Control in A Cosmetic CompanyDocument10 pagesScrutinizing The Viscosity Measures of Two Formulations of Face Cream Light An Application of Statistical Quality Control in A Cosmetic CompanymigueleozbNo ratings yet
- Product Development: A Structured Approach to Consumer Product Development, Design, and ManufactureFrom EverandProduct Development: A Structured Approach to Consumer Product Development, Design, and ManufactureRating: 5 out of 5 stars5/5 (4)
- Chemical Product Engineering and Design: Active Learning Through The Use of Case StudiesDocument5 pagesChemical Product Engineering and Design: Active Learning Through The Use of Case Studiesulfah nur khikmahNo ratings yet
- UNMSM/FQIQ/EPIQ/DAADP/Ingles I/EVP2/2021-II: Chapter 1. Diagrams For Understanding Chemical ProcessesDocument5 pagesUNMSM/FQIQ/EPIQ/DAADP/Ingles I/EVP2/2021-II: Chapter 1. Diagrams For Understanding Chemical ProcessesPiero Guerrero RodríguezNo ratings yet
- Dint A 00096Document36 pagesDint A 00096Harland SitorusNo ratings yet
- Development of Toilet Soap Production Technology FDocument8 pagesDevelopment of Toilet Soap Production Technology FHara alienNo ratings yet
- AIChE Journal Formulating Sunscreen 2011Document17 pagesAIChE Journal Formulating Sunscreen 2011bmvogel95No ratings yet
- Chemical ProcessesDocument103 pagesChemical ProcessesHildemaro CarrasquelNo ratings yet
- Steam Network Design Proposal - Group 16Document20 pagesSteam Network Design Proposal - Group 16Nisal KarunathilakeNo ratings yet
- Key - Activity FINAL PERIODDocument10 pagesKey - Activity FINAL PERIODgeorginaNo ratings yet
- The Use of Lean Manufacturing Practices in Cleaner Production A Systematic ReviewDocument11 pagesThe Use of Lean Manufacturing Practices in Cleaner Production A Systematic ReviewJose Daniel Humanez LopezNo ratings yet
- The Affecting Factors of Reject Bottles in Bottle Washing MachineDocument7 pagesThe Affecting Factors of Reject Bottles in Bottle Washing MachineInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Elfreda Siwes 0000w, JDocument19 pagesElfreda Siwes 0000w, JneemeneelfredaNo ratings yet
- Q1 - Poka Yoke ArticDocument6 pagesQ1 - Poka Yoke Articelioglp1No ratings yet
- Facility Layout and DesignDocument9 pagesFacility Layout and DesignAfia Nazmul 1813081630No ratings yet
- Guide to Selecting Product EPIsDocument40 pagesGuide to Selecting Product EPIsArmand LiviuNo ratings yet
- UN GLOBAL COMPACT WASH_May2021_Risk Assessment Tool_WRC-WA (1)Document163 pagesUN GLOBAL COMPACT WASH_May2021_Risk Assessment Tool_WRC-WA (1)katia ferreiraNo ratings yet
- 17NMCOM002Document92 pages17NMCOM002Apoorva M VNo ratings yet
- Teoria ACVDocument11 pagesTeoria ACVMarcelo Weihmayr da SilvaNo ratings yet
- Evaluation of Detergents and Washing ProcessesDocument14 pagesEvaluation of Detergents and Washing ProcessesKushagradhi DebnathNo ratings yet
- Computer Aided Methods & Tools For Separation & Purification of Fine Chemical & Pharmaceutical ProductsDocument6 pagesComputer Aided Methods & Tools For Separation & Purification of Fine Chemical & Pharmaceutical ProductsJohnathan Ortega MenesesNo ratings yet
- Product Design Chapter 2 AnswersDocument5 pagesProduct Design Chapter 2 AnswersBITS STUDENTNo ratings yet
- Production in SCMDocument12 pagesProduction in SCMSonia LawsonNo ratings yet
- Application of Lean Kaizen in Productivity Improvement and Safety Measures in A Manufacturing IndustryDocument6 pagesApplication of Lean Kaizen in Productivity Improvement and Safety Measures in A Manufacturing IndustryGizachew ZelekeNo ratings yet
- Managing Cosmetics Technologies in Dynamic Environments: November 2014Document19 pagesManaging Cosmetics Technologies in Dynamic Environments: November 2014Samrah QamarNo ratings yet
- Course Outline - DraftDocument2 pagesCourse Outline - DraftAki EspaldonNo ratings yet
- What Is Chemical EngineeringDocument2 pagesWhat Is Chemical EngineeringtalesofseriesNo ratings yet
- Maidin - 2020 - Soap Making Machine Development For Home AppliancesDocument14 pagesMaidin - 2020 - Soap Making Machine Development For Home AppliancesNgọc Nguyễn MinhNo ratings yet
- Design of ExperimentsDocument9 pagesDesign of ExperimentsMohammad AjlouniNo ratings yet
- PSEAsia2013 99 PDFDocument6 pagesPSEAsia2013 99 PDFMostofa RubalNo ratings yet
- PoM Unit - 1Document21 pagesPoM Unit - 1Dharani C KNo ratings yet
- General Background Detergents: Chapter 1: IntroductionDocument5 pagesGeneral Background Detergents: Chapter 1: IntroductionAhmad Al-HawadiNo ratings yet
- Handbook, Now in Its 5th Edition.: Joseph M. Juran - A Man Who Dedicated His Life To Improving The Quality ofDocument14 pagesHandbook, Now in Its 5th Edition.: Joseph M. Juran - A Man Who Dedicated His Life To Improving The Quality ofTooling ganeshNo ratings yet
- University Malaya: KKEK 3156 Plant EngineeringDocument10 pagesUniversity Malaya: KKEK 3156 Plant EngineeringJoeNo ratings yet
- Kaoru Ishikawa View On QualityDocument20 pagesKaoru Ishikawa View On QualityShahmien Seven100% (1)
- Flow ChartDocument2 pagesFlow ChartSanjeeb KafleNo ratings yet
- Chapter 1: Introduction To Company Background 1.1 Background of Company or IndustryDocument4 pagesChapter 1: Introduction To Company Background 1.1 Background of Company or IndustryIzzatyNabihah Zainudin100% (1)
- Interacademic Collaboration Involving Higher Education Institutions in Tlaxcala and Puebla, Mexico. Presented in Collaboration with Université Clermont Auvergne (France): Case Studies of Collaborative, Multidisciplinary Applications.From EverandInteracademic Collaboration Involving Higher Education Institutions in Tlaxcala and Puebla, Mexico. Presented in Collaboration with Université Clermont Auvergne (France): Case Studies of Collaborative, Multidisciplinary Applications.No ratings yet
- Implementation of Waste Assesment Model in Midas SafetyDocument15 pagesImplementation of Waste Assesment Model in Midas Safetytwinkle girlNo ratings yet
- Initial Report Blender Cum RoasterDocument21 pagesInitial Report Blender Cum Roastervinay muleyNo ratings yet
- Analysing Operations Management Problems in PepsiDocument8 pagesAnalysing Operations Management Problems in PepsiAnnerlynn Solano100% (1)
- Formulas, Ingredients and Production of Cosmetics: Technology of Skin- and Hair-Care Products in JapanFrom EverandFormulas, Ingredients and Production of Cosmetics: Technology of Skin- and Hair-Care Products in JapanRating: 4 out of 5 stars4/5 (28)
- Term Paper: Manufacturing SciencesDocument15 pagesTerm Paper: Manufacturing SciencesArpit BajajNo ratings yet
- Most Important Principles and Authors of QualityDocument12 pagesMost Important Principles and Authors of QualityMilena P.No ratings yet
- Shampoo ThicknersDocument8 pagesShampoo ThicknersAbdul SattarNo ratings yet