Professional Documents
Culture Documents
Modul Potensi Kimia Melaka Gemilang SPM 2014
Uploaded by
Cikgu FaizalCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Modul Potensi Kimia Melaka Gemilang SPM 2014
Uploaded by
Cikgu FaizalCopyright:
Available Formats
1
JABATAN PELAJARAN MELAKA
____________________________________________________________
PROGRAM KECEMERLANGAN AKADEMIK
SIJIL PELAJARAN MALAYSIA 2014
CHEMISTRY
MODUL KUMPULAN POTENSI
Modul soalan ini mengandungi 35 halaman bercetak
2
TOPICS THAT CONTAIN THE BASIC CONCEPTS THAT STUDENT SHOULD
MASTER IN CHEMISTRY.
TOPI K-TOPI K YANG MENGANDUNGI KONSEP ASAS KI MIA YANG PERLU
DI KUASAI OLEH PELAJ AR
1. THE STRUCTURE OF THE ATOM.
STRUKTUR ATOM
2. CHEMICAL FORMULAE AND EQUATIONS.
FORMULA DAN PERSAMAAN KIMIA
3. THE PERIODIC TABLE OF ELEMENTS
JADUAL BERKALA UNSUR
4. CHEMICAL BOND
IKATAN KIMIA
MODULE 1
1 Which of the following substances consist of atoms?
Antara bahan-bahan berikut, yang manakah terdiri daripada atom?
A Iron / Besi C Water / Air
B Sodium chloride / Natrium klorida D Oxygen / Oksigen
2 Table 1 shows the melting and boiling points of substances P, Q, R and S.
Jadual 1 menunjukkan takat lebur dan takat didih bagi bahan P, Q, R dan S.
Substance
Bahan
Melting point /
0
C
Takat lebur /
0
C
Boiling point /
0
C
Takat didih /
0
C
P 25 5
Q 50 300
R 256 192
S 10 140
Table 1 / Jadual 1
Which of the following substances is a liquid at room temperature?
Antara bahan-bahan berikut, yang manakah merupakan cecair pada suhu bilik?
A P C R
B Q D S
3
3 Diagram 1 shows the change of the state of matter.
Rajah 1 menunjukkan perubahan keadaan jirim.
Process P
Diagram 1 / Rajah 1
Which of the following is process P?
Antara berikut, yang manakah proses P?
A Freezing / Pembekuan C Boiling / Pendidihan
B Condensation / Kondensasi D Melting / Peleburan
4 When steam changes to water,
Apabila stim bertukar menjadi air,
A density decreases
ketumpatan berkurang
B energy is released
tenaga dibebaskan
C the size of the molecules decreases
saiz molekul berkurang
D the distance between the particles increases
jarak antara zarah-zarah bertambah
5 Which one of the following processes most clearly suggests that matter is made up of
particles in continuous motion?
Antara berikut, proses yang manakah menerangkan dengan jelas bahawa jirim terdiri
daripada zarah-zarah yang sentiasa bergerak?
A Combustion / Pembakaran C Diffusion / Resapan
B Melting / Peleburan D Neutralization / Peneutralan
4
6
What can be deduced from the symbol
27
13
Al?
Apakah yang boleh dideduksikan daripada simbol
27
13
Al?
I The electron arrangement of aluminium atom is 2.8.3
Susunan elektron bagi atom aluminium ialah 2.8.3
II Aluminium atom has 13 protons and 27 neutrons.
Atom aluminium mempunyai 13 proton dan 27 neutron
III Aluminium atom has a proton number of 27 and 14 neutrons.
Nombor proton atom aluminium ialah 27 dan terdapat 14 neutron
IV The total number of proton and neutron of aluminium atom is 27.
Jumlah bilangan proton dan neutron atom aluminium ialah 27
A I and III C I and IV
B II and IV D II and III
7 Diagram 2 shows the electron arrangement of sodium atom.
Rajah 2 menunjukkan susunan elektron bagi atom natrium.
Diagram 2 / Rajah 2
Which is the correct standard representation for the atom? [Nucleon number: 23]
Perwakilan piawai yang manakah betul bagi atom tersebut? [Nombor nucleon : 23]
A
Na
11
23
C
Na
12
23
B
Na
23
12
D
Na
23
11
Na
5
8 Diagram 3 shows the symbol for atom X.
Rajah 3 menunjukkan simbol bagi atom X.
Diagram 3 / Rajah 3
Which of the following represents the electron arrangement of ion X
?
Antara berikut, yang manakah mewakili susunan elektron bagi ion X
-
?
A 2. 8 C 2. 8. 8. 1
B 2. 7 D 2. 6
9 Which of the following pairs of ion has the same of number of electrons?
[Proton number: Li = 3 , O = 8 , F = 9 , Na = 11 , Mg = 12 , Cl = 17 , Ca = 20 ]
Antara pasangan ion berikut, pasangan yang manakah mempunyai bilangan elektron
yang sama?
[Nombor proton: Li = 3 , O = 8 , F = 9 , Na = 11 , Mg = 12 , Cl = 17 , Ca = 20 ]
A Na
+
and Li
+
C Mg
2+
and O
2-
B Cl
-
and F
-
D Ca
2+
and Mg
2+
10 Which of the following isotopes is used to detect the leakage in a gas pipe?
Antara berikut, isotop manakah yang digunakan untuk mengesan kebocoran paip gas?
A Carbon-14 C Cobalt-60
B Iodine-131 D Sodium-24
6
11 Which of the following set of substances contain all molecules?
Set bahan kimia yang manakah yang kesemuanya terdiri daripada molekul?
A Methane, chlorine, aluminium,, zinc nitrate
Metana, klorin, aluminium, zink nitrat
B Glucose, carbon dioxide, ammonia, ethanol
Glukosa, karbon dioksida, ammonia, etanol
C Helium, iron, calcium, nitrogen
Helium, besi, kalsium, nitrogen
D Zinc, nitrogen, copper, magnesium oxide
Zink, nitrogen, kuprum, magnesium oksida
12 The Table 2 shows the change of physical state and change of energy of four
substances.
Jadual 2 menunjukkan perubahan keadaan fizikal dan perubahan tenaga bagi empat
bahan.
Process
Proses
Change of physical state
Perubahan keadaan
fizikal
Name of process
Nama proses
Change of energy
Perubahan tenaga
W Gas to liquid
Gas kepada cecair
Condensation
Kondensasi
Heat is given out
Haba dibebaskan
X Liquid to gas
Cecair kepada gas
Melting
Peleburan
Heat is absorbed
Haba diserap
Y Liquid to solid
Cecair kepada pepejal
Freezing
Penyejukan
Heat is absorbed
Haba diserap
Z Solid to gas
Pepejal kepada gas
Sublimation
Pemejalwapan
Heat is given out
Haba dibebaskan
Table 2 / Jadual 2
Which of the processes given in Table 2 is true of the descriptions given?
Antara proses-proses yang dinyatakan dalam Jadual 2, yang manakah benar?
A W C Y
B X D Z
7
13 Diagram 4 shows the electron arrangement in ions W
-
, X
+
, Y
2-
and Z
2+
.
Rajah 4 menunjukkan susunan elektron dalam ion W
-
, X
+
, Y
2-
dan Z
2+
.
Diagram 4 / Rajah 4
Which of the following shows the correct nucleon number of the ion?
Antara berikut, yang manakah menunjukkan nombor nukleon yang betul bagi ion yang
berkenaan?
Ion
I on
Nucleon number
Nombor nukleon
A W
-
20
B X
+
22
C Y
2-
34
D Z
2+
40
14 Which of the following processes occur when ammonium chloride is heated?
Antara proses-proses berikut, yang manakah akan berlaku apabila ammonium klorida
dipanaskan?
A Condensation
Kondensasi
C Sublimation
Pemejalwapan
B Melting
Peleburan
D Boiling
Pendidihan
X
X
X
X
X
X
W
10n
X
X
X
X
X
X
X
X
X
X
X
12n
X
X
X
X
+
Y
16n
n
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
2-
Z
20n
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
2+
8
15 Diagram 5 shows the heating curve of solid benzoic acid.
Rajah 5 menunjukkan lengkung pemanasan bagi pepejal asid benzoik.
Diagram 5 / Rajah 5
Which statement can be deduced from Diagram 4?
Pernyataan manakah yang boleh dideduksikan daripada Rajah 4?
A No heat is absorbed in the first 2 minutes
Tiada haba diserap dalam 2 minit pertama
B Benzoic acid needs 8 minutes to melt completely
Asid benzoik memerlukan 8 minit untuk melebur selengkapnya
C Benzoic acid undergoes physical changes between 2
nd
minute to 6
th
minute
Asid benzoik mengalami perubahan fizikal di antara minit ke 2 hingga minit ke 6
D The attractive forces between particles of benzoic acid become stronger after 6
minutes.
Daya tarikan antara zarah-zarah asid benzoik menjadi semakin kuat selepas 6
minit.
16 Gypsum, CaSO
4
.2H
2
O is widely used in plastering of ceilings. What is the relative
formula mass of Gypsum?
Gipsum, CaSO
4
.2H
2
O digunakan secara meluas dalam pembuatan siling plaster.
Apakah jisim formula relatif bagi Gipsum?
A 164 C 172
B 272 D 180
9
17 A compound with formula X
2
CO
3
has a relative formula mass of 138. What is the
relative atomic mass of X? [Relative atomic mass : C = 12, O = 16]
Satu sebatian dengan formula X
2
CO
3
mempunyai jisim formula relatif 138. Apakah
jisim atom relatif bagi X? [ Jisim atom relatif : C = 12, O = 16]
A 78 C 69
B 39 D 110
18 A compound of magnesium nitrite contains 72% of magnesium and 28% of nitrogen.
What is the empirical formula of magnesium nitrite?
[Relative atomic mass : N = 14, Mg = 24]
Suatu sebatian magnesium nitrit mengandungi 72% magnesium dan 28% nitrogen.
Apakah formula empirik bagi magnesium nitrit?
[Jisim atom Relatif : N = 14, Mg = 24]
A MgN
2
C Mg
2
N
3
B Mg
2
N D Mg
3
N
2
19 Calculate the number of sulphur atoms present in 206 g of aluminium sulphate,
Al
2
(SO
4
)
3
.
[Given relative formula mass : Al
2
(SO
4
)
3
= 342 ; Avogadros constant = 6 X 10
23
mole
-1
]
Hitung bilangan atom sulfur yang terdapat dalam 206 g aluminium sulfat, Al
2
(SO
4
)
3
.
[ Jisim formula relatif : Al
2
(SO
4
)
3
= 342 ; Pemalar Avogadro = 6 X 10
23
mol
-1
]
A 0.74 x 6 x 10
23
atoms
C 0.79 x 6 x 10
23
atoms
B 1.8 x 6 x 10
23
atoms D 0.6 x 6 x 10
23
atoms
10
20 Diagram 6 shows the set-up of apparatus for an experiment to determine the empirical
formula of magnesium oxide.
Rajah 6 menunjukkan susunan radas bagi eksperimen untuk menentukan formula
empirik magnesium oksida.
Diagram 6 / Rajah 6
Why is the crucible lid opened once in a while during the experiment?
Mengapakah penutup mangkuk pijar dibuka secara berkala semasa eksperimen?
A To avoid explosion
Untuk mencegah letupan
B To cool the magnesium
Untuk menyejukkan magnesium
C To allow oxygen gas to enter the crucible
Untuk membenarkan oksigen memasuki mangkuk pijar
D To see what happen inside the crucible
Untuk melihat apakah yang berlaku di dalam mangkuk pijar
21 Which of the following is not matched correctly?
Antara berikut, yang manakah tidak dipadankan dengan betul?
Substance
Bahan
Formula
Formula
A Calcium ion
Ion kalsium
Ca
2+
B Copper(II) chloride
Kuprum(II) klorida
CuCl
2
C Carbon dioxide
Karbon dioksida
CO
2
D Ammonium ion
Ion ammonium
NH
3
Heat
Haba
Magnesiu
m
Crucible
Mangkuk pijar
Lid
Penutup
11
22 Which of the following statements is true for one mole of a substance?
Antara pernyataan berikut, yang manakah benar mengenai satu mol bahan?
A 1 mol of hydrogen gas contains 6.02 10
23
atoms
1 mol gas hidrogen mengandungi 6.02 10
23
atom
B 1 mol of magnesium contains 6.02 10
23
molecules
1 mol magnesium mengandungi 6.02 10
23
molekul
C 1 mol sodium chloride solution occupies 22.4 dm
3
at s.t.p
1 mol larutan natrium klorida menempati 22.4 dm
3
pada s.t.p
D 1 mol of water contains the same number of molecules as the number of atoms in
12 g carbon-12.
1 mol air mengandungi bilangan molekul yang sama dengan bilangan atom
dalam 12 g karbon-12
23 The chemical equation below shows a reaction between chlorine and iron.
Which of the following is the formula of the product?
Persamaan kimia di bawah menunjukkan tindak balas antara klorin dan besi.
Antara berikut, yang manakah formula bagi hasil yang terbentuk?
3Cl
2
(g) + 2Fe(s) 2
A FeCl
3
C FeO
B FeCl
2
D Fe
2
Cl
24 Diagram 7 shows the set-up of the apparatus to determine the empirical formula of a
metal oxide.
Rajah 7 menunjukkan susunan radas untuk menentukan formula empirik bagi oksida
logam.
Diagram 7 / Rajah 7
Dry hydrogen gas
Gas hidrogen
kering
Metal oxide
Oksida logam
12
Which of the following metal oxides is suitable to be used in Diagram 7?
Antara oksida logam berikut, yang manakah sesuai digunakan dalam Rajah 7?
A Magnesium oxide
Magnesium oksida
C Copper(II) oxide
Kuprum(II) oksida
B Aluminium oxide
Aluminium oksida
D Zinc oxide
Zink oksida
25 The equation below shows the reaction between V and W,
Persamaan di bawah menunjukkan tindak balas di antara V dan W,
V + 2W X + Y
Which of the following statements is true?
Antara berikut pernyataan yang manakah benar?
A V and W are the products of the reaction
V dan W adalah hasil tindak balas
B X and Y are the reactants of the reaction
X dan Y adalah bahan tindak balas
C 1 mole of V react with 1 mole of W to produce 1 mole of X and 1 mole of Y
1 mol V bertindak balas dengan 1 mol W menghasilkan 1 mol X dan 1 mol Y
D 1 mole of V react with 2 moles of W to produce 1 mole of X and 1 mole of Y
1 mol V bertindak balas dengan 2 mol W menghasilkan 1 mol X dan 1 mol Y
26 When copper(II) carbonate is heated , it decomposes to copper(II) oxide and carbon
dioxide gas. The equation for the reaction is
Apabila kuprum(II) karbonat dipanaskan, ia akan terurai kepada kuprum(II) oksida
dan gas karbon dioksida. Persamaan tindak balas ialah
CuCO
3
(s) CuO (s) + CO
2
( g)
13
12.4 g of of copper(II) carbonate decomposes completely. What is the mass of
copper(II) oxide formed ? [ Relative atomic mass : Cu = 64, O = 16, C = 12 ]
12.4 g kuprum(II) karbonat terurai dengan lengkap. Berapakah jisim kuprum(II)
oksida yang terhasil? [ Jisim atom relatif : Cu = 64, O = 16, C = 12 ]
A 8.0 g C 4.4 g
B 44.0 g D 80.0 g
27
Magnesium reacts with oxygen to form magnesium oxide.
Magnesium bertindak balas dengan oksigen untuk menghasilkan magnesium oksida
2Mg + O
2
2MgO
What is the mass of magnesium oxide formed when 2.4 g of magnesium reacts with
excess oxygen? [ Relative atomic mass: Mg = 24 , O = 16]
Berapakah jisim magnesium oksida yang terhasil apabila 2.4 g magnesium bertindak
balas dengan oksigen berlebihan? [ Jisim atom relatif : Mg = 24, O = 16]
A 1.6 g C 4.0 g
B 3.6 g D 8.0 g
28
The following chemical equation shows the reaction between sodium and oxygen.
Persamaan kimia berikut menunjukkan tindak balas antara natrium dan oksigen
nNa(s) + O
2
(g) m
What are the values of n, m and the formula in the box?
Apakah nilai bagi n, m dan formula bagi bahan dalam kotak di atas?
n m Formula
A 2 2 Na
2
O
B 4 2 Na
2
O
C 2 2 NaO
2
D 2 4 NaO
14
29 Diagram 8 shows the set-up of the apparatus used to study the reaction between a
metal oxide and carbon.
Rajah 8 menunjukkan susunan radas yang digunakan untuk mengkaji tindak balas
antara suatu oksida logam dan karbon.
Diagram 8 / Rajah 8
Which of the following metal oxide will turn the lime water cloudy ?
Oksida logam yang manakah akan menyebabkan air kapur menjadi keruh?
A Magnesium oxide
Magnesium oksida
C Calcium oxide
Kalsium oksida
B Aluminium oxide
Aluminium oksida
D Copper(II) oxide
Kuprum(II) oksida
30 Hydrogen peroxide decomposes according to the following equation:
Hidrogen peroksida terurai berdasarkan persamaan berikut:
2 H
2
O
2
2 H
2
O + O
2
One mole of hydrogen peroxide is decomposed. What is the volume of oxygen gas
released? [1 mol of gas occupies 24 dm
3
at room temperature and pressure]
Satu mol hidrogen peroksida telah terurai. Berapakah isipadu gas oksigen yang
terbebas? [1 mol gas menempati 24 dm
3
pada suhu dan tekanan bilik]
A 96 dm
3
C 12 dm
3
B 24 dm
3
D 48 dm
3
Metal oxide and
carbon
Oksida logam dan
karbon
Lime water
Air kapur
15
31 Which of the following elements are in Group 1 in the Periodic Table of Elements?
Antara unsur-unsur berikut, yang manakah merupakan unsur kumpulan 1 dalam
Jadual Berkala Unsur?
A Sodium and Chlorine
Natrium dan Klorin
C Lithium and Hydrogen
Litium dan hidrogen
B Sodium and Magnesium
Natrium dan Magnesium
D Lithium and Potassium
Litium dan Kalium
32 Table 3 shows the elements in Period 3 of the Periodic Table of elements. The
elements can react with oxygen to form oxides.
Jadual 3 menunjukkan unsur-unsur dalam Kala 3 Jadual Berkala Unsur. Unsur-unsur
tersebut boleh bertindak balas dengan oksigen untuk membentuk oksida.
Elements
Unsur
Na
Mg
Al
Si
P
S
Cl
Table 3 / Jadual 3
Which of the following element form oxide that can react with both hydrochloric acid
and sodium hydroxide solution?
Manakah antara unsur-unsur berkenaan boleh membentuk oksida yang boleh
bertindak balas dengan asid hidroklorik dan larutan natrium hidroksida?
A Na C Al
B S D Cl
33 The information below describes the properties of element X
Maklumat di bawah menerangkan sifat-sifat unsur X
Which of the following elements best fits the above properties?
Antara berikut, unsur manakah yang memenuhi sifat-sifat di atas?
A Chlorine
Klorin
C Argon
Argon
B Potassium
Kalium
D Magnesium
Magnesium
Conducts electricity
Soft and shiny
Reacts vigorously with water
Mengkonduksikan elektrik
Lembut dan berkilau
Bertindak balas sangat cergas dengan air
16
34 Which of the following statements best explain the stability of noble gases?
Antara pernyataan berikut, yang manakah menerangkan dengan tepat tentang
kestabilan gas Adi?
A Have octet electron arrangement except helium
Mempunyai susunan elektron oktet kecuali helium
B Can accept, lose or share electron
Boleh menerima, hilang atau berkongsi electron
C Have 8 valence electrons
Mempunyai 8 elektron valens
D Exists as polyatomic gases
Wujud sebagai gas poliatom
35 Atom of element X has a proton number of 13. Where is X located in the Periodic
Table of Elements?
Atom unsur X mempunyai nombor proton 13. Di manakah X terletak dalam Jadual
Berkala Unsur?
Group
Kumpulan
Period
Kala
A 3 13
B 13 3
C 3 3
D 13 2
36 Table 4 shows the proton number of elements S, T, U and V.
Jadual 4 menunjukkan nombor proton bagi unsur S, T, U dan V
Element
Unsur
S T U V
Proton number
Nombor proton
11 14 16 19
Table 4 / Jadual 4
What is the arrangement of elements S, T, U and V in ascending order of atomic size?
Apakah susunan unsur S, T, U dan V mengikut tertib menaik saiz atom?
A S, T, U, V C U, T, S, V
B S, V, T, U D V, U, T, S
17
37 Astatine is below iodine in Group 17 of Periodic Table.
Which of the following statements is true for astatine?
Astatin berada di bawah iodin dalam Kumpulan 17 Jadual Berkala Unsur.
Antara berikut, pernyataan yang manakah benar mengenai astatin?
A Form basic oxide
Membentuk oksida bes
C Solid at room temperature
Pepejal pada suhu bilik
B Exists as monoatom
Wujud sebagai monoatom
D More electronegative than iodine
Lebih elektronegatif dari iodin
38 In the Periodic Table, element X is in group 1 and element Y is in group 17.
The letters used are not the actual symbol of the elements.
Dalam Jadual Berkala, unsur X berada dalam kumpulan 1 dan unsur Y berada dalam
kumpulan 17.
Simbol yang digunakan bukan simbol sebenar unsur berkenaan.
Which of the following chemical equation is true for the reaction between element X
and element Y?
Antara persamaan kimia berikut, yang manakah benar bagi tindak balas antara unsur
X dan unsur Y?
A 2X + Y
2
XY
C 2X + Y X
2
Y
B X + Y
2
XY
2
D X + Y XY
39 Which of the following electron arrangements is found in a noble gas atom?
Antara susunan elektron berikut, yang manakah susunan elektron bagi atom gas adi?
A 1 C 2.7
B 2. 8. 2 D 2. 8
18
40 Which of the characteristics indicates that chromium is a transition element?
Sifat yang manakah menunjukkan bahawa kromium ialah unsur peralihan?
A It can form a basic oxide.
Ia boleh membentuk oksida bes
B It can form coloured solution.
Ia boleh membentuk larutan berwarna
C It can conduct electricity.
Ia boleh mengkonduksi elektrik
D It has a high melting point.
Ia mempunyai takat lebur yang tinggi
41 Table 4 shows the position of two elements, P and Q, in the Periodic Table of
Elements.
Jadual 4 menunjukkan kedudukan dua unsur, P dan Q, dalam Jadual Berkala Unsur.
Elements
Unsur
Group
Kumpulan
Period
Kala
P 1 3
Q 17 3
Which of the following is true about elements P and Q?
Antara berikut, yang manakah benar tentang unsur P dan Q?
A The atoms of P are smaller than the atoms of Q
Atom P lebih kecil daripada atom Q
B Atoms of P have stronger attraction for other electrons
Atom P menarik elektron lebih kuat berbanding Q
C The number of positive charges in the nucleus of P is less than Q
Jumlah cas positif dalam nukleus P kurang daripada Q
D Atoms of Q have a higher tendency than P to release electrons
Atom Q lebih cenderung menderma elektron berbanding atom P
19
42 Diagram 9 shows part of the Periodic Table.
Rajah 9 menunjukkan sebahagian daripada Jadual Berkala.
X
Diagram 9 / Rajah 9
What are the electron arrangements of atoms of element X?
Apakah susunan elektron bagi atom unsur X?
A 2. 6 C 2. 8. 6
B 2. 8 D 2. 8. 8
43 Diagram 10 shows the electron arrangement of ion T.
Rajah 10 menunjukkan susunan elektron bagi ion T.
Diagram 10 / Rajah 10
Where is element T placed in the Periodic Table?
Di manakah kedudukan unsur T dalam Jadual Berkala?
Group
Kumpulan
Period
Kala
A 17 2
B 18 2
C 2 18
D 2 17
20
44 Diagram 11 shows the formation of sodium ion.
Rajah 11 menunjukkan pembentukan ion natrium.
Diagram 11 / Rajah 11
Which of the following statements are true?
Antara berikut, pernyataan yang manakah benar?
I Sodium atom has an unstable electron arrangement.
Atom natrium mempunyai susunan electron yang tidak stabil
II Sodium ion has an octet electron arrangement as in neon atom.
Ion natrium mempunyai susunan electron oktet seperti atom neon
III Sodium atom donates an electron to form a positive charge ion.
Atom natrium menderma elektron untuk membentuk ion bercas positif
IV Sodium atom reacts with non-metals atoms to form ionic compounds.
Atom natrium bertindak balas dengan atom bukan logam untuk membentuk
sebatian ionik
A I and III only C II and III only
B I, III and IV only D I, II, III and IV
21
45 Diagram 12 shows the electron arrangement of a compound formed between atoms X
and Y.
Rajah 12 menunjukkan susunan elektron bagi sebatian yang terbentuk antara atom X
dan Y.
Diagram 12 / Rajah 12
Which of the following statements is true about the compound?
Antara pernyataan berikut, yang manakah benar tentang sebatian tersebut?
A It is an ionic compound
Sebatian tersebut merupakan sebatian ionik.
B The compound is formed by covalent bonds
Sebatian terbentuk melalui ikatan kovalen
C The compound has a high boiling point
Sebatian tersebut mempunyai takat didih yang tinggi
D The compound is formed by electron transfer
Sebatian terbentuk melalui perpindahan elektron
46 Which of the following is a property of covalent compound?
Antara berikut yang manakah sifat sebatian kovalen?
A Conducts electricity in liquid state.
Mengkonduksi elektrik dalam keadaan cecair.
B Dissolves in organic solvents.
Larut dalam pelarut organik.
C Requires a great amount of heat to break the attractive forces between
molecules.
Memerlukan haba yang banyak untuk mengatasi daya tarikan antara molekul.
D Solid covalent compounds consist of particles which are scattered randomly.
Pepejal sebatian kovalen mengandungi zarah-zarah yang tersebar secara
rawak.
X X x
Y x
x
x
x
x
22
47 The proton numbers of a few elements are as shown:
Nombor proton beberapa unsur adalah seperti berikut:
Element
Unsur
Proton Number
Nombor proton
H 1
Na 11
Mg 12
S 16
With the atoms of which element would chlorine atoms combine by sharing electrons?
Atom unsur manakah yang akan bergabung dengan atom klorin secara perkongsian
elektron?
I H III Mg
II Na IV S
A I only
I sahaja
C II and III only
II dan III sahaja
B II only
II sahaja
D I and IV only
I dan IV sahaja
48 The number of valence electrons of atoms X and Y are 2 and 7 respectively.
Which of the following chemical formulae and types of bonding are true for the
compound formed between X and Y?
Bilangan elektron valens bagi atom X dan Y adalah 2 dan 7 masing-masing.
Antara berikut, formula kimia dan jenis ikatan yang manakah benar bagi sebatian
yang terbentuk antara X dan Y?
Chemical Formula
Formula Kimia
Type of Bonding
J enis I katan
A XY
2
ionic
B XY
2
covalent
C X
2
Y ionic
D X
2
Y covalent
23
49 Diagram 13 shows the electron arrangement of atoms P, Q and R.
Rajah 13 menunjukkan susunan elektron bagi atom P, Q dan R.
Diagram 13 / Rajah 13
R can react with P and Q to form two different compounds. What are the formulae of
the compounds formed?
R boleh bertindak balas dengan P dan Q untuk membentuk dua sebatian yang
berbeza. Apakah formula sebatian-sebatian yang terbentuk?
P and R
P dan R
Q and R
Q dan R
A P
2
R QR
2
B P
2
R QR
C PR QR
2
D PR
2
QR
2
P Q
R
24
50 Diagram 14 shows the electron arrangement of a carbon dioxide molecule.
Rajah 14 menunjukkan susunan elektron bagi molekul karbon dioksida.
Which of the following is true?
Antara berikut, yang manakah benar?
A Each oxygen atom contributes one electron for sharing.
Setiap atom oksigen menyumbang satu elektron untuk dikongsi.
B Four double covalent bonds are formed in a carbon dioxide molecule.
Empat ikatan kovalen ganda dua terbentuk dalam satu molekul karbon dioksida
C One carbon atom contributes four electrons to be shared by two oxygen atoms.
Satu atom karbon menyumbang empat elektron untuk dikongsi bersama dua
atom oksigen.
D One carbon atom requires two electrons to achieve the octet electron
arrangement.
Satu atom karbon memerlukan dua elektron untuk mencapai susunan elektron
oktet.
Diagram 14 / Rajah 14
O
C
O
25
MODULE 2
1. Diagram 1 shows part of the Periodic Table of Elements.
Rajah 1 menunjukkan sebahagian daripada Jadual Berkala Unsur.
Na Cl Ar
K Cu
Diagram / Rajah 1
(a) (i) State the name of the element represented by the symbol Cu in Diagram 1.
Nyatakan nama bagi unsur yang diwakili dengan simbol Cu dalam Rajah 1.
..
[1 mark]
(ii) Cu is a transition element.
State one special characteristic of the transition elements.
Cu ialah satu unsur peralihan.
Nyatakan satu sifat istimewa unsur peralihan itu.
..
[1 mark]
(b) Which element is very stable and not reactive? Explain your answer.
Unsur yang manakah sangat stabil dan tidak reaktif?Terangkan jawapan anda.
...
...
...
[3 marks]
(c) Na and K react with water to produce alkaline solution.
Which element more reactive when react with water? Explain your answer.
Na dan K bertindak balas dengan air menghasilkan larutan beralkali.
Unsur yang manakah lebih reaktif apabila bertindak balas dengan air.
Terangkan jawapan anda
...
...
...
[3 marks]
26
(d) (i) Na and Cl in the same period. Which element is bigger atomic size?
Na dan Cl dalam kala yang sama. Unsur yang manakah mempunyai saiz atom
yang lebih besar?
..
[1 mark]
2 Table 2 shows the diagram of electron arrangement of carbon atom, oxygen atom and
sodium atom.
Jadual 2 menunjukkan gambar rajah susunan elektron bagi atom karbon, atom oksigen
dan atom natrium.
Carbon atom
Atom karbon
Oxygen atom
Atom oksigen
Sodium atom
Atom natrium
Table / Jadual 2
(a) Write the electron arrangement for carbon atom.
Tuliskan susunan elektron bagi atom karbon.
.
[1 mark]
(b) Carbon combines with oxygen to form a compound.
Karbon bergabung dengan oksigen untuk membentuk suatu sebatian.
(i) What is the type of the compound formed?
Apakah jenis sebatian yang terbentuk?
[1 mark]
(ii) Draw the diagram of electron arrangement of this compound.
Lukiskan gambar rajah susunan elektron bagi sebatian tersebut.
[2 mark]
C
O Na
27
(iii)State one physical property of the compound.
Nyatakan satu sifat fizik bagi sebatian tersebut.
[1 mark]
(c) Oxygen combines with sodium to form another compound.
Oksigen bergabung dengan natrium membentuk satu lagi sebatian.
(i) What is the name of the compound formed?
Apakah nama bagi sebatian yang terbentuk?
[1 mark]
(ii) Write the chemical equation for the reaction between oxygen and sodium.
Tuliskan persamaan kimia bagi tindak balas antara oksigen dan natrium.
[2 marks]
(iii)The compound formed has a high melting point. Explain why.
Sebatian yang terbentuk mempunyai takat lebur yang tinggi. Terangkan
mengapa.
[2 marks]
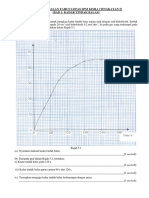
3 Graph 1 shows the temperature against time when solid P is heated.
Graf 1 menunjukkan graf suhu melawan masa bagi pemanasan pepejal P.
Graph 1[Graf 1]
t
1
Temperature /
o
C
Suhu /
o
C
Time / s
Masa /s
t
2
t
3
t
4
t
5
28
83
136
200
28
(a) Based on Graph 1 ,
Berdasarkan Graf 1,
(i)
(ii)
What is meant by melting point ?
Apakah yang dimaksudkan dengan takat lebur ?
................................................................................................................................
...............................................................................................................................
[1 mark]
State the boiling point of substance P
Nyatakan takat didih bahan P
[1 mark]
(iii) Explain why the temperature remains constant from time t
1
to t
2
.
Terangkan kenapa suhu tidak berubah dari masa t
1
hingga t
2
.
................................................................................................................................
................................................................................................................................
[2 marks]
(b) Diagram 3.1 and Diagram 3.2 shows the apparatus set up of two experiments
Rajah 3.1 dan Rajah 3.2 menunjukkan susunan radas bagi dua eksperimen
Purple colour of potassium manganate(VII) crystal
spread throughout water
Warna ungu kalium manganat(VII) merebak ke seluruh
air
Diagram 3.1 [Rajah 3.1]
After 3 days
Selepas 3 hari
Purple colour of potassium manganate(VII) crystal s
pread throughout the gel
Warna ungu kalium manganat(VII) merebak ke seluruh agar-
agar
Potassium manganate(VII) crystals
Hablur kalium manganat(VII)
After 1 hour
Selepas 1 jam
Potassium manganate(VII) crystals
Hablur kalium manganat(VII)
Water
Gel
Agar-agar
29
Diagram 3.2 [Rajah 3.2]
(i) State the name of the process involved in the experiments
Nyatakan nama proses yang terlibat dalam ke dua-dua eksperimen
....................................................................................................................................
[1 mark]
(ii) State the type of particle present in potassium manganate(VII) crystals.
Nyatakan jenis zarah yang terdapat dalam hablur kalium manganat(VII)
[1 mark]
(iii)Based on Diagram 3.1 and Diagram 3.2, explain the differences in the observation by
using kinetic theory of matter.
Berdasarkan Rajah 3.1 dan Rajah 3.2, terangkan perbezaan dalam pemerhatian
dengan menggunakan teori kinetik jirim.
....................................................................................................................................
[3 marks]
4(a) Diagram 4 shows the standard representation of two isotopes of chlorine atoms.
Rajah 4 menunjukkan perwakilan atom bagi dua isotop atom klorin.
Diagram 4
Rajah 4
(i) Based on Diagram 4, state the meaning of isotope.
Berdasarkan Rajah 4, nyatakan maksud isotop.
..............................................................................................................................
..............................................................................................................................
[2 marks]
Cl
35
17
Cl
37
17
30
(ii) Determine the number of neutrons in
Tentukan bilangan neutron dalam
Cl
35
17
:
Cl
37
17
:.
[2 marks]
(iii) State one use of chlorine in our daily lives.
Nyatakan satu kegunaan klorin dalam kehidupan seharian.
..
[1 mark]
(b) X is a substance that has melting point of 43
o
C and boiling point of 89
o
C.
X adalah satu bahan yang mempunyai takat lebur 43
0
C dan takat didih 89
0
C.
(i) Sketch a graph of temperature against time when substance X is heated from
30
0
C to 80
0
C.
Lakarkan graf suhu melawan masa apabila bahan X dipanaskan daripada 30
0
C hingga 80
0
C.
[2 marks]
(ii) Explain the arrangement of particles in substance X at :
Terangkan susunan zarah dalam bahan X pada :
30
0
C :
80
0
C :
.
[2 marks]
31
5.
(a)
Diagram 5.1 shows an apparatus set-up to determine the empirical formula of magnesium oxide.
Rajah 5.1 menunjukkan susunan radas untuk menentukan formula empirik magnesium oksida.
Why is it necessary to clean the magnesium with sand paper before the weighing process?
Mengapakah magnesium perlu dibersihkan menggunakan kertas pasir sebelum proses menimbang?
.....................................................................................................................................
[1 mark]
Table 5.2 shows the results for the experiment to determine the empirical formula of magnesium
oxide.
Jadual 5.2 menunjukkan keputusan bagi satu eksperimen untuk menentukan formula empirik bagi
magnesium oksida.
Mass of crucible + lid
Jisim mangkuk pijar + penutup
28.24 g
Mass of crucible + lid + magnesium ribbon
Jisim mangkuk pijar + penutup + pita magnesium
30.64 g
Mass of crucible + lid + magnesium oxide
Jisim mangkuk pijar + penutup + magnesium oxide
32.24 g
Table 5.2
Jadual 5.2
Heat
Dipanaskan
Magnesium ribbon
Pita magnesium
Diagram5.1
Rajah 5.1
32
(b)
Based onTable 5.2, complete Table 5.3 and determine the empirical formula of magnesium oxide.
Berdasarkan Jadual 5.2, lengkapkan Jadual 5.3 dan seterusnya tentukan formula empirik bagi
magnesium oksida:
[Relative atomic mass: O = 16, Mg = 24]
[Jisim atom relatif: O = 16, Mg = 24]
Element
Unsur
Mg O
Mass (g)
Jisim
Number of moles
Bilangan mol
Simplest ratio of moles
Nisbah ringkas bilangan mol
Empirical formula
Formula empiric
Table 5.3
Jadual 5.3
[4 marks]
(c) What must be done to ensure that the reaction has completed?
Apakah yang perlu dilakukan untuk memastikan tindak balas telah lengkap?
..
[1 mark ]
(d) Write the chemical equation for the reaction in the experiment.
Tuliskan persamaan kimia bagi tindak balas dalam eksperimen itu.
.................................................................................................................................
[2marks]
(e) Can the empirical formula of copper (II) oxide be determined using this same method? Give the
reason why.
Bolehkah formula empirik bagi kuprum(II) oksida ditentukan menggunakan cara yang sama?
Berikan alasannya .
....................................................................................................................................
[2marks]
33
6 Diagram 6 shows the set up apparatus of an experiment to determine the empirical
formula of copper oxide.
Rajah 6 menunjukkan susunan radas eksperimen untuk menentukan formula empiric
bagi kuprum oksida.
Diagram 6
Rajah 6
(a) What is the meaning of empirical formula?
Apakah maksud formula empirik?
.......
[1 mark]
(b) (i) Name an acid and a metal that can be used to prepare hydrogen gas in
this experiment.
Namakan suatu asid dan logam yang boleh digunakan untuk menyediakan
gas hidrogen dalam eksperimen ini.
[1 mark]
ii)
Write a balanced chemical equation for the reaction between the acid and
the metal in (b) (i).
Tuliskan persamaan kimia yang seimbang untuk tindak balas yang
berlaku di antara asid dan logam di (b) (i).
....
[1 mark]
Copper(II) oxide
Kuprum(II) oksida
Heat
Panaskan
Anhydrous calcium chloride, CaCl
2
Kalsium klorida kontang, CaCl
2
Hydrogen gas
Gas hidrogen
Porcelain dish
Mangkuk
porselin
34
Table 6 shows the results of an experiment carried out by a student.
Jadual 6 menunjukkan keputusan eksperimen yang dilakukan oleh pelajar.
Mass of combustion tube + porcelain dish
Jisim tiub pembakaran + mangkuk porselin
30.24g
Mass of combustion tube + porcelain dish + copper (II) oxide
Jisim tiub pembakaran + mangkuk porselin + kuprum(II)
oksida
32.26g
Mass of combustion tube + porcelain dish + copper
Jisim tiub pembakaran + mangkuk porselin + kuprum
31.86g
Table 6
Jadual 6
(c)(i) Calculate the number of moles of copper in this reaction.
[Relative atomic mass : Cu = 64]
Kirakan bilangan mol kuprum dalam tindak balas ini.
[Jisim atom relatif : Cu= 64]
[2 mark]
(ii) Calculate the number of moles of oxygen in this reaction.
[Relative atomic mass : O = 16]
Kirakan bilangan mol oksigen dalam tindak balas ini.
[Jisim atom relative: O= 16]
[2 mark]
(d)(i) Determine the empirical formula of copper oxide.
Tentukan formula empirik kuprum oksida.
35
[2 mark]
(ii) Name another metal oxide with empirical formula that can be determined using
the same method.
Namakan suatu oksida logam yang lain di mana formula empiriknya ditentukan
dengan kaedah yang sama.
..
[1 mark]
(e) M is a reactive metal. Suggest a method to determine the empirical formula of
the oxide of M.
M merupakan suatu logam reaktif. Cadangkan satu kaedah untuk menentukan
formula empirik bagi oksida M.
...............................................................................................................................
[1 mark]
KERTAS SOALAN TAMAT
Sumbangan: Panel-Q, Majlis Guru Cemerlang Negeri Melaka
1
JABATAN PELAJARAN MELAKA
____________________________________________________________
PROGRAM KECEMERLANGAN AKADEMIK
SIJIL PELAJARAN MALAYSIA 2014
CHEMISTRY
MODUL KUMPULAN POTENSI
Modul soalan ini mengandungi 5 halaman bercetak
JAWAPAN
2
SKEMA JAWAPAN MODUL PROGRAM KECEMERLANGAN AKADEMIK
SPM 2014
MODULE 1
Nombor Jawapan Nombor Jawapan
1 A 26 A
2 D 27 C
3 D 28 B
4 B 29 D
5 C 30 C
6 C 31 D
7 D 32 C
8 A 33 B
9 C 34 A
10 D 35 B
11 B 36 D
12 A 37 C
13 D 38 A
14 C 39 D
15 C 40 B
16 C 41 C
17 B 42 C
18 D 43 A
19 B 44 D
20 C 45 B
21 D 46 B
22 D 47 D
23 A 48 A
24 C 49 B
25 D 50 C
3
MODULE 2
1 (a) (i) Copper 1
(ii) Form coloured ion or compound//Colour of ion/compound is blue//
Has different oxidation number in compound// oxidation number
+2 and +1 in its compound//Form complex ions// Act as a catalyst
// CuSO
4
is catalyst in the reaction between zinc and acid.
1
(b) Ar
Atom of Ar achieve octet electron arrangement
Atom do not accept , donate or share electron with other atom
1
1
1
(c) K
Atomic size K is bigger // Valence electron of atom K further
from nucleus Force of attraction between valence electron and nucleus
weaker//
Atom K easier to release electron
1
1
1
(d) Na 1
TOTAL 9
2 (a) 2.4 1
(b) (i) Covalent 1
(ii)
[Number of atom combined: 1 carbon and 2 oxygen]
[Electron arrangement with 2 double covalent bonds]
1
1
(iii) Low melting/boiling point//cannot conduct electricity//dissolve in
organic solvent//not dissolve in water
1
(c) (i) Sodium oxide
4Na + O
2
2 Na
2
O
[Correct formulae of reactants and product]
[Balanced equation]
1
1
1
(ii) Strong force of attraction between oppositely charged ions.
Need a lot of energy to overcome the forces.
1
1
TOTAL 10
C O O
4
Question Description Marks
3 (a) (i) [Able to give a definition correctly]
The temperature at which solid turns to liquid
1
.....1
(ii) [Able to state the boiling point correctly]
136
0
C
1
.....1
(iii) [Able to explain the reasons correctly]
Heat energy is absorbed and
used to overcome the force of attraction between particles
// Heat is absorbed to overcome the intermolecular force between
molecule
r: ion/atom
1
1
.....2
(b)
(i) [Able to state the process correctly ]
Diffusion
1 ....1
(ii) [Able to state the type of particle correctly ]
Ion
1
....1
(iii) [Able to explain the observations correctly]
- Potassium manganate(VII)is made up of tiny particles
- The spaces between particles in gel are smaller than in water//vice
versa
- Potassium manganate (VII) particles diffuse slower in gel // vice
versa
1
1
1
.3
9
4 4(a) i) Same element
that have same proton number but different nucleon number
1
1
ii) Cl-35:18
Cl-37:20
1
1
iii) Used in water treatment to kill microorganisms/germs 1
(b) i) Temperature(
0
C)
43
Time(s)
1. labeled correct axis with unit
2. correct graph
1
1
ii) At 30
0
C : the particles are arraged closely packed in orderly
manner
At 80
0
C: the particles are arranged closely packed but not in
orderly manner
1
1
TOTAL
9
5
KERTAS SOALAN TAMAT
Sumbangan: Panel-Q, Majlis Guru Cemerlang Negeri Melaka
5(a) To remove the oxide layer 1
(b)
Element
Unsur
Mg O
Mass (g)
Jisim
2.4 1.6
Number of moles
Bilangan mol
0.1 0.1
Simplest ratio of moles
Nisbah ringkas bilangan
mol
1 1
Empirical formula
Formula empiric
MgO
1
1
1
1
(c) Repeat the heating, cooling and weighing process
until a constant weight is obtained
1
1
(d) 2Mg + O
2
2MgO
Correct Reactants and products ---------------1mark
Balanced equation ---------------1mark
2
(e) No, because copper is unreactive metal // does not react easily with oxygen 2 11
6(e) Formula that shows the simplest ratio of the number of atoms for each
element in the compound.
1
(b)(i) Zink and hydrochloric acid 1
(ii) Zn + 2HCl ZnCl
2
+ H
2
1
(c)(i) 1. Able to calculate the mass of copper
2. Able to calculate the no of mol of copper
1
1
(ii) 1. Able to calculate the mass of copper
2. Able to calculate the no of mol of copper
1
1
d(i)) Simplest ratio
Ratio of molles
1
1
(ii) Lead (I) oxide, Silver oxide 1
(e) Memanaskan logan tersebut dengan oksigen 1. 11
You might also like
- Kimia-Ujian Mac 2014Document3 pagesKimia-Ujian Mac 2014Damit Jaffar Mohd ThaniNo ratings yet
- Modul Masyhur Kimia 2022 PDFDocument65 pagesModul Masyhur Kimia 2022 PDFOnesue RedcloudyNo ratings yet
- Kimia SPM Kimia k2 Set 2Document15 pagesKimia SPM Kimia k2 Set 2api-3841296No ratings yet
- Faqs Kimia SPM t4 2015Document10 pagesFaqs Kimia SPM t4 2015Faridah Othman100% (4)
- Kimia k2 JPWPP 2023Document32 pagesKimia k2 JPWPP 2023Rosdianah AbdullahNo ratings yet
- Final Kimia DLP T5Document29 pagesFinal Kimia DLP T5nory msNo ratings yet
- Latih Tubi Sains Pt3Document7 pagesLatih Tubi Sains Pt3Anonymous fBxwaWRCNo ratings yet
- Exercise Chem f4 c7Document5 pagesExercise Chem f4 c7Rohani YusofNo ratings yet
- 3 Formula Dan Persamaan KimiaDocument20 pages3 Formula Dan Persamaan KimiawannwaNo ratings yet
- Kimia Tingkatan 4 Bab 3Document8 pagesKimia Tingkatan 4 Bab 3Vicknesh RamanaiduNo ratings yet
- 2019 - Modul Tudingan SPM19Document31 pages2019 - Modul Tudingan SPM19Zaza Mawar100% (1)
- Alkana Dan AlkenaDocument4 pagesAlkana Dan AlkenaHaris AffendiNo ratings yet
- Kertas 2 Pahang 2017Document30 pagesKertas 2 Pahang 2017Bryan Wong0% (1)
- Latihan Konsep Mol2Document1 pageLatihan Konsep Mol2Arbayana AmbranNo ratings yet
- Modul Perfect Score SBP Chemistry SPM 2014 - Modul Pecutan - Modul X A PlusDocument219 pagesModul Perfect Score SBP Chemistry SPM 2014 - Modul Pecutan - Modul X A PlusCikgu Faizal100% (3)
- Sel KimiaDocument2 pagesSel KimiamarziahNo ratings yet
- Kertas 2 Pep Pertengahan Tahun Ting 4 Terengganu 2018 - Soalan PDFDocument20 pagesKertas 2 Pep Pertengahan Tahun Ting 4 Terengganu 2018 - Soalan PDFfizaali87No ratings yet
- Puas KimiaDocument20 pagesPuas KimiaKOH JEE MENG MoeNo ratings yet
- Trial Negeri Sembilan Chemistry Pra SPM 2013 SET 1 K1 - K2 - K3 - Question - SchemeDocument0 pagesTrial Negeri Sembilan Chemistry Pra SPM 2013 SET 1 K1 - K2 - K3 - Question - SchemeCikgu FaizalNo ratings yet
- Nota Kimia Tingkatan 4 Bab 8Document21 pagesNota Kimia Tingkatan 4 Bab 8Aleya NurNo ratings yet
- Modul PDPR 4.0 Kimia T4 2021Document9 pagesModul PDPR 4.0 Kimia T4 2021Hafizasharin OthmanNo ratings yet
- Modul Kolaborasi SBP-SBP Johor 2019 PDFDocument28 pagesModul Kolaborasi SBP-SBP Johor 2019 PDFSiti Hajar Abd HamidNo ratings yet
- Sebatian KarbonDocument88 pagesSebatian KarbonNana DiyanaNo ratings yet
- Sebatian KarbonDocument88 pagesSebatian KarbonAiman NurrazyNo ratings yet
- Formula Kimia Tingkatan 4Document16 pagesFormula Kimia Tingkatan 4Wenn Winnona100% (6)
- Kertas 1 Kimia Tingkatan 4 DwibahasaDocument13 pagesKertas 1 Kimia Tingkatan 4 Dwibahasaafida0511100% (1)
- Latihan Persamaan Kimia Dis 2016Document6 pagesLatihan Persamaan Kimia Dis 2016Mohd Jamalil Azam Mustafa100% (1)
- Modul Teknik Menjawab Sains SPM 2023Document51 pagesModul Teknik Menjawab Sains SPM 2023Jowez Lee100% (1)
- Kimia Bahagian B Dan CDocument72 pagesKimia Bahagian B Dan CSMK SULTAN SALAHUDDIN ABDUL AZIZ SHAH Moe0% (1)
- Modul Straight A+ Kimia 2021Document27 pagesModul Straight A+ Kimia 2021TAMIL KODINo ratings yet
- BAB 4 Sebatian KarbonDocument50 pagesBAB 4 Sebatian KarbonChu Wai SengNo ratings yet
- Bab 2Document3 pagesBab 2Noor Azizah ZakariaNo ratings yet
- Latihan JMR & JFRDocument2 pagesLatihan JMR & JFRRohani YusofNo ratings yet
- Kima F4Document16 pagesKima F4Shin Obi100% (1)
- Kedah Trial Fizik Kertas 2 SPMDocument22 pagesKedah Trial Fizik Kertas 2 SPMfarahibbNo ratings yet
- SPMDocument181 pagesSPMMuhammad Hazwan Rahman100% (3)
- IT Chem F5 Topical Test 4 (BL)Document10 pagesIT Chem F5 Topical Test 4 (BL)Hajar Norasyikin Abu Bakar0% (1)
- Latihan SPM Kimia (Kadar Tindak Balas) 21Document2 pagesLatihan SPM Kimia (Kadar Tindak Balas) 21Meen Leen0% (1)
- Soalan Dan Skema Ujian 1 Bio 2022Document9 pagesSoalan Dan Skema Ujian 1 Bio 2022SHARIFAH BINTI MOHAMAD HADI MoeNo ratings yet
- Oxidation and Reduction (KIMIA SPM)Document29 pagesOxidation and Reduction (KIMIA SPM)Jachinta JuliusNo ratings yet
- Tindak Balas RedoksDocument3 pagesTindak Balas Redoksmkanwars100% (5)
- Sebatian KarbonDocument99 pagesSebatian KarbonRozi Bt YusofNo ratings yet
- 4.2 (C) Kedudukan Hidrogen Dalam Siri Kereaktifan LogamDocument2 pages4.2 (C) Kedudukan Hidrogen Dalam Siri Kereaktifan LogamDanish AhmadNo ratings yet
- Tindak Balas Pengoksidaan Dan PenurunanDocument6 pagesTindak Balas Pengoksidaan Dan Penurunanmkanwars100% (3)
- BAB 4 Jadual Berkala UnsurDocument2 pagesBAB 4 Jadual Berkala UnsurzaizazmkNo ratings yet
- Jus A-Bhg B - 4-Bab 4,5Document25 pagesJus A-Bhg B - 4-Bab 4,5Haida BakriNo ratings yet
- Bab Jirim Dan Bahan Ting 4 LatihanDocument2 pagesBab Jirim Dan Bahan Ting 4 Latihantumirah86No ratings yet
- T4 Bab 3 Formula & Persamaan Kimia (Lat)Document9 pagesT4 Bab 3 Formula & Persamaan Kimia (Lat)残桥灬断雪100% (1)
- Senarai Eksperimen Dan Aktiviti Tingkatan 4 Dan 5Document5 pagesSenarai Eksperimen Dan Aktiviti Tingkatan 4 Dan 5lhmoo50% (2)
- 2.5 Kesan Daya: Fizik Tingkatan 4Document31 pages2.5 Kesan Daya: Fizik Tingkatan 4sadyehNo ratings yet
- Uk1 Kimia f4Document24 pagesUk1 Kimia f4Izz KhawarizmiNo ratings yet
- p1 Oti 1 TG 5 Chemistry 2011Document34 pagesp1 Oti 1 TG 5 Chemistry 2011Jaaizah JaafarNo ratings yet
- K2 KedahDocument29 pagesK2 Kedahryder1man6433No ratings yet
- Bengkel Pecutan Kimia 2023Document31 pagesBengkel Pecutan Kimia 2023sitizabidahnawayiNo ratings yet
- PKSM f4Document8 pagesPKSM f4NurImanNo ratings yet
- Bab 03 Kompilasi K1 Percubaan 2019 Susun - Ok - RawDocument22 pagesBab 03 Kompilasi K1 Percubaan 2019 Susun - Ok - RawakmarfadzilNo ratings yet
- Struk TurDocument12 pagesStruk TurSiva GuruNo ratings yet
- Soalan Kimia T4 2020Document12 pagesSoalan Kimia T4 2020Nor Hafizah Bt Mohd AliNo ratings yet
- Modul Kimia Tingkatan 4Document7 pagesModul Kimia Tingkatan 4Ain FarhaniNo ratings yet
- Modul Smart Chem 122 WPKL PDFDocument565 pagesModul Smart Chem 122 WPKL PDFThung Ling75% (4)