Professional Documents
Culture Documents
Accelerated Exposure Tests of Painted Steels With Different Surface Preparations of Steel Substrate PDF
Uploaded by
José Avendaño0 ratings0% found this document useful (0 votes)
39 views6 pagesThis study examined corrosion characteristics of Painted Steels with different surface preparations. 9-mm thick 70 150 mm steel plates were made of two types of structural steels. The painted specimens were exposed into the S6-cycle corrosion environment for 251 days.
Original Description:
Original Title
Accelerated Exposure Tests of Painted Steels With Different Surface Preparations of Steel Substrate.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis study examined corrosion characteristics of Painted Steels with different surface preparations. 9-mm thick 70 150 mm steel plates were made of two types of structural steels. The painted specimens were exposed into the S6-cycle corrosion environment for 251 days.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
39 views6 pagesAccelerated Exposure Tests of Painted Steels With Different Surface Preparations of Steel Substrate PDF
Uploaded by
José AvendañoThis study examined corrosion characteristics of Painted Steels with different surface preparations. 9-mm thick 70 150 mm steel plates were made of two types of structural steels. The painted specimens were exposed into the S6-cycle corrosion environment for 251 days.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 6
ACCELERATED EXPOSURE TESTS OF PAINTED STEELS WITH DIFFERENT
SURFACE PREPARATIONS OF STEEL SUBSTRATE
In-Tae Kim
1
, Yoshito Itoh
2
, Shigenobu Kainuma
3
and Yoshihisa Kadota
4
ABSTRACT : This study examined corrosion characteristics of painted steels with different surface
preparations. Accelerated corrosion tests were performed on the painted steels using A Combined
Cyclic Corrosion Test Instrument. The combined cyclic test condition adopted in the research was the
S6-cycle, which is applicable for simulating atmospheric exposure conditions. 9-mm thick 70 150
mm steel plates were made of two types of structural steels, J apan Industrial Standard (J IS) SM490A
and SMA490AW. The surfaces of the steel plates were girt-blasted with No.50 grit specified in J IS S-
G50. In each set, 9 of 12 plates were pre-corroded by the accelerated test, and then they were surface-
treated mechanically by a disc grinder in three ways, i.e. complete removal, slight removal, and no
removal of corrosion productions on the corroded surfaces. The 12 steel plates were painted with
multilayer paint films except 20 70 mm rectangular substrate surface. Cross-scribed lines reaching
the steel substrate were made in the painted surface.
The painted specimens were exposed into the S6-cycle corrosion environment for 251 days (about S6-
1000 cycles). The surface geometry, gloss, and thickness of the paint films were measured at the
exposure times of 0, 67, 141, and 251 days. After the accelerated tests, paint films and corrosion
productions were removed mechanically and chemically, and then surface geometry and thickness loss
of the steels were also measured. Based on blistering area of paint films and thickness loss of the steel
substrates, the effect of the surface preparations on the durability of painted steels was discussed.
KEYWORDS: Corrosion, Painted Steels, Surface Preparation, Durability
1. INTRODUCTION
Corrosion is one of the most important causes of deterioration for steel structures. Organic and
metallic coatings have been widely used to prevent corrosion attacks, and to maintain the functional
ability of the steel structures to bear loads. In addition, coatings are also used to preserve cosmetic
appearance, which is often a major concern for structures exposed to public view. The coatings in
atmospheric environments are deteriorated, and periodic recoating is necessary for steel structures to
prevent environmental attacks and to extend their service life. Because the recoating work lead to
increase in lifecycle cost, durability evaluation of recoating becomes one of the most important issues
in maintenance of old steel structures.
The durability of coating systems has been often evaluated by atmospheric exposure tests. Although
they allow field examinations, it is takes long time to obtain any deterioration data of the coating
systems. In addition, it is difficult to obtain fundamental information since various environmental
factors, such as temperature, humidity, flying salt and carbon dioxide affect corrosion process in each
1
Research Associate, Nagoya University, Japan
2
Professor, Nagoya University, Japan
3
Associate Professor, Gifu University, Japan
4
Graduate Student, Nagoya University, Japan
2
test site. In these reasons, accelerated exposure tests in a laboratory are employed to obtain the
fundamental data and to complement the data of the field exposure tests.
In recent years, Fujiwara (Hiroshi et al. 1997) performed on accelerated exposure tests of 7 sets of
accelerated test conditions, called SS, S6, DS, J ASO, NS, seawater-NS, and ASTM-cycles. Based on
the results of painted steels filed exposure tests in 5 sites in J apan, he presented the correlation
between laboratory and field tests and proposed that S6-cycle test conditions is applicable for
simulating the field exposure tests. The S6-cycle was proposed by the Ministry of International Trade
and Industry and was specified in J IS. Itoh (Yoshito et al. 2002) performed on laboratory accelerated
tests of unpainted steel plates, and proposed accelerating factors of the S6-cycles against field
exposure tests using weight of flying salt.
This study performed laboratory accelerated tests conforming to the S6-cycle test condition to
examine corrosion characteristics and durability evaluation of painted steels with different surface
preparations of steel substrate. Four sets of surface preparations, grit-blast cleaning and hand-tool
cleaning of complete, slight, and no removal of rusts, were used in the experiment. Comparing
blistering area of paint films and thickness loss of steel substrate, the effect of the surface preparation
on the durability of the painted steels is discussed.
2. EXPERIMENT PROCEDURE
2.1 Equipment of Experiment
A Combined Cyclic Corrosion Test Instrument made by SUGA TEST INSTRUMENTS Co., Ltd. was
used in the research. This equipment can operate automatically the conditions of atomizing of salt
water, temperature, and humidity in arbitrary order and combination. This has an environmental
chamber of 2000 1000 500 mm, in which 188 test specimens of 70 150 mm can be arranged.
The S6-cycle test condition adopted in the experiment is shown in Figure 1.
2.2 Test Specimen Preparation
The fabrication process of test specimens and the geometry of the specimen are shown in Figure 2 and
Blast surface preparation of steel plates
(SM490W and SMA490WA)
Pre-corrosion (30 days)
3 types of surface
preparation
C-4 Painting C-3 Painting
4 sets of painted test specimens
Figure 2. Process in fabrication of test specimens
Atomizing of 5%
salt water
30 2 , 98%
0.5hr
Wetting
30 2 , 95%
1.5hr
Drying
50 2 , 20%
2.0hr
Drying
30 2 , 20%
2.0hr
Figure 1. Condition of accelerated environment cycle (S6-cycle)
Figure 3. Dimension and configurations of test
specimens (unit in mm)
1 cycle (6.0 hr)
70
50
20
150
9
55
55
20
20
Unpaintedregion
Paintedregion
Cross-scribed
region
Boundary
region
Scribedline
3
3, respectively. Two types
of steel plates, called
normal structural steel
plates and weathering
structural steel plates,
were made of J apan
Industrial Standard (J IS)
SM490A and SMA490AW
steels, respectively. In
each type, 12 steel plates
of 70 150 9 mm were
prepared. They were grit-
blasted by No.50 grit
specified in J IS S-G50. 9
of 12 steel plates in each
type were pre-corroded by
the S6-cycle corrosion
tests for 30 days, and then they were treated mechanically by a disc grinder in three ways, i.e.
complete removal of all visual rusts, slight removal of rusts, which remains tight residues of rusts in
the bottoms of corrosion pits, and no removal of rusts in each for 3 corroded plates.
All steel plates were painted with multilayer paint films having different functions, called C-3 or C-4
painting systems, except 20 70 mm rectangular region of steel substrate. Total thickness of paint
films is between 0.3 to 0.4 mm. Cross-scribed lines of 0.3mm width, which reached the steel substrate,
were made in the painted surface using a cutter knife. The specimen edges were protected with an
extra thickness of paint films in order to prevent corrosion attack in these parts. The details of the test
specimens are summarized in Table 1.
2.3 Test Procedure and Measurement
The test specimens were exposed in the environmental chamber of the S6-cycle corrosion conditions
during 251 day (about S6-1000 cycles). The thickness, gloss, and blistering area of paint films were
measured at exposure times of 0, 67, 147, and 251 days. After the accelerated tests, corrosion
productions and paint films on the steel substrates were removed by boiling with ammonium citric and
thioureas, and then the geometry of steel surface under paint films was also measured by A Laser
Focus Measuring Instrument.
3. EXPERIMENTAL RESULTS
3.1 Classification of Specimen Surface
In the research, paint films were scribed to create a well-defined defect, and resistance is judged by the
degree of corrosion attack at the scribe lines. An unpainted region was also prepared to determine the
degree of corrosion attack at the boundary of painted and unpainted regions. As shown in Figure 3,
the test surface was divided into three regions, painted region(0 X 20 mm), cross-scribed region
(20 X 90 mm), and boundary region (90 X 150 mm). In the painted region, deterioration of the
paint films was invisible to the naked eye.
3.2 Cross-scribed Region
1) Blistering of the paint film
Corrosion originated at the scribed lines, undercut the paint film/steel substrate interface, and moved
out from the lines. As a result of this process, blistering of paint film was produced. Typical surfaces
Table 1. Details of test specimens
Symbol Number Surface preparation degree*
Pre-
corrosion
Steel type
NL4 3 Grit blast cleaning (Level4)
NL3 3 Hand tool cleaning (Level3)
NL2 3 Hand tool cleaning (Level2)
NL1 3 No cleaning (Level1)
Normal
structural
steel
(SM 490A)
WL4 3 Grit blast cleaning (Level4)
WL3 3 Hand tool cleaning (Level3)
WL2 3 Hand tool cleaning (Level2)
WL1 3 No cleaning (Level1)
Weathering
structural
steel
(SMA
490AW)
* Level 3, 2, and 1 indicate the surface conditions that removal all visible corrosion
productions, that slight removal of rust, remaining tight residues of rust in the
bottoms of corrosion pits, and no removal corrosion productions, respectively.
4
of the cross-scribed regions
at 251 days are shown in
Table 2. Blistering of the
paint films in the NL4
specimen is observed near
the scribe lines. On the
other hand, corrosion in the
other specimens moved out
from the lines, and then the
blistering is widened far
away from the lines.
The geometry of the
painted surfaces was measured at 0.5-mm interval in both width and length direction on the surface
using a Laser Focus Measuring Instrument. From these data, blistering area was calculated, and its
mean is plotted against to the testing time in Figure 4. When the testing time increases, the blistering
area also increases gradually, while the increase in the NL4 and WL4 specimens are little. The
difference in the blistering area due to the surface preparation degree becomes clear as the testing time
increases.
Mean and mean s data of the area for 251 days is shown in Figure 5. The blistering area increases in
this order of Level 4 (NL4 or WL4), 3 (NL3 or WL3), 2 (NL2 or WL2) and 1 (NL1 or WL1).
Comparing the blistering area, it is observed that the blistering of paint films depends on the surface
preparation degree: corrosion initiation and propagation in blast-cleaned painted steels is limited near
the defects, and that in hand-tool cleaned steels drastically increases.
2) Deterioration of the painted steels
The corrosion resistance in painted steels is usually characterized by the degree of corrosion attack at
Table 2. Blistering in the cross-scribed region
No corrosion NL4 NL3 NL2 NL1
Figure 4. Increase in blistering area Figure 5. Blistering area at 251 days
(a) Normal steels (SM490A) (b) Weathering steels (SMA490WA)
Figure 6. Deterioration curves of painted steels
Testing time (day)
0 50 100 150 200 250 300
B
l
i
s
t
e
r
i
n
g
a
r
e
a
o
f
p
a
i
n
t
f
i
l
m
(
m
m
2
)
0
1000
2000
3000
4000
5000
NL4
WL4
NL3
WL3
NL2
WL2
NL1
WL1
B
l
i
s
t
e
r
i
n
g
a
r
e
a
o
f
p
a
i
n
t
f
i
l
m
(
m
m
2
)
0
1000
2000
3000
4000
5000
NL4 NL3 NL2 NL1 WL4 WL3 WL2 WL1
Normal Steel Weathering Steel
Testing time (day)
0 1000 2000 3000 4000
B
l
i
s
t
e
r
i
n
g
a
r
e
a
o
f
p
a
i
n
t
f
i
l
m
(
m
m
2
)
0
1000
2000
3000
4000
5000
6000
NL4
NL3
NL2
NL1
NL4
NL3
NL2
NL1
Testing time (day)
0 1000 2000 3000 4000
B
l
i
s
t
e
r
i
n
g
a
r
e
a
o
f
p
a
i
n
t
f
i
l
m
(
m
m
2
)
0
1000
2000
3000
4000
5000
6000
WL4
WL3
WL2
WL1
WL4
WL3
WL2
WL1
5
scribed lines. In addition, adhesion between paint
films and steel substrates is the most important
property of organic painting because if it fails, all
other paint properties become worthless. Herein,
based on the blistering area of paint films resulted
from loss of the adhesion, deterioration degree of
the painted steels is evaluated. The data of the
blistering area were fitted by Gompertzs growth
regression curves,
t
c
ab y = , as shown in Figure 6,
where 4900 mm
2
is the area of the cross-scribed
region. The application of the Gompertzs curve
for evaluating deterioration of paint films was
theoretically confirmed by Nishimura (Akira et al. 1985). He presented that long-time deterioration of
paint films can be estimated using corroded area under the films in early stage of the deterioration
process.
The testing time for the same blistering area tends to shorten in the order of Level 4, 3, 2 and 1
specimens, respectively, while those for the Level 2 and Level 1 are almost equal. For example, when
the blistering area is 2000 mm
2
, the testing time is 250 days for the NL2 and NL1 specimens, 350 days
for the NL3 specimens, and 1200 days for NL4 specimens. From comparison of the regression curves
obtained from the present data, it is observed that blistering area of painted steels depends on the
degree of the surface preparations, while it is almost same when the rusts remained on the substrate
after the surface preparation.
3) Thickness loss
After removal of paint films and rusts, the geometry of the surfaces was measured. From these data,
corrosion depth (thickness loss) in the three regions, painted region, cross-scribed region, and
boundary region, were calculated. In this calculation, average thickness of the paint region, where no
corrosion attack was observed, was taken as the thickness of each specimen. Based on the thickness in
each specimen, thickness loss in the cross scribe region was calculated. Mean thickness loss tend to
increase in the order of Level 4, 3, 2, and 1. However it is below 0.06 mm for the normal steels, N-
series specimens, and below 0.25 mm for the weathering steels, W-series specimens. In maximum
thickness loss, the NL4 and WL4 specimens show the smallest thickness loss in each set, and the other
specimens in each set show almost the same mean values. It is noted that mean thickness loss in
unpainted steel plates is 0.2 to 0.25 mm (Kainuma et al. 2002).
3.3 Boundary Region of Painted and unpainted regions
1) Blistering area
Blistering area at 251 days in the boundary region is plotted in Figure 7. It tends to increase in the
order of Level 4, 3, 2, and 1 similar to that in the cross-scribed region.
Figure 7. Blistering area in boundary region
Figure 8. Average thickness loss in an AP2 specimen
B
l
i
s
t
e
r
i
n
g
a
r
e
a
o
f
p
a
i
n
t
f
i
l
m
(
m
m
2
)
0
500
1000
1500
2000
Upside
Downside
NL4 NL3 NL2 NL1 WL4 WL3 WL2 WL1
Normal Steel Weathering Steel
0 20x10
3
40x10
3
60x10
3
80x10
3
100x10
3
120x10
3
140x10
3
A
v
e
r
a
g
e
l
o
s
s
i
n
t
h
i
c
k
n
e
s
s
(
1
0
-
3
m
m
)
1000
800
600
400
200
0
-200
Cross-scribed region Boundary region
Painted region
Cross-scribed line
Unpainted region
6
2) Thickness loss
Mean and mean s corrosion depths in each
specimen were measured. The mean thickness loss
in the width direction is plotted in Figure 8.
Thickness loss under paint film is occurred in the
cross-scribed region and the boundary region. It is
invisible in the painted region. The mean thickness
loss maximizes at the side of paint films along the
boundary-lines of paint and upside/ bottom side of
the unpainted region.
The maximum and mean thickness loss in the
boundary region of each specimen is shown in
Figure 9. Difference in the average thickness due
to the surface preparation is not clear, while the maximum thickness loss tends to reduce in the order
of Level 4, 3, 2, and 1. The maximum loss in the NL4 and WL4 is 1.5 to 3.5 mm, and that of the
others is 0.7 to 2.0 mm.
4. SUMMARY
This study performed the corrosion characteristics of painted steels with different surface preparations
of steel substrate. The surfaces of the steel plates were surface-treated in four ways, and they were
painted with multilayer paint films. In this procedure, unpainted region and cross-scribed lines were
made in the painted surface. The painted steels were exposed in the S6-cycle accelerated test condition
during 251days.
From blistering area of paint films and thickness loss in steel substrate, the effect of the degree of
surface preparations on deterioration of paint discontinuities, cross-scribed lines and boundary of
painting and unpainting regions was discussed. Deterioration curves of four sets of test specimens
were also presented.
5. REFERENCES
Fujiwara, H. and Tahara, Y. (1997), Research on the correlativity of outdoor exposure test of painting
test piece with corrosion test for steel bridge painting, Journal of Structural Mechanics and
Earthquake Engineering, No. 570/I-40, 129-140.
Itoh, Y., Iwata, A., and Kainuma, S. (2002), Accelerated exposure tests of environmental durability
for steels and the estimation of acceleration coefficient, Journal of Structural Engineering,
JSCE,Vol. 48A, 1021-1029.
Kainuma, S., Hoshomi, N., Kim, I.T., and Itoh, Y. (2002), Fatigue behavior of corroded out-of-plane
gusset welded joints, Proceedings of 2
th
International Conference on Advances in Structural
Engineering and Mechanics (ASEM02), Busan.
Funke, W. (1981), Blistering of paint films and filiform corrosion, Progress in Organic coatings,
Vol. 9, 29-46.
Denny, A.J . (1992), Principles and prevention of corrosion, Prentice Hall International, Inc., Singapore.
Funke, W. (1997), Problems and progress in organic coatings science and technology, Progress in
Organic coatings, Vol. 31, 5-9.
Masuko, N. (1981), Corrosion initiation and propagation under paint films, Anticorrosion
Engineering (Bousyoku Gizyutsu), Vol. 30, 699-704.
Nishimura, S. and Shimada, K. (1985), Theoretical analysis of deterioration behavior of paint film in
steel structures, Bridge and foundation engineering, No.5, 17-21.
Figure 9. Thickness loss in boundary region
T
h
i
c
k
n
e
s
s
l
o
s
s
(
m
m
)
0.5
1.5
2.5
3.5
0.0
1.0
2.0
3.0
4.0
NL4 NL3 NL2 NL1 WL4 WL3 WL2 WL1
Normal Steel Weathering Steel
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Surface PreparationDocument8 pagesSurface PreparationimyparkarNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Corrosion Inhibitors - Principles, Mechanisms and Applications PDFDocument16 pagesCorrosion Inhibitors - Principles, Mechanisms and Applications PDFleonardoNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Integard 475HSDocument2 pagesIntegard 475HSJosé AvendañoNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Use of Organic Corrosion Inhibitors in High Performance CoatingsDocument29 pagesUse of Organic Corrosion Inhibitors in High Performance CoatingsJosé AvendañoNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Use of Organic Corrosion Inhibitors in High Performance CoatingsDocument29 pagesUse of Organic Corrosion Inhibitors in High Performance CoatingsJosé AvendañoNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Surface Preparation of Concrete (Floors)Document3 pagesSurface Preparation of Concrete (Floors)José AvendañoNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Tale of Two Contractors - The Hidden Staggering Costs of InefficiencyDocument6 pagesA Tale of Two Contractors - The Hidden Staggering Costs of InefficiencyJosé AvendañoNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- A Hard Assignment - Getting A Good Read On Moisture in ConcreteDocument11 pagesA Hard Assignment - Getting A Good Read On Moisture in ConcreteJosé AvendañoNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Abrasive Selection Requires Evaluation of Needs Cost and ProductivityDocument4 pagesAbrasive Selection Requires Evaluation of Needs Cost and ProductivityJosé AvendañoNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- An Experimental Study of Bolted Shear ConnectionsDocument245 pagesAn Experimental Study of Bolted Shear ConnectionsJosé AvendañoNo ratings yet
- Advanced Paints Help Improve IAQDocument6 pagesAdvanced Paints Help Improve IAQJosé AvendañoNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Applicator Training Bulletin - Introduction To Plural-Component SprayDocument3 pagesApplicator Training Bulletin - Introduction To Plural-Component SprayJosé AvendañoNo ratings yet
- Why It's A Mistake To Reuse Old Coating SpecsDocument2 pagesWhy It's A Mistake To Reuse Old Coating SpecsJosé AvendañoNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Writing Safety Into Your Coating SpecificationDocument5 pagesWriting Safety Into Your Coating SpecificationJosé AvendañoNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Coatings Specifications, Good, Bad or UglyDocument3 pagesCoatings Specifications, Good, Bad or UglyJosé AvendañoNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- A Hard Assignment - Getting A Good Read On Moisture in ConcreteDocument11 pagesA Hard Assignment - Getting A Good Read On Moisture in ConcreteJosé AvendañoNo ratings yet
- What Is CorrosionDocument4 pagesWhat Is CorrosionmkccmNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Tightrope - Identifying Limiting Conditions For Coatings SpecificationDocument4 pagesTightrope - Identifying Limiting Conditions For Coatings SpecificationJosé AvendañoNo ratings yet
- Achieving Efficiencies With Coatings and Linings Work in Gulf Coast EnvironmentsDocument4 pagesAchieving Efficiencies With Coatings and Linings Work in Gulf Coast EnvironmentsJosé Avendaño100% (1)
- Writing Safety Into Your Coating SpecificationDocument5 pagesWriting Safety Into Your Coating SpecificationJosé AvendañoNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Problems Caused by The Lack of Clarity and Definition in Coating SpecificationsDocument4 pagesProblems Caused by The Lack of Clarity and Definition in Coating SpecificationsJosé AvendañoNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Condition Survey - The Backbone of A Good Coating SpecificationDocument4 pagesCondition Survey - The Backbone of A Good Coating SpecificationJosé AvendañoNo ratings yet
- Methods and Pitfalls in Selecting Coating Systems For SpecificationDocument2 pagesMethods and Pitfalls in Selecting Coating Systems For SpecificationJosé AvendañoNo ratings yet
- Aesthetic Repairs To Concrete Without CoatingsDocument3 pagesAesthetic Repairs To Concrete Without CoatingsJosé AvendañoNo ratings yet
- Research News Blistering in Resinous Systems On Concrete Surfaces Types Causes and Preventive MeasuresDocument11 pagesResearch News Blistering in Resinous Systems On Concrete Surfaces Types Causes and Preventive MeasuresJosé AvendañoNo ratings yet
- Corrosion Control Plan For BridgesDocument34 pagesCorrosion Control Plan For BridgesJosé AvendañoNo ratings yet
- The Basics of - Solvents and Thinners PDFDocument20 pagesThe Basics of - Solvents and Thinners PDFJosé Avendaño100% (3)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- MS 2021-2022Document106 pagesMS 2021-2022Ege Arda AkyürekNo ratings yet
- احمد ابراهيمDocument25 pagesاحمد ابراهيمAhmd amynNo ratings yet
- Separators SizingDocument16 pagesSeparators SizingRonald GeorgeNo ratings yet
- Physical Science With Earth Science NotebookDocument341 pagesPhysical Science With Earth Science NotebookJanice Read100% (1)
- Why Tight-Binding Theory?: Walter A. HarrisonDocument5 pagesWhy Tight-Binding Theory?: Walter A. HarrisonzoehdiismailNo ratings yet
- G200Document4 pagesG200Gul Hassan Akhunzada100% (2)
- Me6301 Engineering Thermodynamics Nov Dec 2014.Document3 pagesMe6301 Engineering Thermodynamics Nov Dec 2014.BIBIN CHIDAMBARANATHANNo ratings yet
- Jahangirabad Instiute of Technology Barabanki Department of Mechanical EngineeringDocument23 pagesJahangirabad Instiute of Technology Barabanki Department of Mechanical EngineeringMuhammad ImranNo ratings yet
- CHEM 135 Exam 2 F15 KeyDocument7 pagesCHEM 135 Exam 2 F15 KeyMikeNo ratings yet
- Register of Pesticides March 26 2020 Active IngredientDocument26 pagesRegister of Pesticides March 26 2020 Active Ingredient20 Võ Xuân KỳNo ratings yet
- Nabakem PCB Insulating Coating AgentDocument4 pagesNabakem PCB Insulating Coating Agentsutrisno00No ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Notes+4 +ATP,+Water+and+Inorganic+IonsDocument5 pagesNotes+4 +ATP,+Water+and+Inorganic+IonsSyeda Wardah NoorNo ratings yet
- Pipeline 2009 Vol21 01Document12 pagesPipeline 2009 Vol21 01hozhabrNo ratings yet
- Annex A 2007Document6 pagesAnnex A 2007ChemicalB0yNo ratings yet
- IEEMA Circular Aug 2020Document2 pagesIEEMA Circular Aug 2020AMARENDRA SINo ratings yet
- Class 8 Revised SyllabusDocument5 pagesClass 8 Revised SyllabusAman AmanNo ratings yet
- Sys Bio TextDocument25 pagesSys Bio TextAlpahNo ratings yet
- Chapter 15 Fluid and Chemical BalanceDocument47 pagesChapter 15 Fluid and Chemical BalanceIntan FirmallahNo ratings yet
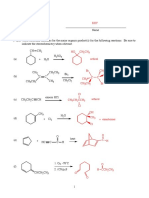
- MT Lab Mini Project Groups - 3!2!2022Document6 pagesMT Lab Mini Project Groups - 3!2!2022Pavan ChinnaNo ratings yet
- ManufacturingDocument2 pagesManufacturingBarbieBarbieNo ratings yet
- Sample Preparation, Gas Chromatography-Mass Spectrometry, and Data AnalysisDocument2 pagesSample Preparation, Gas Chromatography-Mass Spectrometry, and Data AnalysisAndrés MárquezNo ratings yet
- Sreeraj Gopi (editor), Augustine Amalraj (editor), Shintu Jude (editor) - High-Resolution Mass Spectroscopy for Phytochemical Analysis_ State-of-the-Art Applications and Techniques-Apple Academic PresDocument271 pagesSreeraj Gopi (editor), Augustine Amalraj (editor), Shintu Jude (editor) - High-Resolution Mass Spectroscopy for Phytochemical Analysis_ State-of-the-Art Applications and Techniques-Apple Academic PresAna JuliaNo ratings yet
- The Basics of Reading A Spark Plug - Honda-TechDocument19 pagesThe Basics of Reading A Spark Plug - Honda-Techdinamik2tNo ratings yet
- A3.01 Union List of Food Additives Approved For Use in FoodDocument33 pagesA3.01 Union List of Food Additives Approved For Use in FoodBesian OsmaniNo ratings yet
- Pages FromThakore, Shuchen B. - Bhatt Introduction To Process Engineering and DesignDocument191 pagesPages FromThakore, Shuchen B. - Bhatt Introduction To Process Engineering and DesignAhmed HassanNo ratings yet
- HSSC-II PHYSICS HALF SYLLABUS (16-To-20) April 2021Document4 pagesHSSC-II PHYSICS HALF SYLLABUS (16-To-20) April 2021Heaven ColoursNo ratings yet
- Structural Steel DesignDocument108 pagesStructural Steel DesignMilomir Gavrilovic77% (13)
- Foamysense n60k PolymerDocument4 pagesFoamysense n60k Polymerthanhviet02072000No ratings yet
- Aspen Exchanger Design and Rating Shell & Tube V9Document1 pageAspen Exchanger Design and Rating Shell & Tube V9MAYANK AGRAWALNo ratings yet
- USP 191 Identification Tests-General - Chemical Equations - by - Jude Daval-SantosDocument17 pagesUSP 191 Identification Tests-General - Chemical Equations - by - Jude Daval-Santosjude_daval_santos100% (5)