Professional Documents
Culture Documents
UV-Visible Spectroscopy Report
Uploaded by
amy0%(1)0% found this document useful (1 vote)
2K views7 pagesUV-Visible Spectroscopy Report
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentUV-Visible Spectroscopy Report
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0%(1)0% found this document useful (1 vote)
2K views7 pagesUV-Visible Spectroscopy Report
Uploaded by
amyUV-Visible Spectroscopy Report
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 7
Amy Tran 12R
UV-Visible Spectroscopy Iron in Dietary Supplements
Introduction
Ultraviolet-visible spectroscopy (UV/Vis) refers to the absorption spectroscopy in the ultraviolet-
visible spectral region. Many molecules absorb ultraviolet or visible radiation as they move between
energy levels. The wavelength of radiation that is absorbed directly affects the perceived colour of the
chemicals involved and is related to molecular structure. Different molecules absorb radiation of
different wavelengths; therefore an absorption spectrum can be used to qualitatively identify
compounds.
However, UV/Vis spectroscopy is mainly used in the quantitative analysis of compounds. When a
substance absorbs visible light, it appears coloured. The human eye does not see the colour that is
absorbed by the sample; however, what is seen is the complement of the absorbed colour. For
example, a solution of copper(II) sulphate appears blue because the absorption energy comes from the
orange region of the visible spectrum.
Iron is an essential human nutrient, as it has many roles within the body. A lack of iron can lead to the
development of iron deficiency anaemia. To combat this, many people use iron dietary supplements in
order to help maintain healthy levels of iron. To detect iron with the use of UV/Vis spectroscopy, the
reaction between Fe
3+
and thiocyanate irons (SCN
-
) must occur. This reaction gives an intensely red-
coloured product that can be used as a qualitative test to determine the presence of Fe
3+
. However,
most of the iron in dietary supplements is in its ferrous form and must be oxidised to the ferric form
with the use of hydrogen peroxide (H2O2). This is to ensure that the iron can be sufficiently bonded
with the thiocyanate irons, giving the red colour of the solution.
Aim
To determine the mass of Fe
3+
in a sachet of a dietary supplement using UV-Visible Spectroscopy.
Hypothesis
It is hypothesised that the mass of Fe
3+
found by using UV-visible spectroscopy is the same as the
mass of Fe
3+
stated on the sachet of the dietary supplement.
Materials
2.000 x 10
-4
M Fe
3+
standard solution
4 M HNO3 solution
10% KSCN solution
10% H2O2 solution
Iron dietary supplement (5mg/25mL)
Distilled water
5 x 25 mL volumetric flasks
250 mL volumetric flask
2 x small beakers
6 x Pasteur pipettes
6 x cuvettes
Autopipette
Spectrophotometer
Spectrometer
Safety goggles
Lab coat
Amy Tran 12R
Method
Part 1: Preparation of the calibration curve
1. The beaker labelled iron standard solution was rinsed with a small amount of the standard
solution and then 40 mL of the solution was placed into the beaker.
2. Five 25 mL volumetric flasks were labelled with numbers 1 to 5.
3. An autopipette was used to transfer the amounts of iron standard solution listed in the table
to the flasks.
4. The 4M HNO3 and 10% KSCN solutions were added to each of the flasks.
5. The solutions were mixed by stoppering and shaking, then the colour variations in the flasks
were checked by eye.
Solution Fe
3+
solution 4 M HNO3
solution
10% KSCN
solution
Distilled
water
Fe
3+
concentration
1 0 mL 2 mL 2 mL To the line 0 M
2 1 mL 2 mL 2 mL To the line 0.000008 M
3 2 mL 2 mL 2 mL To the line 0.000016 M
4 3 mL 2 mL 2 mL To the line 0.000024 M
5 4 mL 2 mL 2 mL To the line 0.000032 M
6. 5 cuvettes were filled with each of the solutions using a clean Pasteur pipette each time and
were arranged in order of concentration.
Part 2: Frequency of light absorbed
1. A spectrophotometer was used to record the spectrum of the solution in flask 4 over a range of
approx. 400 700 nm. (refer to spectrum included)
Part 3: Determining the iron content of the supplement
(i) Preparation of the sample solution
1. The contents of the sachet were emptied into a small beaker.
2. An autopipette was used to add 1.0mL of this liquid into a 250 mL volumetric flask.
3. 1 mL of 10% H2O2 solution was added to the flask.
4. 10 mL of 4M HNO3 and 10 mL of 10% KNCS was added to the solution.
5. The flask was made up to the mark with distilled water using a clean plastic pipette.
(ii) Analysis of the stock solution
1. The sample was transferred to a cuvette and the absorbance was measured at 473.0 nm (the
wavelength of maximum absorbance determined in part 2)
Part 4: Amount of light absorbed
1. The spectrometer was set to 473.0 nm.
2. The absorbance of each solution was measured. (refer to Table 1)
3. Using this information, a calibration curve was plotted. (refer to Graph 1)
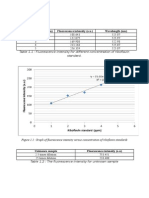
Results
Table 1: The concentration of Fe
3+
in each solution with the corresponding absorbance
Solution Fe
3+
concentration (M) Absorbance
1 0 0
2 0.000008 0.026
3 0.000016 0.109
4 0.000024 0.144
5 0.000032 0.205
Sample unknown 0.098
Amy Tran 12R
Graph 1: Calibration curve of the absorbance against concentration of Fe
3+
Spectrum 1: Absorbance spectrum (attached)
1. What is the iron concentration in the sachet solution (in the 250 mL flask)?
16 x 10
-6
M (according to the graph)
2. Assuming the sachet contains exactly 25.00 mL of iron supplement, what is the mass of iron
in the sachet?
3. Calculate the mass of Fe3(PO4)2 in the sachet.
0
0.05
0.1
0.15
0.2
0.25
0 0.000005 0.00001 0.000015 0.00002 0.000025 0.00003 0.000035
A
b
s
o
r
b
a
n
c
e
Concentration of Fe
3+
(M)
Graph 1: Calibration Curve
Amy Tran 12R
Discussion
1. Briefly describe the method used in your analysis. A diagram illustrating the various steps
involved might be helpful.
2. Briefly describe the principles of operation and the major components of the spectrometer
you used. A labelled diagram might be helpful.
3. Are there other parts of the instrument (aside from the sample) that could absorb light from
your light source? Has this affected your results? Why/why not?
Aside from the sample, no other parts of the instrument could absorb light from the light
source. If there was a part of the instrument that could absorb light, then the end results
would be different, making the amount of iron determined using UV/Vis would be different to
what was stated by the manufacturer.
4. Was the analytical procedure quantitative or qualitative, or both? Explain fully.
The analytical procedure was quantitative because it was used to determine the concentration
of Fe
3+
in the dietary supplement in terms of molarity.
5. What is the iron content as stated by the manufacturer?
The iron content as stated by the manufacturer was 5mg/25mL.
Standard solutions
were prepared
with varying
concentrations of
Fe
3+
.
Sample solution
was prepared.
Sample and
standard
solutions
were
transferred
into cuvettes.
A
spectrophotometer
was used to find the
absorption
spectrum.
A spectrometer was used
to find the absorption of
each of the samples.
From these results, a
calibration curve was
determined.
Using the
calibration curve,
the concentration of
Fe
3+
in the dietary
supplement was
found.
Amy Tran 12R
6. What uncertainties (or errors) were involved in the procedure?
The nitric acid and potassium thiocyanate were not added to the flask with the 0 M Fe
3+
(flask
1). The contents of this flask were used to calibrate the spectrometer to 0 M concentration.
Fortunately, the results of the procedure were unaffected despite the nitric acid and
potassium thiocyanate not being present and the calibration curve produced was as expected.
7. Were there any unexpected results?
All the results were as expected and there were no unexpected results.
8. What are some other applications or uses of UV-visible spectroscopy that you investigated?
UV/Vis can be used in clinical analysis, measuring the concentrations of specific substances in
body fluids such as urine or blood. For example, the haemoglobin content and sugar levels in
blood can be found by using UV/Vis. In addition to this, like the experiment conducted,
UV/Vis can be utilised to identify the presence of metal ions; even if the metal ion itself is not
coloured, it is possible to be analysed if it is converted into a coloured compound. For
example, finding the amount of calcium in urine can be found using UV-Vis if an organic
complexing agent (eg. arsenazo III) is reacted with it to form a highly coloured liquid.
9. Why is iron particularly suited to this form of analysis? Do you think it would be detected by
the other instruments used in this workshop?
Iron is particularly suited to this form of analysis because UV/Vis is routinely used in
analytical chemistry for the quantitative determination of different substances like transition
metal ions. They can be detected because the solutions of these metal ions are often coloured.
The detection of iron could also be done by Atomic Absorption Spectroscopy (AAS) because it
is also a type of spectroscopy therefore meaning it is able to detect iron ions in a solution.
Conclusion
The amount of iron in a 25mL sachet of iron dietary supplement according the manufacturer is 5mg.
By using UV/Vis spectroscopy, the dietary supplement was tested and it was determined that there is
5.44mg of iron in the supplement. This result therefore supports the hypothesis that the mass of iron
found by using UV-Vis is the same as the mass of iron as stated on the sachet of the dietary
supplement. Five standard solutions were made with increasing concentrations of Fe
3+
with the
addition of nitric acid and potassium thiocyanate in order to make the solution a red colour. Then the
iron from the dietary supplement was prepared by oxidising the Fe
2+
ions to Fe
3+
using hydrogen
peroxide, then nitric acid and potassium thiocyanate were added to make this solution red as well. The
standard solutions and sample were then transferred into cuvettes, and an absorption spectrum was
found using a spectrophotometer and the solution in cuvette 4. The absorption spectrum clearly
showed the wavelength where the maximum amount was absorbed (473.0 nm), allowing us to use the
spectrometer to find the absorbance of each of the standard solutions and the sample. From these
results, a calibration curve was made and the concentration of the sample was able to be found.
The reason as to why there was a 0.44mg difference between the final result and the mass stated on
the sachet is likely to be because the manufacturer found it unnecessary to have the mass of iron in the
sachet to have a decimal point when their users are likely not to mind the miniscule difference.
Alternatively, another reason as to who there was a difference is because of inaccurate results. The
accuracy of the results could be improved if there werent any time restrictions, therefore allowing
more time to prepare more standard solutions and, in turn, a more accurate calibration curve.
In conclusion, it was learned that UV/Vis has many uses, i.e. finding the concentration of metal ions in
a compound. Finally, a greater understanding of the principles and applications of UV/Vis was gained
from the outcome of this experiment.
Amy Tran 12R
Risk Assessment
Preparation/Provision
of
Acute Hazards Control Measures First Aid
Nitric acid
It is not combustible,
but can enhance
combustion of other
substances.
Gives off
irritating/toxic fumes.
If inhaled, it can cause
sore throat, coughing,
burning sensation,
headache, shortness of
breath and labored
breathing.
Can cause serious skin
burns with yellow
discolouration.
If it comes into
contact with eyes, it
can cause redness,
pain and burns.
If ingested, it can
cause abdominal pain,
sore throat, burning
sensation, shock or
collapse and vomiting.
Ensure the acid is well
away from flammable
substances and any
combustibles or
organic chemicals.
Keep in a well-
ventilated room that is
cool and dry.
Wear safety gear (lab
coat, gloves, protective
goggles) and do not
eat, smoke or drink
during work.
In case of fire, do not
use foam. Keep cool
by spraying with
water.
If inhalation occurs,
treat with fresh air
and rest in a half-
upright position.
Artificial respiration
may be needed. Refer
for medical attention.
If there is exposure to
skin, remove
contaminated clothing
and rinse skin with
plenty of water. Refer
for medical attention.
If there is contact with
eyes, rise with plenty
of water for several
minutes and refer for
medical attention.
If ingested, do not
induce vomiting. Rest
and give one or two
glasses of water to
drink. Refer for
medical attention.
Potassium thiocyanate It is not combustible,
but gives off irritating
fumes/gases in a fire.
If inhaled, it can cause
coughing.
If ingested, it can
cause confusion,
convulsions, nausea,
vomiting and
weakness.
Keep separated from
strong oxidants in a
dry, well-closed area.
Wear safety gear (lab
coat, gloves, protective
goggles) and do not
eat, smoke or drink
during work.
In case of fire, use the
appropriate
extinguishing
medium.
If inhaled, treat with
fresh air and rest.
If there is exposure to
skin, remove
contaminated clothing
and rinse skin with
plenty of water.
If there is contact with
eyes, rise with plenty
of water for several
minutes and refer for
medical attention.
If ingested, rinse
mouth. Give a slurry
of activated charcoal
in water to drink.
Refer for medical
Amy Tran 12R
attention.
Hydrogen peroxide It is not combustible,
but it can ignite
combustible materials.
There is a risk of fire
on contact with heat
or metal catalysts.
Inhalation can cause
sore throat, cough
dizziness, headache,
nausea and shortness
of breath.
If it comes into
contact with skin, it is
corrosive and causes
white spots, redness,
skin burns and pain,
If it comes into
contact with eyes, it is
corrosive and causes
redness, pain, blurred
vision and severe deep
burns.
If ingested, it can
cause abdominal pain,
abdominal distention,
nausea and vomiting.
Ensure the acid is well
away from
combustibles,
reducing agents and
hot surfaces.
Store in a cool, dark
area in vented
containers separated
from combustible and
reducing agents, food,
strong bases and
metals. Wear safety
gear (lab coat, gloves,
protective goggles)
and do not eat, smoke
or drink during work.
In case of fire, use
water in large
amounts (water
spray).
If inhalation occurs,
treat with fresh air
and rest in a half-
upright position. Refer
for medical attention.
If there is exposure to
skin, remove
contaminated clothing
and rinse skin with
plenty of water and
rinse again. Refer for
medical attention.
If there is contact with
eyes, rise with plenty
of water for several
minutes and refer for
medical attention.
If ingested, rinse
mouth, do not induce
vomiting and refer for
medical attention.
You might also like
- I'd Give My Life For You - Miss SaigonDocument6 pagesI'd Give My Life For You - Miss Saigonamy25% (4)
- I'd Give My Life For You - Miss SaigonDocument6 pagesI'd Give My Life For You - Miss Saigonamy25% (4)
- Preparation of Turkey Red OilDocument16 pagesPreparation of Turkey Red OilHimanshu Jha73% (11)
- EDTA Titrations: An Introduction to Theory and PracticeFrom EverandEDTA Titrations: An Introduction to Theory and PracticeRating: 2.5 out of 5 stars2.5/5 (3)
- Physics Precision Measurement ReportDocument7 pagesPhysics Precision Measurement ReportBramwel Mbogo78% (76)
- Exp 5 Gas ChromatographyDocument8 pagesExp 5 Gas Chromatographylebogang75% (4)
- Determination of Fluoride Concentration Using Ion Selective ElectrodeDocument7 pagesDetermination of Fluoride Concentration Using Ion Selective ElectrodeAmanda WangNo ratings yet
- Spectrophotometric Determination of Iron in Vitamin TabletsDocument13 pagesSpectrophotometric Determination of Iron in Vitamin TabletsSophie CroninNo ratings yet
- CO2 Depressurisation OLGADocument8 pagesCO2 Depressurisation OLGAMaheshNo ratings yet
- 1 4 Bookmark A Crash Course in Forces MotionDocument2 pages1 4 Bookmark A Crash Course in Forces Motionapi-115513756No ratings yet
- Maintenance of Mining MachineryDocument22 pagesMaintenance of Mining Machineryjorgeluis2000100% (1)
- Spectrophotometric Determination of IronDocument2 pagesSpectrophotometric Determination of IronNiaz Ali KhanNo ratings yet
- Acid/Base Titration LabDocument5 pagesAcid/Base Titration LabDavid GrahamNo ratings yet
- Experiment 1: Potentiometric TitrationDocument11 pagesExperiment 1: Potentiometric TitrationJoni Ilagan100% (1)
- Lab Report To Determine The Concentration Using GC-MSDocument9 pagesLab Report To Determine The Concentration Using GC-MSSamuel Ogeda OtienoNo ratings yet
- Atomic Absorption SpectrosDocument9 pagesAtomic Absorption Spectrosamirul azhar80% (10)
- EXPERIMENT 3: AAS Determination of Calcium in Commercial Supplement TabletsDocument11 pagesEXPERIMENT 3: AAS Determination of Calcium in Commercial Supplement Tabletsdjambulaziz100% (3)
- Lightweight UavDocument149 pagesLightweight Uavvb corpNo ratings yet
- Determination of Riboflavin by Fluorescence SpectrophotometryDocument8 pagesDetermination of Riboflavin by Fluorescence SpectrophotometryNajwa ZulkifliNo ratings yet
- High Performance Liquid Chromatography (HPLC), Method DevelopmentDocument4 pagesHigh Performance Liquid Chromatography (HPLC), Method DevelopmentAmirul Azhar100% (9)
- Discussion On Potentiometric TitrationsDocument16 pagesDiscussion On Potentiometric TitrationsKcirtap Zketh60% (5)
- Cyclic Voltammetry Lab ManualDocument3 pagesCyclic Voltammetry Lab ManualGourav DasNo ratings yet
- Electrolytic ConductanceDocument8 pagesElectrolytic Conductancevijaye36100% (1)
- Argentometric - Titration of ChlorideDocument13 pagesArgentometric - Titration of Chloridenurhidayat71100% (1)
- Determination of Copper by AASDocument18 pagesDetermination of Copper by AASscarmathor9092% (50)
- Polarography: Principles and Applications of the Electroanalytical TechniqueDocument20 pagesPolarography: Principles and Applications of the Electroanalytical TechniqueRiya Das100% (1)
- 10-Lab-10Spectrophotometric Determination of PhosphatDocument4 pages10-Lab-10Spectrophotometric Determination of PhosphatHoang Huong Tra33% (3)
- Spectrophotometric Determination of IronDocument7 pagesSpectrophotometric Determination of IronJoseph PelaeloNo ratings yet
- Potentiometric Titration of Cerium SolutionDocument4 pagesPotentiometric Titration of Cerium SolutionValentin-AngeloUzunov100% (1)
- Liquid Chromatography Lab ReportDocument4 pagesLiquid Chromatography Lab ReportOmar Alkhadra100% (1)
- Analysis of Aspirin - Infrared (Ir) Spectroscopy and Melting Point DeterminationDocument15 pagesAnalysis of Aspirin - Infrared (Ir) Spectroscopy and Melting Point DeterminationMahmoud ElshahawyNo ratings yet
- How To Design R.C. Flat Slabs Using Finite Element AnalysisDocument16 pagesHow To Design R.C. Flat Slabs Using Finite Element Analysisocenkt100% (10)
- GC MSDocument9 pagesGC MShyutoyrNo ratings yet
- Reaction Rate and Rate Constant of The Hydrolysis of Ethyl Acetate With Sodium HydroxideDocument5 pagesReaction Rate and Rate Constant of The Hydrolysis of Ethyl Acetate With Sodium HydroxideAmyNo ratings yet
- Lab Report HPLCDocument8 pagesLab Report HPLCafifiNo ratings yet
- Thermal Analysis of Albendazole Investigated by HSM, DSC and FTIRDocument8 pagesThermal Analysis of Albendazole Investigated by HSM, DSC and FTIRElvina iskandarNo ratings yet
- Title Uv-Vis Determination of An Unknown Concentration Kmno SolutionDocument4 pagesTitle Uv-Vis Determination of An Unknown Concentration Kmno SolutionMuhammad Amirul AfifiNo ratings yet
- chm510 Exp2Document10 pageschm510 Exp2May LeeNo ratings yet
- Experiment 1 The Potentiometric Titration of Hydrogen PeroxideDocument10 pagesExperiment 1 The Potentiometric Titration of Hydrogen PeroxideAfiqah SamanNo ratings yet
- Amino Acid TitrationDocument9 pagesAmino Acid TitrationuğurNo ratings yet
- 2-14 Determination of The Dissociation Constant of Weak AcidsDocument3 pages2-14 Determination of The Dissociation Constant of Weak Acidsdbroncos78087100% (6)
- UV-Vis Determination of KMnO4 ConcentrationDocument5 pagesUV-Vis Determination of KMnO4 ConcentrationMustafidzul Mustapha56% (9)
- Complexometric TitrationDocument4 pagesComplexometric TitrationWendell Kim LlanetaNo ratings yet
- 4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFDocument27 pages4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFWanqing HeNo ratings yet
- Experiment 5 Determination of Caffeine 2020Document4 pagesExperiment 5 Determination of Caffeine 2020FYNo ratings yet
- Determination of Lead in Anchovies by Cold Vapour Generation Atomic Absorption SpectrometryDocument30 pagesDetermination of Lead in Anchovies by Cold Vapour Generation Atomic Absorption SpectrometryIbrahim Muhamad100% (2)
- Determination of Vitamin CDocument2 pagesDetermination of Vitamin CWalwin HareNo ratings yet
- UV-VIS Experiment AS230Document8 pagesUV-VIS Experiment AS230Qamarul IzzatNo ratings yet
- Inorganic Lab1Document50 pagesInorganic Lab1Mohamed YasinNo ratings yet
- The Gravimetric Determination of Sulfate in A Soluble SampleDocument6 pagesThe Gravimetric Determination of Sulfate in A Soluble SampleKris CruzNo ratings yet
- Heat of ReactionDocument8 pagesHeat of ReactionNece Jean Tagam83% (6)
- NaBH4 Reduction of Cyclohexanone to Cyclohexanol (87Document8 pagesNaBH4 Reduction of Cyclohexanone to Cyclohexanol (87hahadindongNo ratings yet
- Preparation and Standardisation of Base and Acid SolutionDocument6 pagesPreparation and Standardisation of Base and Acid Solutionhasifah abdaziz80% (5)
- Simultaneous determination of chromium and manganeseDocument35 pagesSimultaneous determination of chromium and manganeseVatra ReksaNo ratings yet
- Introduction to Spectrophotometry: Principles, Techniques and ApplicationsDocument19 pagesIntroduction to Spectrophotometry: Principles, Techniques and Applicationsabhinav_ramana100% (1)
- Experiment 5 Analysis of Chlorpyrifos in Water Using Solid-Phase Extraction (SPE) and Gas Chromatography-Electron Capture Detector (GC-ECD)Document8 pagesExperiment 5 Analysis of Chlorpyrifos in Water Using Solid-Phase Extraction (SPE) and Gas Chromatography-Electron Capture Detector (GC-ECD)NUR IZZATI OTHMAN BASRINo ratings yet
- S Determination of Caffeine in BeveragesDocument5 pagesS Determination of Caffeine in BeveragesVioleta Grigoras100% (1)
- Experiment 6Document7 pagesExperiment 6sajithNo ratings yet
- Instrumental Analytical Methods Experiment 1 - Flame-Photometric AnalysisDocument3 pagesInstrumental Analytical Methods Experiment 1 - Flame-Photometric Analysisapi-23518718950% (2)
- Riboflavin Concentration Fluorescence SpectroscopyDocument5 pagesRiboflavin Concentration Fluorescence SpectroscopySyahriezan HaminNo ratings yet
- CV LabDocument9 pagesCV LabtworedpartyhatsNo ratings yet
- Conductometric TitrationDocument5 pagesConductometric TitrationChayon Mondal100% (1)
- Objectives: FIGURE A: Example of Coordination CompoundsDocument7 pagesObjectives: FIGURE A: Example of Coordination CompoundsNurul izzatiNo ratings yet
- Exp4 chm456Document8 pagesExp4 chm456Mawar AhmadNo ratings yet
- Ioron Determination in WaterDocument6 pagesIoron Determination in WaterGobe JamNo ratings yet
- Analytical ChemistryDocument8 pagesAnalytical ChemistryNaheed AwanNo ratings yet
- Atomic Absorption SpectrosDocument7 pagesAtomic Absorption SpectrosDavid Joram MendozaNo ratings yet
- Exp.7 Quantitative Analysis of IronDocument10 pagesExp.7 Quantitative Analysis of Ironhadiyaharif10No ratings yet
- Photosynthesis PracDocument4 pagesPhotosynthesis PracamyNo ratings yet
- Acid Content of VinegarDocument5 pagesAcid Content of VinegaramyNo ratings yet
- Comparing Masses of Reactants and ProductsDocument4 pagesComparing Masses of Reactants and ProductsDaniel TriumbariNo ratings yet
- A2 Nuclear Models LiqDrop FermiGasDocument19 pagesA2 Nuclear Models LiqDrop FermiGasAbdul RehmanNo ratings yet
- Samuel Glasstone - Thermodynamics For Chemists PDFDocument532 pagesSamuel Glasstone - Thermodynamics For Chemists PDFRimmon Singh100% (2)
- NCHRP RPT 242 PDFDocument85 pagesNCHRP RPT 242 PDFDavid Drolet TremblayNo ratings yet
- 9702 w04 QP 4Document16 pages9702 w04 QP 4api-3706826No ratings yet
- Catalog Whatman 2018.compressedDocument214 pagesCatalog Whatman 2018.compressedRakha Milan BachtiarNo ratings yet
- KIT-DISSOLVED OXYGEN CHEMets® Refills, ULR CHEMets®-KITDocument2 pagesKIT-DISSOLVED OXYGEN CHEMets® Refills, ULR CHEMets®-KITSorinNo ratings yet
- Offshore Pipeline Hydraulic and Mechanical AnalysesDocument25 pagesOffshore Pipeline Hydraulic and Mechanical AnalysesEslam RedaNo ratings yet
- Ricapito-1 PbLi-T DatabaseDocument16 pagesRicapito-1 PbLi-T DatabaseSasa DjordjevicNo ratings yet
- The Chronology Protection ConjectureDocument4 pagesThe Chronology Protection ConjectureKrisNo ratings yet
- Metodo Epa 18Document40 pagesMetodo Epa 18Luis TrejoNo ratings yet
- Effect of Speration in Modified BitumenDocument12 pagesEffect of Speration in Modified BitumenyadavameNo ratings yet
- Radiation Heat Transfer in Combustion Systems - Viskanta and Menguc PDFDocument64 pagesRadiation Heat Transfer in Combustion Systems - Viskanta and Menguc PDFXamir Suarez Alejandro100% (1)
- M.Prasad Naidu MSC Medical Biochemistry, PH.D Research ScholarDocument31 pagesM.Prasad Naidu MSC Medical Biochemistry, PH.D Research ScholarDr. M. Prasad NaiduNo ratings yet
- Isaacs. Differential GamesDocument13 pagesIsaacs. Differential GamescrovaxIIINo ratings yet
- Bhavans Public School, Doha - Qatar: Model Question Paper 2016-17 MathematicsDocument4 pagesBhavans Public School, Doha - Qatar: Model Question Paper 2016-17 MathematicsSanthosh KrishnanNo ratings yet
- Cluster Level Question Bank (Ahmedabad & Gandhinagar Cluster)Document33 pagesCluster Level Question Bank (Ahmedabad & Gandhinagar Cluster)manpreetsingh3458417No ratings yet
- How Does DCPIP WorkDocument3 pagesHow Does DCPIP WorkIsaac LeeNo ratings yet
- The Weighted Histogram Analysis Method (WHAM) : Michael AndrecDocument14 pagesThe Weighted Histogram Analysis Method (WHAM) : Michael AndrecWilliam AgudeloNo ratings yet
- Math 115 HW #9 Solutions PDFDocument7 pagesMath 115 HW #9 Solutions PDFHyan Gontijo0% (1)
- Energy Balance Untuk Teknik KimiaDocument19 pagesEnergy Balance Untuk Teknik Kimiamelisa amaliaNo ratings yet
- Direct Determination of The Flow Curves of NoDocument4 pagesDirect Determination of The Flow Curves of NoZaid HadiNo ratings yet