Professional Documents
Culture Documents
CDU Overhead Multiple Corrosion
Uploaded by
rvkumar61100%(1)100% found this document useful (1 vote)
640 views7 pagesMultiple Corrosion activity in Crude unit overhead corrosion
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentMultiple Corrosion activity in Crude unit overhead corrosion
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
100%(1)100% found this document useful (1 vote)
640 views7 pagesCDU Overhead Multiple Corrosion
Uploaded by
rvkumar61Multiple Corrosion activity in Crude unit overhead corrosion
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 7
Multiple corrosion mechanisms in
crude distillation overhead system
W
ith declining crude
quality and the high proft
potential of opportunity
crudes, refners continue to face a
diffcult balancing act when controlling
corrosion: determining the optimum
combination of crude blends, unit
operations, corrosion-control programs
and unit maintenance in order to achieve
the greatest return on investment for
the refnery. Understanding the source
of corrosion is critical. It is only after the
root cause of the corrosion is properly
identifed that viable mitigation
solutions can be selected based on the
refners unique circumstances and
short- and long-term goals.
The Baker Petrolite TopGuard
overhead corrosion-control
program from Baker Hughes Inc is a
comprehensive, engineering-based
program designed to meet refners
proft objectives in the most cost-
effective way possible. Working
closely with each refner, Baker
Hughes provides the knowledge
required to effectively manage the
corrosive impact of specifc crude
blends and operating conditions. The
following case study provides a detailed
summary of the troubleshooting
efforts and the methods implemented
to successfully reduce the threat of
corrosion-related failures at a refnery
in Canada.
History of corrosion incidents
More than four years ago, problematic
episodes of corrosion occurred in the
overhead condensing system of the #3
crude unit atmospheric tower at Irving
Oil in Saint John, New Brunswick,
Canada. Corrosion occurred in three
separate locations in the overhead, with
each location experiencing a different
Extensive troubleshooting efforts determine distinct corrosion mechanisms
simultaneously attacking multiple areas of an atmospheric tower overhead system
GEorGE DuGGan and ranDy rEcHtiEn Baker Hughes
LionEL robErts Irving Oil
mechanism of attack. Although
uncommon, there are industry
examples of the simultaneous
occurrence of different corrosion
mechanisms in a single overhead
system.
1
For this particular system, the primary
sources of corrosion were strongly
related to unit operating conditions,
contaminant levels in the crude and,
ultimately, contaminant levels in the
tower overhead itself. The refnery
processed blends of either sweet or
sour crudes in blocked operation. These
alternating crude slates, combined with
seasonal variations in tower operations,
produced a wide range of corrosive
environments in the overhead.
In particular, spikes in overhead
hydrochloric acid (HCl) concentration
increased the formation potential of
ammonium chloride (NH
4
Cl) salt and
made pH control of overhead drum
water more diffcult. During sour
crude processing, increased levels
of hydrogen sulphide (H
2
S) in the
overhead produced preferential attack
on copper-based equipment. Higher
system temperatures and higher fow
rates during some operating modes
created localised zones in which
velocity-accelerated corrosion was
prevalent.
The variations in system conditions
required more diligence on the part of
operators, inspectors and corrosion-
control engineers to address the
problems. Multiple analytical and
monitoring techniques were required
to identify the cause of the corrosion
mechanism and to develop appropriate
mitigation options. To this end,
efforts were conducted to correlate
operational changes with periods of
www.digitalrefning.com/article/1000598 PTQ Q3 2009 43
Off gas
Sour water
Naphtha product
Pelief valves
|nhibitor Ammonia
Peflux
water
wash
T 22001
E 22001 A/B
E 22027 A/H
D 22001
Figure 1 Schematic of atmospheric overhead system
electrical resistance (ER) probes
and weight loss coupons at several
locations in the overhead. Specifcally,
a total of seven monitoring devices
were installed as follows: the main
overhead vapour line (x 1); the E-22001
A/B inlets (x 4); and the E-22001 A/
B outlets (x 2). In addition, frequent
UT measurements were taken by the
refnerys inspection department. In
general, the rates measured at these
locations were within acceptable
ranges. However, there were occasional
periods of unacceptably high corrosion
rates (>0.25 mm/year [>10 mpy]) at
these locations. The periods of high
corrosion rates usually correlated to
variations in operating modes and/or
switches in crude blends.
The corrosion activity in the
atmospheric tower overhead was most
severe from late 2004 through late
2006. During this period, the refnery
processed several different crudes of
varying sulphur content. Typically,
the unit was operated in blocked
operation: several days of sweet crude
and then several days of sour crude,
and so on. Crude blends with sulphur
contents below 1.0% were considered
sweet. In addition to variations in
crude sulphur, the tower was operated
on seasonal cycles (summer vs winter
operation). These seasonal modes
covered a relatively wide range of
tower operations, particularly in terms
of tower top temperature and overhead
fow rate. Table 1 summarises the
typical conditions under each of the
four primary operating modes. There
were three distinct areas of the overhead
that experienced periods of excessive
corrosion activity:
Pressure relief valves at the tower
top
E-22001 A/B tube bundles
E-22001 A/B outlet elbows.
Details of the corrosion mechanisms
increased corrosion activity. The Baker
Petrolite Ionic Model was employed
to calculate amine-hydrochloride salt
formation temperatures and to defne
safe operating envelopes. Detailed
compositional analyses of scale deposits
and metallurgical analyses of weight
loss coupons were also performed.
Traditional methods for measuring
metal loss rates provided insight into
the magnitude of corrosion activity
as well.
system overview
The #3 crude unit atmospheric
distillation tower overhead (Figure 1)
comprises a set of two parallel shell-
and-tube exchangers (E-22001 A/B)
that are vertically oriented. In these
exchangers, process vapours exchange
heat with cold crude oil. The exchanger
outlet streams are combined and then
fed to a set of eight air coolers (E-22027
A-H). The vapour/liquid mixture from
the air coolers is separated in the D-
22001 drum. Naphtha from the drum is
divided between both the tower refux
and the product. A portion of sour
water from the drum is continuously
recycled to the overhead vapour line
for use as wash water.
The E-22001 A/B exchanger tube
bundles are constructed of 70/30
copper/nickel alloy (UNS C71500).
The remaining overhead equipment is
constructed of carbon steel. The existing
corrosion-control program includes
an oil-soluble inhibitor injected into
the overhead vapour line via a refux
carrier. Neutralisation is provided
by an aqueous ammonia solution
(approximately 20% concentration),
which is injected into the overhead
line via the water wash. Currently, the
ammonia injection rate is adjusted to
maintain a nominal target pH range of
66.5 in the overhead drum. During the
periods of corrosion activity discussed
herein, the drum was typically operated
at a pH of 7.0 or higher.
Corrosion rate monitoring was
measured via a combination of
44 PTQ Q3 2009 www.digitalrefning.com/article/1000598
Chloride in D-2200l drum water, ppm
75
l00
l25
l50
l75
200
225
250
275
300
325
350
50
30 40 50 60 70 80 90 l00 ll0 l20 l30 l40 l50 l60 l70 l80 20
m
p
p
,
r
e
t
a
w
m
u
r
d
l
0
0
2
2
-
D
n
i
a
i
n
o
m
m
A
Range of typical operations
104 C 110 C 113 C 116 C
107 C
Figure 2 NH
4
Cl salt formation temperatures at tower top (winter-sour operations)
operating mode tower top temp stripping steam to tower naphtha refux, bpd naphtha product, bpd total naphtha, bpd
Winter-sour 129C (264F) 11 022 kg/hr (24 300 lb/hr) 38 100 23 000 61 100
Winter-sweet 148C (298F) 11 340 kg/hr (25 000 lb/hr) 36 800 30 000 66 800
Summer-sour 146C (294F) 14 379 kg/hr (31 700 lb/hr) 29 960 27 400 57 360
Summer-sweet 152F (306F) 13 926 kg/hr (30 700 lb/hr) 28 000 33 300 61 300
Note: For these operating modes, tower top pressures ranged from 234241 kPa (3435 psia)
typical unit conditions for each operating mode
table 1
and associated mitigation steps for
each of these areas are provided in the
following sections.
corrosion mechanism 1:
ammonium chloride deposition
background
Beginning in late 2004, there were
indications of corrosion activity in
several of the pressure relief valves
(PSV) located at the top of the
distillation tower. These PSVs were
connected along a common manifold
that was uninsulated. UT measurements
revealed corrosion rates in the range of
0.51.0 mm/year (2040 mpy) in this
area. Measured corrosion rates were at
the high end of this range during the
winter modes of operation. In addition,
radiography (x-ray) measurements
indicated the build-up of deposits on
the PSV internals and in the manifold
piping. These monitoring results
suggested that a corrosive deposit,
most likely an NH
4
Cl salt, was forming
in the PSV header.
troubleshooting efforts
In an effort to confrm the damage
mechanism, electrolyte-based process
simulation modelling (Baker Petrolite
Ionic Model)
2,3
was performed at tower
top conditions. In particular, the salt
formation temperature of NH
4
Cl was
determined using the average
concentration of HCl and ammonia in
the D-22001 drum water under each
operating mode. As shown in Table 2,
the tower top temperatures were higher
than the calculated salt formation
temperatures for all operating
conditions. These results suggested that
salt formation was not favoured at bulk
stream temperatures and average
contaminant levels.
However, there were two additional
effects that needed to be considered:
locally colder temperatures on the PSV
header wall, and variations in HCl
and ammonia concentrations. Further
modelling efforts were then conducted
to examine salt formation over a wider
range of operations and contaminant
levels. A plot of these results for the
winter-sour mode is given in Figure
2. Each curve (isotherm) represents
the phase boundary for NH
4
Cl salt
formation at the tower top temperature
indicated. The typical ranges of HCl
and ammonia in the D-22001 drum
water are represented by the shaded
region.
Guidelines for avoiding localised
salt deposition in the upper sections of
towers have been developed by Baker
Hughes. These guidelines require that
the bulk tower top temperature be at
least 14C (25F) higher than the salt
formation temperature. In this way,
the tower is protected against shock
cooling caused by cold refux as it enters
the tower. However, for uninsulated
piping in cold weather climates, the
guidelines call for a more stringent
minimum temperature differential of
28C (50F).
As shown in Figure 2, the range of
NH
4
Cl formation temperatures given
by the shaded region is approximately
104114C (219237F). This salt
formation temperature range is only
1525C (2745F) below the tower top
temperature of 129C (264F). As such,
these temperature differentials are less
than the recommended minimum of
28C (50F) for cold weather climates.
Considering higher rates of metal
loss were detected during winter
operation and the PSV manifold was
uninsulated, these results confrmed
that NH
4
Cl deposition and its
associated under-salt mechanism were
the source of corrosion in this area.
Mitigation
Once the mechanism had been properly
identifed, several mitigation options
were considered. These mitigation
options included the following:
Increasing the tower top
temperature
Installing insulation or heat tracing
on the PSV manifold
Decreasing overhead HCl levels
Periodically injecting water to the
manifold.
Increasing the tower temperature
was ruled out because of its adverse
effects on tower operation and desired
product compositions. Fearing that too
much damage had already occurred, the
refner replaced the PSV header in early
2006. The new header was installed
with blind fanged connections and
included heat tracing and insulation.
In addition, the new header had been
water washed during post-2006 unit
shutdowns to ensure any salts that may
have deposited were removed.
About a year after the new PSV
header was installed, another key
operational change was implemented.
In early 2007, a caustic addition
program was initiated, in which caustic
was injected into the desalted crude
at a rate of about 3 ppmw (1lb/1000
bbl). With this caustic application
now in place, the levels of HCl in the
overhead drum water are typically
2030 ppmw, but prior to caustic use
HCl levels were always greater than
60 ppmw. As shown in Figure 2, this
reduction in overhead HCl greatly
minimised the potential for NH
4
Cl
formation at the tower top.
To monitor NH
4
Cl formation potential
on an ongoing basis, a customised
version of the Baker Petrolite Ionic
Model Field Monitor was created. This
tool, which resides with the local feld
staff, is an extension of the Ionic Model.
4
Using operating variables as input, the
monitoring tool determines salt
formation on a frequent (even daily)
basis. As such, the monitoring tool
serves as an early warning system to
alert the refner should NH
4
Cl formation
potential increase when crude types or
tower conditions change.
results
As a result of the mechanical and
chemical mitigation steps employed,
metal loss at the PSV manifold has
been stopped. There is no indication of
table 2
operating mode tower top temp nH
4
cl formation temp
Winter-sour 129C (264F) 111C (232F)
Winter-sweet 148C (298F) 104C (219F)
Summer-sour 146C (294F) 124C (255F)
Summer-sweet 152C (306F) 116C (240F)
nH
4
cl salt formation temperatures for each operating mode
46 PTQ Q3 2009 www.digitalrefning.com/article/1000598
corrosion activity from the most recent
UT measurements in these areas. These
positive results have been confrmed
by the on-site monitoring tool that
continues to be used to track tower
operations.
corrosion mechanism 2:
hydrogen sulphide attack on copper
background
In early 2005, during the same time that
corrosion issues in the PSVs were being
addressed, there were also corrosion
concerns at the E-22001A/B exchangers.
Corrosion rates, as measured by 70/30
Cu/Ni weight loss coupons located
at the exchanger inlets, ranged from
0.250.60 mm/year (1024 mpy). To
determine the corrosion activity on
the exchanger shells and inlet piping,
carbon steel coupons were also installed
at the exchanger inlets. The measured
rates on the carbon steel coupons were
consistently below 0.13 mm/year (5
mpy). The monitoring indicated that
corrosion activity on carbon steel was
lower than that on 70/30 Cu/Ni at the
operating conditions of the exchangers.
Assuming that the inlet coupons
were a reasonable representation of the
metal loss rate on the tubes themselves,
the expected life of the existing bundles
(which had been newly installed in
2003) was less than fve years. Prior to
the new installation, the previous set of
bundles had been in service for eight
years. It should be noted that, during
the previous eight-year run, crude
sulphur levels were lower than those
during post-2003 operation. There was
a conjecture, then, that higher levels of
overhead H
2
S and/or sulphur-oxygen
(SO
x
) species were contributing to the
attack on the bundles.
troubleshooting efforts
To better identify the mechanism of
attack, the Ionic Model was performed
at the exchanger conditions for all
operating modes. The modelling
focused on three key areas:
Performance of the existing water
wash
The pH profle produced by the
ammonia neutraliser injection
Vapour velocities at the exchanger
inlets.
The existing water wash is injected via
atomising spray nozzles at a consistent
rate of 756 litres/minute (200 gallons/
minute). This rate is nearly twice
that recommended by the simulation
modelling. At all points downstream
of the water wash injection, the pH
of the aqueous phase was above 6.0.
Modelling under all operating modes
produced equivalent results. Therefore,
neither NH
4
Cl salt deposition (from
inadequate water washing) nor low
pH aqueous corrosion was a likely
mechanism in the exchangers.
The vapour velocities at the exchanger
inlets ranged from 1518 m/sec (5060
ft/sec). There was no visual indication
to suggest a velocity component to
the corrosion observed on the Cu/Ni
coupons, although these velocities are
at the upper end of typical concern
levels.
3,5
Additional troubleshooting tech-
niques were needed to confrm these
modelling results and to identify the
cause of this corrosion. As part of this
effort, metallurgical analysis was
conducted on a coupon removed from
the system. Figure 3 shows a coupon
that had been installed at the E-22001A
inlet in late 2004. The measured
corrosion rate on this coupon was 0.38
mm/year (15 mpy). Using scanning
electron microscopy (SEM), a cross-
sectional, magnifed view of the coupon
was generated (Figure 4). In addition to
the unaffected base metal, the analysis
revealed two distinct scale layers.
Electron dispersion spectroscopy
(EDS) techniques were used to
determine the composition of the base
metal and of the two scale layers. These
48 PTQ Q3 2009 www.digitalrefning.com/article/1000598
Figure 3 70/30 copper/nickel coupon located in E-22001a inlet line
Figure 4 Scale layers present in copper/nickel coupon (400x magnifcation)
base metal (EDs 03) inner scale layer (EDs 02) outer scale layer (EDs 01)
Copper, wt% 74 4 73
Nickel, wt% 25 64 <1
Iron, wt% <1 1 <1
Sulphur, wt% 31 26
Elemental analyses of copper/nickel coupon scales
table 3
elemental composition results are
summarised in Table 3. As shown, the
nature of the scale layers is markedly
different: the inner layer was
predominantly nickel sulphide, while
the outer layer was composed almost
exclusively of copper sulphide. As
shown in Figure 4, the inner layer has a
tighter, more cohesive structure than
the outer layer. Therefore, it is reasonable
to conclude that the nickel sulphide
layer provided some protection against
attack, while the amorphous copper
sulphide scale did not.
To identify any water-soluble species
present, ion chromatography (IC) was
performed on a water extract of the
scale. As shown in Table 4, the scale
contained chlorides, sulphates and
organic acids, but very little ammonia
and no other amines. Given that it is
common to measure percentage levels
of chlorides and amines in overhead
system scales, these low levels
indicated that amine-hydrochloride
salt corrosion was not the mechanism
of attack. Note also that the pH of the
water extract was nearly neutral. The
pH of a water extract from a scale that
contains chloride salts is typically less
than 4.5. The scale analyses confrmed
the modelling results, which precluded
both under-salt and low pH aqueous
corrosion as viable mechanisms.
The most revealing piece of data
was the high proportion of copper
sulphide in the outer scale layer, which
suggested a preferential attack on
copper. Corrosion of copper alloys in
aqueous sulphide environments has
been noted in the industry.
6
Research in
this area indicates that copper corrosion
can be accelerated in sour systems,
particularly when the aqueous phase
approaches neutral or even alkaline
pH.
7
The conclusion here was that the
inlet coupons (as well as the exchanger
bundles themselves) were subject to
accelerated attack via this aqueous
sulphide mechanism. The rate of attack
was almost certainly more pronounced
during the processing of sour crude
blends, as well as during those times
when system pH was at the high end of
the range (above 6.5).
Mitigation
The main focus of mitigation efforts was
improved pH control. When ammonia
is used as an overhead neutraliser, it is
common to encounter periods of both
high and low pH excursions. As would
be expected for this overhead, operation
outside of the desired drum pH target
range was a frequent occurrence.
Other operational factors made pH
control more challenging. Switching
www.digitalrefning.com/article/1000598 PTQ Q3 2009 49
from one crude blend to another led to
short-term periods of impaired desalter
effciency. Also, some of the routinely
processed crudes were suspected to
contain organically bound chlorides.
As such, HCl levels in the overhead
would rise sharply during a crude
switch. These higher HCl levels often
caused the drum pH to drop below 5.0,
and then, after the ammonia injection
rate was adjusted, the drum pH would
often rise above 8.0.
To address the pH excursions, several
mechanical and operational changes
were implemented. First, in late 2005,
the refner installed an on-line pH meter
at the overhead drum. The measured
output from the meter was connected to
the refnerys distributed control system
(DCS) and the drum pH was displayed
on all control monitors. In this way,
operators who were responsible for
adjustments to the ammonia injection
rate were continually aware of the
drum pH. In addition, an improved
plan for addressing the problems
of crude switches was devised. For
example, ammonia injection rates were
often increased prior to crude switches
in anticipation of lower drum pHs from
increased overhead HCl levels.
4.0
4.5
5.0
5.5
6.0
6.5
7.0
7.5
8.0
8.5
9.0
9.5
3.5
1anuary 05 - December 06
On-line pH
analyser
installed
Lower pH
target
range
Lower pH
target
range
H
p
r
o
t
a
l
u
m
u
c
c
A
Figure 5 D-22001 drum water pH (2005 and 2006)
chloride, ppm sulphate, ppm Formic, ppm acetic, ppm Propionic, ppm ammonia, ppm pH, 1% soln
160 390 200 64 3 3 6.8
Water-soluble species in copper/nickel coupon scale
table 4
Electron dispersion
spectroscopy
techniques were used
to determine the
composition of the
base metal and of the
two scale layers
Lastly, the drum pH target range
was lowered in a step-wise fashion
during 2006. In this way, the pH at the
exchanger inlets was correspondingly
lowered in the hopes of minimising
the preferential attack on the copper
bundles. The results of these changes
on drum pH are shown in Figure 5.
While pH excursions still occurred, the
overall effect was a much higher
percentage of operation within the
desired target ranges.
results
The primary purpose for improving pH
control was the reduction of corrosion
on the exchanger bundles. To this
end, the mitigation steps were very
successful. Since the installation of the
pH meter, the measured corrosion rates
on the inlet coupons have decreased
by a factor of 23. As shown in Figure
6, metal loss rates are now typically
below 0.15 mm/year (6 mpy). These
results indicate that the attack on copper
has been reduced and that the expected
life of the exchanger bundles has now
been increased.
Building on the success of earlier
efforts, the refner recently enhanced
the pH control program. Specifcally,
the adjustment of ammonia injection
rates is now automated. The output
signal from the pH meter is used to
actuate the ammonia injection pump
so that the system is now a closed loop.
Although the system has only been
in place for a few months, the results
are very positive: pH control is nearly
always within range and corrosion rates
at the exchanger inlets remain low.
corrosion mechanism 3:
velocity-accelerated corrosion
background
In addition to the PSVs and the E-
22001A/B inlets, a third region of the
overhead was affected by corrosion
during this same period. Unacceptably
high metal loss was occurring at the
E-22001A/B outlets. Measurements
from ER probes and UT monitoring
indicated attack at the outlet elbows
of the exchangers. Measurements on
piping areas adjacent to the elbows did
not indicate activity. The corrosion rates
at the elbows ranged from 0.250.75
mm/year (1030 mpy). The area of
attack was localised at the outer radii of
both elbows. To prevent the possibility
of failure, the refnery welded external
patches on the elbows in late 2004 and
then reinforced the patches again in
early 2005.
troubleshooting efforts
A thorough review of operating
conditions was conducted to identify
those variables that may have correlated
with the periods of highest corrosion
activity at the exchanger elbows. This
effort revealed several key fndings.
First, during all operating modes, total
overhead fow rates were found to be
10% above design rates. Second, there
was a direct correlation between
measured corrosion rates and outlet
elbow temperatures. As shown in Table
1, the tower top temperature was
typically higher during summer
operation. Therefore, the entire
overhead (including the exchanger
outlets) was generally operating hotter
and at higher rates during these
periods.
In addition, there was an inverse
correlation between measured
corrosion rates and drum pH. That is,
during low pH excursions, corrosion
rates were higher at the outlet elbows.
The review of operating data helped to
Sweet crude, winter
Sour crude, summer
Sweet crude, summer
Sour crude, winter
Temperature, C
5
l0
l5
20
25
30
35
40
45
50
55
60
65
70
75
80
0
82 86 84 88 90 92 94 96 98 l00 l02 l04 l06 80
c
e
s
/
m
,
y
t
i
c
o
l
e
v
r
o
p
a
v
Range of typical operations
Figure 7 Vapour velocities at E-22001 outlet elbows for each operating mode
0.05
0.l0
0.l5
0.20
0.25
0.30
0.35
0.40
0.45
0.50
0.55
0.60
0.65
0.00
5
0
n
a
1
5
0
r
a
M
5
0
y
a
M
5
0
l
u
1
5
0
t
c
O
6
0
n
a
1
6
0
r
p
A
6
0
l
u
1
6
0
v
o
N
7
0
n
a
1
7
0
r
a
M
7
0
v
o
N
8
0
b
e
P
8
0
y
a
M
8
0
l
u
1
On-line pH
analyser
installed
y
p
m
m
,
e
t
a
r
n
o
i
s
o
r
r
o
C
Figure 6 Corrosion rates on E-22001 inlet coupons (20052008)
50 PTQ Q3 2009 www.digitalrefning.com/article/1000598
confrm what was already suspected:
the corrosion at the elbows was more
pronounced under conditions that
promote acidic aqueous corrosion
(higher temperatures and lower pH).
Given that the attack at the elbows
was localised to the outer radii, there
was a strong suspicion that a velocity-
accelerated mechanism was involved.
Corrosion control in areas of high
vapour velocity is always a challenge.
Higher velocities produce higher shear
stresses at the pipe wall. These localised
forces remove the partially protective
corrosion product scale at the wall
and expose fresh metal to more attack.
In addition, the stresses at the wall
place a greater burden on the ability
of inhibitors to flm the surface. Even
under mild pH regimes, high vapour
velocities can promote accelerated
metal loss rates.
Vapour velocities at the elbows were
calculated for all operating modes
(Figure 7). As shown, depending upon
operating mode and outlet temp-
eratures, there is a wide range of
possible vapour velocities. For typical
exchanger outlet temperatures of 89
99C (192210F), vapour velocities can
range from 737 m/sec (23122 ft/sec).
A typical concern level for vapour
velocities in these environments is
about 15 m/sec (50 ft/sec). For a
signifcant portion of the units
operating time, vapour velocities
exceeded this threshold level. In fact,
during periods of summer operation,
vapour velocities at the elbows were
often well above 30 m/sec (100 ft/sec).
Mitigation
There were several options used
to minimise the velocity effects at
the elbows. First, it must be noted
that the mitigation options already
discussed for corrosion mechanisms
1 and 2 provided some beneft here
as well. The injection of caustic to the
desalted crude helped to reduce HCl
levels in the overhead. The installation
of the pH meter resulted in fewer
low pH excursions and better pH
control overall.
To address the specifc issues at
the elbows, the refner set an upper
temperature limit of 102C (215F)
at the exchanger outlets. If system
temperatures exceed this limit, unit
operation is adjusted to meet the
temperature target. In addition, the
refner is considering the installation
of new piping with a larger diameter
to reduce the vapour velocities at the
exchanger outlets. If this piping is
installed, the temperature limit can be
relaxed and less frequent operational
adjustments will be required.
Lastly, as described earlier, because
the on-site monitoring tool can calculate
vapour velocities at any location in the
system, it has provided critical feedback
on changes in corrosion potential as a
function of variations in operations.
results
Improvements in pH control and
adherence to recommended operating
temperature limits have greatly
reduced the corrosion activity at the
outlet elbows. Current metal loss rates
as measured by ER probes at the outlets
typically range from 0.050.15 mm/year
(26 mpy). These results agree with UT
measurements taken in the same areas.
conclusions
Although the simultaneous occurrence
of multiple corrosion mechanisms in
this overhead system was problematic,
the issues were not insoluble. The
end result of this TopGuard program
implementation was a corrosion-control
strategy designed to manage even the
most diffcult corrosion challenges.
The cause of each mechanism, to one
degree or another, was found to be a
function of crude types and operating
conditions. Only after the source of each
mechanism was properly identifed via
modelling and analytical techniques
could mitigation steps be employed. As
detailed here, and as is frequently the
case, no single mitigation step could
address all of the problems. Instead, a
combination of operational mechanical
and monitoring options was required
to minimise corrosion activity and
improve unit reliability.
Reproduced with permission from NACE
International, Houston, Texas. All rights
reserved. Paper no. 09332 presented at the
March 2009 NACE Corrosion Conference and
Expo. 2009 NACE International.
TopGuard (TOPGUARD) is a trademark of Baker
Hughes Incorporated.
The authors would like to thank Baker Hughes
Account Representative James Titus and Baker
Hughes Canadian Fuel Additives Manager Paul
Winters for their assistance in generating the
data used in this study.
references
1 Saab M, Dias O, Faqeer F, Damage mechanisms
and corrosion control in a crude unit overhead
line, NACE Corrosion 2005, paper 566.
2 Duggan G, Rechtien R, Application of Ionic
equilibria process simulation for atmospheric
distillation overhead systems, NACE Corrosion
1998, paper 586.
3 Giesbrecht W, Duggan G, Controlling salt
corrosion, Hydrocarbon Engineering, November
2007.
4 Lack J, Stay on top of your corrosion control
strategy, Hydrocarbon Engineering, October
2008.
5 Gutzeit J, Problems with injection facilities
for process additives or water wash, NACE
Corrosion 1996, paper 591.
6 Sharma S, Reaction of copper and copper
oxide with H
2
S, J. Electrochem. Society, 127,
January 1980.
7 Lenglet M, Lopitaux J, et al, Analysis of
corrosion products formed on copper in Cl
2
/
H
2
S/NO
2
exposure, J. Electrochem. Society, 142,
November 1995.
George Duggan is Manager, Industrial Corrosion
Control, for Baker Hughes Incorporated in
Sugar Land, Texas. Duggan has a BSChE from
the University of Missouri.
Email: George.Duggan@BakerHughes.com
randy rechtien is a Senior Technical Support
Engineer for Baker Hughes Incorporated in St
Louis, Missouri. Rechtien holds a BSChE from
Rice University, Texas.
Email: Randy.Rechtien@BakerHughes.com
Lionel roberts is a Technical Manager for Irving
Oil Refning G.P. in Saint John, New Brunswick,
Canada. Roberts holds a BSc in metallurgical
engineering from the Technical University of
Nova Scotia (now Dalhousie).
Email: lionel.roberts@irvingoil.com
52 PTQ Q3 2009 www.digitalrefning.com/article/1000598
Links
More articles from the following
categories:
corrosion/Fouling control
You might also like
- Navistar Diagnostic Trouble Codes EGES395 - DTCDocument8 pagesNavistar Diagnostic Trouble Codes EGES395 - DTCjpablop1278% (9)
- Naphthenic Acid Corrosion Risk Assessment and MitigationDocument24 pagesNaphthenic Acid Corrosion Risk Assessment and MitigationSampat100% (3)
- Upstream Process Engineering Course: 4. SeparationDocument46 pagesUpstream Process Engineering Course: 4. SeparationReza SalimiNo ratings yet
- Crude Distillation UnitsDocument32 pagesCrude Distillation Unitsmoujahed100% (1)
- Corrosion Prevention Yanbu RefineryDocument10 pagesCorrosion Prevention Yanbu RefineryOmid Hn100% (1)
- 05-Crude Unit - Corrosion Control TechnologyDocument55 pages05-Crude Unit - Corrosion Control TechnologyJosé Fernando TerronesNo ratings yet
- Sulphuric Acid - Process EngineeringDocument12 pagesSulphuric Acid - Process EngineeringChaitanya Potti100% (1)
- Pump SizingDocument7 pagesPump Sizingrvkumar61No ratings yet
- Pump SizingDocument7 pagesPump Sizingrvkumar61No ratings yet
- Distillation Tower Internals InstallationDocument10 pagesDistillation Tower Internals Installationrvkumar61No ratings yet
- Radar Absorbent MaterialDocument15 pagesRadar Absorbent Materialisa_krizNo ratings yet
- High TAN CrudesDocument7 pagesHigh TAN Crudesmanassk100% (1)
- Effective Corrosion Control Techniques For Crude Unit OverheadsDocument17 pagesEffective Corrosion Control Techniques For Crude Unit OverheadsNishat M PatilNo ratings yet
- Corrosion in Cdu LectureDocument36 pagesCorrosion in Cdu LectureashrafsaberNo ratings yet
- APP NOTE 01 Crude Unit OverheadDocument2 pagesAPP NOTE 01 Crude Unit OverheadDaniele CirinaNo ratings yet
- Condensate Return CEPDocument8 pagesCondensate Return CEPrvkumar61No ratings yet
- Cdu Presentation 13 07 2010Document38 pagesCdu Presentation 13 07 2010mujeebmeharNo ratings yet
- Valero Energy Benicia RefineryDocument34 pagesValero Energy Benicia RefineryonkarNo ratings yet
- DESALTERSDocument48 pagesDESALTERSPRET1971100% (1)
- Crude DistillationDocument35 pagesCrude DistillationraisNo ratings yet
- Section 3: Desalters: Reduced Crude Unit Corrosion. at The High Temperatures Found in Crude UnitDocument48 pagesSection 3: Desalters: Reduced Crude Unit Corrosion. at The High Temperatures Found in Crude Unitrvkumar61No ratings yet
- Mercer 9100Document16 pagesMercer 9100dtmgoNo ratings yet
- UOP Proper Design NHT Combined Feed Exchanger Equipment PaperDocument9 pagesUOP Proper Design NHT Combined Feed Exchanger Equipment Paperpiolinwalls100% (1)
- Improve FCCU Operations Using ChemicalDocument7 pagesImprove FCCU Operations Using Chemical3668770No ratings yet
- Vapor Line Corrosion CDU OHDocument16 pagesVapor Line Corrosion CDU OHTruth SeekerNo ratings yet
- Resolving Process Distillation Equipment OpportunitiesDocument13 pagesResolving Process Distillation Equipment Opportunitiesrvkumar61No ratings yet
- Crude Overhead DesignDocument5 pagesCrude Overhead DesignDaniele CirinaNo ratings yet
- Cdu Overhead Afc Failure Rev1Document286 pagesCdu Overhead Afc Failure Rev1rajivNo ratings yet
- Key Process Considerations For Caustic Treatment in CDUDocument4 pagesKey Process Considerations For Caustic Treatment in CDUVenkatesh Kumar RamanujamNo ratings yet
- Damage Mechanism in Refinery AreaDocument83 pagesDamage Mechanism in Refinery AreaKevin PradanaNo ratings yet
- Crude Unit Corrosion Control: Larry R White 281-363-7742Document45 pagesCrude Unit Corrosion Control: Larry R White 281-363-7742Salinas Salcedo Jorge Karol0% (1)
- Case Studies and Best Practices of Refinery Caustic Injection Systems PDFDocument8 pagesCase Studies and Best Practices of Refinery Caustic Injection Systems PDFSalem Garrab100% (1)
- Boil Off Gas QatarDocument4 pagesBoil Off Gas Qatarrvkumar61No ratings yet
- Ammonium Bi-Sulphide Corrosion in HydrocrackersDocument5 pagesAmmonium Bi-Sulphide Corrosion in HydrocrackersiarzuamNo ratings yet
- Hydroprocessing Units CorrosionDocument49 pagesHydroprocessing Units CorrosionAvinawNo ratings yet
- Exhanger Leakages in VDU - ModifiedDocument14 pagesExhanger Leakages in VDU - ModifiedJay LawsonNo ratings yet
- Crude Distiller RBI Intro (Updated)Document31 pagesCrude Distiller RBI Intro (Updated)harrinsonf100% (2)
- Distillation Rev41Document137 pagesDistillation Rev41rvkumar61No ratings yet
- CDUDocument4 pagesCDUmohamedyoussef1No ratings yet
- MAT-32 Managing Chlorides PDFDocument24 pagesMAT-32 Managing Chlorides PDFVictor Doan100% (1)
- Troubleshoot in Heat Exchangers HP 1996Document5 pagesTroubleshoot in Heat Exchangers HP 1996piolinwalls100% (1)
- 050 Meteorology (JAA ATPL Theory)Document700 pages050 Meteorology (JAA ATPL Theory)Sarah Schroeder100% (2)
- Crude Oil DesaltingDocument25 pagesCrude Oil DesaltingNaumanNo ratings yet
- Challenges Crude ProcessingDocument17 pagesChallenges Crude ProcessingAnonymous msVFzaNo ratings yet
- White Paper On Liquid Hydrocarbon Drop Out in Natural Gas InfrastructureDocument30 pagesWhite Paper On Liquid Hydrocarbon Drop Out in Natural Gas InfrastructureHans MuellerNo ratings yet
- Ammonium Chloride Corrossion in RefineryDocument12 pagesAmmonium Chloride Corrossion in RefinerySudarshan GopalNo ratings yet
- High Capacity Tray Reverse FlowDocument11 pagesHigh Capacity Tray Reverse Flowrvkumar61No ratings yet
- Crude Unit Corrosion and Corrosion ControlDocument14 pagesCrude Unit Corrosion and Corrosion Controlparmindarrana86% (7)
- Minimizing Corrosion in Refinery PTQDocument5 pagesMinimizing Corrosion in Refinery PTQjimbob8888No ratings yet
- Acrylic Acid ProductionDocument10 pagesAcrylic Acid Productionstavros7No ratings yet
- 17-08-30 Corrosion Monitoring Solutions For Hydroprocessing UnitsDocument8 pages17-08-30 Corrosion Monitoring Solutions For Hydroprocessing UnitsthangNo ratings yet
- Best Practice: Saudi Aramco Desktop StandardsDocument19 pagesBest Practice: Saudi Aramco Desktop Standardssethu1091100% (4)
- Column Pressure ControlDocument11 pagesColumn Pressure Controlrvkumar61No ratings yet
- Salt Fouling FCCDocument6 pagesSalt Fouling FCCVenkatesh Kumar RamanujamNo ratings yet
- Corrosion in CDUDocument5 pagesCorrosion in CDUelgawadhaNo ratings yet
- HSR 1.63 (For HYSYS V10.0)Document4 pagesHSR 1.63 (For HYSYS V10.0)Ba Jun Thối0% (1)
- 2017-14-07 Continuous Corrosion Monitoring of Crude Unit OverheadsDocument16 pages2017-14-07 Continuous Corrosion Monitoring of Crude Unit OverheadsDavid Cruz ZamoraNo ratings yet
- Cycle Chemistry CommissioningDocument11 pagesCycle Chemistry CommissioningKrishnan Mani100% (1)
- A Complete Analysis of Your Reformer-SynetixDocument9 pagesA Complete Analysis of Your Reformer-SynetixhendraokasNo ratings yet
- How To Check A DrawingDocument3 pagesHow To Check A DrawingSouparna DuttaNo ratings yet
- Nitrogen BlanketingDocument21 pagesNitrogen Blanketingrvkumar61No ratings yet
- Refinery OverviewDocument79 pagesRefinery Overviewchikukotwal100% (1)
- Naphthenic Acid CorrosionDocument6 pagesNaphthenic Acid Corrosionbkmuduli100% (1)
- Hydrocarbons DewDocument37 pagesHydrocarbons DewPasquale CutriNo ratings yet
- Description of Damage: 5.1.1.1 Amine Corrosion 5.1.1.1.1Document5 pagesDescription of Damage: 5.1.1.1 Amine Corrosion 5.1.1.1.1Ajmi HmidaNo ratings yet
- Amine Best Practices GuideDocument63 pagesAmine Best Practices GuideJerold100% (2)
- Contributors To Refinery FlaringDocument3 pagesContributors To Refinery Flaringrvkumar61No ratings yet
- Debottlenecking Refineries Through Comprehensive Feedstock PretreatmentDocument20 pagesDebottlenecking Refineries Through Comprehensive Feedstock PretreatmentAnonymous msVFzaNo ratings yet
- Ammonia StrippingDocument4 pagesAmmonia StrippingDattatraya GutteNo ratings yet
- Ethylene & ACF PDFDocument48 pagesEthylene & ACF PDFSubrato Saha100% (2)
- DHDS ProcessDocument9 pagesDHDS ProcessSandeep ChallaNo ratings yet
- BS 01916-3-2009Document34 pagesBS 01916-3-2009pacoNo ratings yet
- 5 - IsomDocument72 pages5 - IsomAn Lê TrườngNo ratings yet
- VCF CalculationDocument2 pagesVCF Calculationrvkumar61No ratings yet
- 1566016Document6 pages1566016Yudhistira Perdana PutraNo ratings yet
- MECCOCT18-12509: Managing Corrosion Challenges in Crude Overhead Condensers With Intermittent Water WashDocument8 pagesMECCOCT18-12509: Managing Corrosion Challenges in Crude Overhead Condensers With Intermittent Water WashMikeNo ratings yet
- MECCOCT18-12509: Managing Corrosion Challenges in Crude Overhead Condensers With Intermittent Water WashDocument8 pagesMECCOCT18-12509: Managing Corrosion Challenges in Crude Overhead Condensers With Intermittent Water WashMikeNo ratings yet
- Conocophillips Response To The Saturate Gas Plant Fire and Explosion IncidentDocument9 pagesConocophillips Response To The Saturate Gas Plant Fire and Explosion IncidentvengielNo ratings yet
- 32 Productivity Increase in A PeirceSmith Convert 153013Document14 pages32 Productivity Increase in A PeirceSmith Convert 153013amirlpNo ratings yet
- (2005) Control Strategy For A CTDocument4 pages(2005) Control Strategy For A CTCristianNo ratings yet
- Carryover 2Document11 pagesCarryover 2Waleed EmaraNo ratings yet
- CDU 03 CO ControlDocument19 pagesCDU 03 CO Controlrvkumar61No ratings yet
- Interactive Excel Dashboards: by Mynda TreacyDocument22 pagesInteractive Excel Dashboards: by Mynda Treacyrvkumar61No ratings yet
- Quiz For Heat Exchanger Selection and Design: T E C H N O L O G YDocument10 pagesQuiz For Heat Exchanger Selection and Design: T E C H N O L O G Yrvkumar61No ratings yet
- Ultimate Capacity FractionatorsDocument26 pagesUltimate Capacity Fractionatorsrvkumar61No ratings yet
- Exploration Tools and Methods SlidesDocument15 pagesExploration Tools and Methods Slidesrvkumar61No ratings yet
- API-Mechanical Seal-Piping Plan Booklet-LORES-4C-MAR2016 PDFDocument90 pagesAPI-Mechanical Seal-Piping Plan Booklet-LORES-4C-MAR2016 PDFrvkumar61No ratings yet
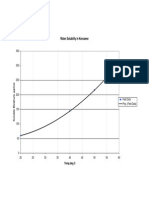
- Kero Water Solubility - UOPDocument1 pageKero Water Solubility - UOPrvkumar61No ratings yet
- Lecture 5 - Redox Reactions, Latimer and Frost DiagramsDocument50 pagesLecture 5 - Redox Reactions, Latimer and Frost DiagramsDaksh GuptaNo ratings yet
- CTS+ Configuration For PI 7.3Document23 pagesCTS+ Configuration For PI 7.3RaQNo ratings yet
- FenestrationDocument14 pagesFenestrationKing AravindNo ratings yet
- Thermo 27 45 1 29Document18 pagesThermo 27 45 1 29Danerys Targaryan0% (1)
- Super Technologies Limited Price List (Updated 14-06-2020)Document21 pagesSuper Technologies Limited Price List (Updated 14-06-2020)Shania RoopnarineNo ratings yet
- FT8 Hinson Tips For HF DXersDocument80 pagesFT8 Hinson Tips For HF DXersIp CamNo ratings yet
- Scope and Sequence of Math CurriculumDocument2 pagesScope and Sequence of Math CurriculumFaisal MunirNo ratings yet
- Sardar Raja College of Engineering Department of Electrical and Electronics Engineering Micro Lesson PlanDocument4 pagesSardar Raja College of Engineering Department of Electrical and Electronics Engineering Micro Lesson PlanKarthi SathyaNo ratings yet
- Laplace Transformation TableDocument1 pageLaplace Transformation TableDjNo ratings yet
- Influence of Botrytis Cinerea InfectionDocument11 pagesInfluence of Botrytis Cinerea InfectionEmmanuel BonninNo ratings yet
- Unit 6 Chapter 1 Parallel Programming Tools Cuda - ProgrammingDocument28 pagesUnit 6 Chapter 1 Parallel Programming Tools Cuda - ProgrammingPallavi BhartiNo ratings yet
- Service Menu: Operación de SistemasDocument8 pagesService Menu: Operación de Sistemasjuan castaedaNo ratings yet
- QUCM CCM Application ManualDocument30 pagesQUCM CCM Application ManualibanvegaNo ratings yet
- Virtual Memory and Demand PagingDocument50 pagesVirtual Memory and Demand PagingPrakash SinghNo ratings yet
- Using Scoria As Fine Aggregate in Lightweight Mortar and ConcreteDocument14 pagesUsing Scoria As Fine Aggregate in Lightweight Mortar and ConcreteFoolad GharbNo ratings yet
- Non Concurrent ForcesDocument6 pagesNon Concurrent ForcesLeah Rivera0% (1)
- Electromagnetic FlowmeterDocument10 pagesElectromagnetic FlowmeterAjjay KumarNo ratings yet
- Oscillating Universe TheoryDocument1 pageOscillating Universe TheoryArabella BasilioNo ratings yet
- Granlund Software Help: RiuskaDocument14 pagesGranlund Software Help: RiuskaObi-Wan KenobiNo ratings yet
- Lab Report #1 - SpectrophotometryDocument2 pagesLab Report #1 - Spectrophotometrybabyduckiez65% (23)
- Experiment - 4: Aim of The ExperimentDocument18 pagesExperiment - 4: Aim of The ExperimentANISH KUMARNo ratings yet
- Fourth Floor PlanDocument1 pageFourth Floor Planvenkatalakshmi natarasanNo ratings yet
- Faulty TRXDocument2 pagesFaulty TRXvaibhav151284No ratings yet