Professional Documents
Culture Documents
Use of Renewable Adsorbent (Peanut Husk) For The Treatments of Textile Waste Water
Uploaded by
chemistryjournalOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Use of Renewable Adsorbent (Peanut Husk) For The Treatments of Textile Waste Water
Uploaded by
chemistryjournalCopyright:
Available Formats
JOURNAL OF CHEMISTRY AND CHEMICAL SCIENCES
An International Open Free Access, Peer Reviewed Research Journal
www.chemistry-journal.org
ISSN 2229-760X (Print)
ISSN 2319-7625 (Online)
Abbr: J. Chem. & Cheml. Sci.
2014, Vol.4 (4): Pg.156-163
Use of Renewable Adsorbent (Peanut Husk) for the
Treatments of Textile Waste Water
Mahmudur Rahman Idris1, Md Shamim Alam2 and Md Wahidur Rahman3
1
M.Phil Student, Department of Physical Chemistry,
B.Sc, M.Sc, Dept of Chemistry,
University of Dhaka, Dhaka, BANGLADESH
2,3
M.Sc (Textile Engineering).
Maulana Vashani University of Science & Technology
M.Sc (Env. Management) Independent University, BANGLADESH.
(Received on: August 29, 2014)
ABSTRACT
Colored textile effluents represent severe environmental
problems as they contain mixture of chemicals, auxiliaries and
dyestuffs of different classes and chemical constitutions.
Elimination of dyes in the textile wastewater by conventional
wastewater treatment methods is very difficult. At present, there is
a growing interest in using inexpensive and potential materials for
the adsorption of reactive dyes1. Peanut husk has been reported
to be a potential media to remove color from wastewater. In this
study, Peanut husk were used as an adsorbent. The results
showed that the selected bio adsorbents have good potential for
removal of reactive dyes from textile effluent.
Keywords: Effluent, Temperature, Treatment, Adsorbent,
Biochemical oxygen demand, Chemical oxygen demand, DO,
Wastewater.
INTRODUCTION
The textile industry is one of the
most
complicated
industries
among
manufacturing
industry.
Wastewater
treatment is one of the major problems faced
by textile manufacturers2. Wastewater from
the textile industry can contain a variety of
polluting substances including dyes. The
Color is the first contaminant to be
recognized in the wastewater and has to be
removed before discharging into water
bodies or on land. The presence of very
small amounts of dyes in water (less than 1
Journal of Chemistry and Chemical Sciences Vol. 4, Issue 4, 31 October, 2014 Pages (156-191)
157
Mahmudur Rahman Idris, et al., J. Chem. & Cheml. Sci. Vol.4 (4), 156-163 (2014)
ppm for some dyes) is highly visible and
affects
the
aesthetic
merit,
water
transparency and gas solubility in lakes,
rivers and other water bodies. Dyes,
however, are more difficult to treat because
of their synthetic origin and mainly complex
aromatic molecular structures3.
Adsorption is an effective method of
lowering the concentration of dissolved dyes
in the effluent resulting in color removal.
Other means of dye removal such as
chemical oxidation, coagulation and reverse
osmosis are generally not feasible due to
economic considerations (Tsai et al., 2001).
The adsorption process is one of the most
efficient methods to remove dyes from
effluent. The process of adsorption has an
edge over the other methods due to it sludge
free clean operation and complete removal
of dyes even from dilute solution. Pea nut
husk are the newly thought adsorbents
because of their extended surface area,
micro porous structure, high adsorption
capacity and high degree of reactivity4. Pea
nut husk are totally new adsorbents for
waste water treatment. For that reason I have
used these adsorbents for wastewater
treatment.
Wastewater characteristics versus different textile process
Journal of Chemistry and Chemical Sciences Vol. 4, Issue 4, 31 October, 2014 Pages (156-191)
Mahmudur Rahman Idris, et al., J. Chem. & Cheml. Sci. Vol.4 (4), 156-163 (2014)
EXPERIMENTAL
METHODS
MATERIALS
&
158
parameter meter, Electronic balance,
Vacuum Oven, Magnetic/ Hotplate stirrer,
Incubator, Refrigerator
List of total materials
Experimental Methods:
Chemicals & others, Sunfix red
Reactive dye, Hydrochloric acid, Sodium
hydroxide, Hydrogen per Oxide, Sodium
carbonate, Acetic acid, Distilled water.
Bio-adsorbents & Waste Water Collection
The bio-adsorbent used for this
study were the pea nut husk which was
collected from Bhola, Bangladesh.
The sample waste water was
collected from Givency Group (Wet
Processing Unit), Gazipur, Bangladesh.
Preparation of standard solution:
A stock solution of Sunfix Red dye
was prepared by dissolving a mixture of 0.1
g of dye in a 1000 mL volumetric flask
followed by dilution up to the mark addition
of de-ionized water. Dye test solution was
prepared through proper dilution of the stock
solution to the desired concentration. Deionized water was used to prepare all of the
solution in this study.
Instruments
Adsorbent preparation
Glassware and apparatus, UV-Vis
Spectrophotometer,
Portable
multi-
Pea nut husk was prepared in the
following steps.
Adsorption and Analytical Procedures
including the amount of adsorbents (2.5 g),
initial sample concentration for waste water
and dye solutions 100 mL and 10 mg/L
respectively, contact time 180 minutes and
pH 7 were evaluated. After the adjustment
Pea nut husk (2.5 g) was added to
the beaker. The adsorption experiments were
carried out in beakers. Adsorption factors
Journal of Chemistry and Chemical Sciences Vol. 4, Issue 4, 31 October, 2014 Pages (156-191)
159
Mahmudur Rahman Idris, et al., J. Chem. & Cheml. Sci. Vol.4 (4), 156-163 (2014)
of pH to the desired value with 0.01 M HCL
and 0.01 M NaOH solutions, the sample
solution was stirred using a magnetic stirrer.
The adsorption from the aqueous solution
was studied. After the desired contact period
for each batch experiment, the aqueous
phases were separated from the materials,
and the dye concentration of dye was
measured
using
a
UV-Vis
Spectrophotometer.
Determination of physical and chemical
characteristics of wastewater
Dissolved oxygen shows the ability
of the stream to purify itself through
biochemical process. Oxygen is dissolved in
most waters in varying concentrations. The
Dissolved Oxygen of waste water sample
was measured by taking 50 mL of
wastewater in a 100 mL beaker and
immersing the electrode of portable multi
parameter meter (Sension 153,HACH,USA)
into the sample.
Analysis: It was determined by digital DO
meter or by conventional trite-metric
method.
Total Solids
Total solids were obtained by evaporating 50
mL waste water sample in a beaker. After
evaporating the sample the solid residues
were dried.
Analysis: It was determined by conventional
method.
Total Dissolved Solids (TDS)
Total dissolved solids contents of
water and waste water is defined as the
residue left upon evaporation at 103C to
105C. Total Dissolved Solids was obtained
by evaporating 50 mL filtered waste water
sample in a beaker. After evaporating the
sample the solid residues were dried.
Analysis: It was determined
digital TDS meter.
Dissolved Oxygen (DO)
by using a
Total Suspended Solids
Total Suspended Solids was
obtained by deducting Total Dissolved
Solids from Total solids.
Biological Oxygen Demand (BOD)
Bio-chemical oxygen demand tests
show the amount of molecular oxygen
required by bacteria to reduce the
carbonaceous materials. The determination
of DO of a sample before and after five days
incubation at 20C is the basic of BOD
determination.
Analysis: It was determined by Winklers
method of 5 day BOD test.
Chemical Oxygen Demand (COD)
COD test shows the oxygen
equivalent of the organic matter that can be
oxidized by using a strong oxidizing agents
e.g. potassium dichromate in acidic solution,
at elevated temperature, for two and half
hour. It indicates the amount of oxygen
required to oxidize the carbonaceous matter.
Analysis: It was determined by closed/open
refluxed trite-metric method.
Journal of Chemistry and Chemical Sciences Vol. 4, Issue 4, 31 October, 2014 Pages (156-191)
Mahmudur Rahman Idris, et al., J. Chem. & Cheml. Sci. Vol.4 (4), 156-163 (2014)
pH
160
Graphical representation of different
parameters
pH is a term used universally to
express the intensity of the acidic or alkaline
condition of solution. It is a measure of
hydrogen ion concentration or more
precisely the hydrogen ion activity.
From the above result it is clearly
shown that the pH, BOD, COD, TS,
TDS,TSS has reduced & DO has increased
which is remarkable.
100
Analysis of samples by UV-Vis
Spectrophotometer
The amounts of dye onto the
adsorbents were determined by measuring
the absorbance of dye after batch experiment
by UV-Vis Spectrophotometer. The sample
were analyzed against a calibration curve
prepare by standard solution of dye.
90
Removal efficiency
Analysis: It was determined by digital pH
meter.
80
70
60
50
20
40
60
80
100
120
140
160
180
200
Time
Figure 1: Effect of contact time for Peanut husk
94
92
Waste water parameter before & after
treatment by adsorbent (Peanut Husk)
Removal efficiency
90
RESULTS & DISCUSSIONS
88
86
84
82
Before
treatment
PH
Dissolved oxygen
( mg/L)
Biological Oxygen
Demand(mg/L)
Chemical Oxygen
Demand (mg/L)
Total Solids
(mg/L)
Total Dissolved
Solid( mg/L)
Total
Suspended
Solids ( mg/L)
11
6.8
After
Treatment
by
Peanut Hust
7
9.2
80
78
0
10
15
20
25
30
Concentration
Figure 2: Effect of dye concentration for Peanut
husk
100
98
45.4
992
310
3444
2210
3224
2080
220
125
90
Removal efficiency
Parameter
80
70
60
50
40
2
10
pH
Figure-3: Effect of pH for Peanut husk
Journal of Chemistry and Chemical Sciences Vol. 4, Issue 4, 31 October, 2014 Pages (156-191)
12
161
Mahmudur Rahman Idris, et al., J. Chem. & Cheml. Sci. Vol.4 (4), 156-163 (2014)
100
by Peanut husk is shown in the figure
(figure-b). From these figures it is proved
that as the initial dye concentration
increased, the adsorption capacity decreased.
From the graph, it is clear that Peanut husk
has higher adsorptive capacity at 10 ppm..
Removaal efficiency
90
80
70
60
Figure 3: Effect of pH variation
50
0
10
15
20
25
30
35
Adsorbent (g/L)
Figure 4: Effect of Adsorbent variation for Peanut
husk
Figure 1: Effect of contact time
The contact time between pollutant
and the adsorbent is of significant
importance in the wastewater treatment by
adsorption. A rapid uptake of pollutants and
establishment of equilibrium in short period
signifies the efficiency of that adsorbate for
its use in wastewater treatment. Available
adsorption studied in literature reveal that
the uptake of the adsorbate species is fast at
the initial stages of the contact period, and
therefore, it becomes slower near the
equilibrium. The effects of contact time for
the adsorption of Sunfix red was studied for
a period of 210 min and the results are
shown in the above figures(figure- a). It
showed that the dye removal was rapid at a
certain time then the rate was decreased after
saturation. In case of figure, Pea nut husk
showed highest absorptive capacity at 150
minutes.
Figure 2: Effect of initial dye
concentration (Batch process)
The effect of initial Sunfix Red
reactive dye concentration for their removal
The pH of the solution affects the
surface charge of the adsorbents as well as
the degree of ionization of different
pollutants. Change of pH also affects the
adsorptive process through dissociation of
functional groups on the adsorbent surface
active sites. Consequently, this leads to a
shift in reaction kinetics and adsorption
equilibrium. The effect of pH of sunfix red
on Peanut husk is shown in the figure
above(figure- c). In graph, Peanut husk
showed the maximum dye adsorption 97 %
at pH 7
Figure 4: Effect of adsorbent dosage
(Batch process):
The effects of peanut husk dosage
on the removal of sunfix red are shown in
(Fig. 4) . The percentage removal increased
with the adsorbent dosage up to a certain
limit then it reached to the constant value.
Pea nut husk showed the maximum dye
adsorption 98 % at adsorbent of 25 g/L.
It was found from the graph that
the removal of dye by Pea nut husk
adsorbents increases with an increase in
the adsorbent dosage (w) initially and,
thereafter, becomes constant after some
value of w. This value is taken as the
optimum dosage w. The increase in
adsorption with the adsorbent dosage can
Journal of Chemistry and Chemical Sciences Vol. 4, Issue 4, 31 October, 2014 Pages (156-191)
Mahmudur Rahman Idris, et al., J. Chem. & Cheml. Sci. Vol.4 (4), 156-163 (2014)
be attributed to the availability of greater
surface area and larger number of
adsorption sites. At w < optimum, the
adsorbent surface becomes saturated with
dye particle and the residual dye
concentration in the solution is large. With
an increase in w, the dye removal increases
due to increased amount of adsorbent
CONCLUSION
In this research work, the removal
percentage of reactive dyes from the textile
waste water was carried out by the
application of bio adsorbents Peanut husk).
When the initial pH is 7, initial
concentration is 10 ppm and temperature is
45C then the maximum amount of the
reactive dyes adsorbed by Peanut husk.
98% removal of dye was found at adsorbent
mass of 25 gm/L, pH 7 and 180 minutes of
contact time by Peanut Husk.
De colorizations process is not
specific and depends upon many factors.
Although there are lots of adsorbents which
can act as a substitute for the expensive
commercial activated carbon but complete
replacement is not possible. The factors
which favors the selection of Peanut husk
are its low cost, widespread presence and
organic composition which shows strong
affinity for some selected dyes. In spite of
the
scarcity
of
consistent
cost
information, the widespread uses of lowcost
adsorbents
in
industries
for
wastewater treatment applications today
are strongly recommended due to their
local availability, technical feasibility,
engineering
applicability,
and
cost
effectiveness.
162
The adsorption can be influenced by
a number of factors, such as, adsorbent
mass, contact time, agitation speed,
temperature and pH etc. Hence, there is a
need to optimize these factors to maximize
the treatment efficiency of Pea nut husk and
minimize the treatment cost for textile
wastewater5.
If low-cost adsorbents perform
well in removing heavy metals and colors
at low cost, they can be adopted and widely
used in industries not only to minimize
cost
inefficiency, but also improve
profitability.
REFERENCES
1. R.S.Mane, V.N. Bhusari, Removal of
Colour (dyes) from textile effluent by
adsorption using Orange and Banana
peel, ISSN: 2248-9622, International
Journal of Engineering Research and
Applications, Vol. 2, Issue 3, pp.19972004 May-Jun (2012).
2. H. Najafi and H. R. Movahed ,
Improvement of COD and TOC reactive
dyes in textile wastewater by
coagulation chemical material, ISSN
16845315, African Journal of Biotechnology, Vol. 8 (13), pp. 3053-3059, 6
July (2009),
3. Thomas E. (Tom) Schultz, Biological
wastewater treatment, International
Journal of Environmental Science, pp.
3, October (2005).
4. Rahman Muhammad Bozlur, Shinichi
Shibata, C. Siddiqua Farah Diba and
Magali Uono, Low Cost Biodegradable
Adsorbent Material for the Removal of
Dissolved
Dyes
from
Aqueous
Solutions, ISSN: 1793-8236, IACSIT
International Journal of Engineering
Journal of Chemistry and Chemical Sciences Vol. 4, Issue 4, 31 October, 2014 Pages (156-191)
163
Mahmudur Rahman Idris, et al., J. Chem. & Cheml. Sci. Vol.4 (4), 156-163 (2014)
and Technology, Vol.2, No.5, October
(2010).
5. Piyush Malaviya, Textile Wastewater
Treatment Using Sawdust as Adsorbent,
ISSN: 2277-1948, International Journal
of Environmental Sciences, Vol.2 No.3.
Pp. 110-113, (2013).
Journal of Chemistry and Chemical Sciences Vol. 4, Issue 4, 31 October, 2014 Pages (156-191)
You might also like
- Method Statement For Lifting WorksDocument12 pagesMethod Statement For Lifting WorksRachel Flores85% (26)
- Environmental Water: Advances in Treatment, Remediation and RecyclingFrom EverandEnvironmental Water: Advances in Treatment, Remediation and RecyclingNo ratings yet
- Bartos P. J., Glassfibre Reinforced Concrete - Principles, Production, Properties and Applications, 2017Document209 pagesBartos P. J., Glassfibre Reinforced Concrete - Principles, Production, Properties and Applications, 2017Esmerald100% (3)
- Dye Removal ProposalDocument15 pagesDye Removal ProposalnorsiahNo ratings yet
- Pamet and PasmethDocument4 pagesPamet and PasmethBash De Guzman50% (2)
- Design and Details of Elevated Steel Tank PDFDocument10 pagesDesign and Details of Elevated Steel Tank PDFandysupaNo ratings yet
- CHAPTER 8 f4 KSSMDocument19 pagesCHAPTER 8 f4 KSSMEtty Saad0% (1)
- Effective Removal and Recovery of Fast Green FCF Dye From Wastewater Using Green AdsorbentDocument12 pagesEffective Removal and Recovery of Fast Green FCF Dye From Wastewater Using Green AdsorbentSandip LabadeNo ratings yet
- The Use of Rice Straw As An AdsorbantDocument4 pagesThe Use of Rice Straw As An AdsorbantgobinathdpiNo ratings yet
- Congo Red Dye PDFDocument6 pagesCongo Red Dye PDFkiranpatil1014532No ratings yet
- 2 Response Surface Optimization of Dye Removal Using Waste Prawn ShellsDocument8 pages2 Response Surface Optimization of Dye Removal Using Waste Prawn ShellssaiNo ratings yet
- Physico-Chemical and Adsorption Studies of Activated Carbon From Agricultural WastesDocument8 pagesPhysico-Chemical and Adsorption Studies of Activated Carbon From Agricultural WasteszannubaqotrunnadhaNo ratings yet
- Biosorption Behavior of Basic Red 46 and Violet 3 by Dead Pleurotus Mutilus From Single - and Multicomponent SystemsDocument13 pagesBiosorption Behavior of Basic Red 46 and Violet 3 by Dead Pleurotus Mutilus From Single - and Multicomponent SystemsChern YuanNo ratings yet
- Calibration of Congo Red ColorDocument12 pagesCalibration of Congo Red ColorJelena MitrovicNo ratings yet
- Removal of Yellow 2G Dye From Aqueous Solutions Using Activated Carbon Prepared From Mosambi and Cotton An Agricultural WasteDocument6 pagesRemoval of Yellow 2G Dye From Aqueous Solutions Using Activated Carbon Prepared From Mosambi and Cotton An Agricultural WasteInternational Organization of Scientific Research (IOSR)No ratings yet
- Bezerra 2018Document38 pagesBezerra 2018Simon OkwareNo ratings yet
- Decolorization and Removal of Textile and Non-Textile DyesDocument8 pagesDecolorization and Removal of Textile and Non-Textile DyesSema SelçukNo ratings yet
- Turkjchem 45 551Document15 pagesTurkjchem 45 551Arpit MehtaNo ratings yet
- Comparative Decolorization of Dyes in Textile Wastewater Using Biological and Chemical TreatmentDocument5 pagesComparative Decolorization of Dyes in Textile Wastewater Using Biological and Chemical TreatmentsnowhuliNo ratings yet
- Methodsx: Chamira Dilanka Fernando, Preethi SoysaDocument9 pagesMethodsx: Chamira Dilanka Fernando, Preethi SoysaColpos BiancaNo ratings yet
- A Comparative Evaluation For Adsorption of Dye On Neem Bark and Mango Bark PowderDocument9 pagesA Comparative Evaluation For Adsorption of Dye On Neem Bark and Mango Bark PowderdivNo ratings yet
- Determination of Aniline Degraded From Sudan I in Cloths: (Penentuan Anilin Yang Terurai Daripada Sudan I Dalam Kain)Document9 pagesDetermination of Aniline Degraded From Sudan I in Cloths: (Penentuan Anilin Yang Terurai Daripada Sudan I Dalam Kain)Khải Nhi LêNo ratings yet
- 3.isca RJCS 2015 017Document5 pages3.isca RJCS 2015 017Igbinwole Kehinde SolomonNo ratings yet
- Chemical Catalytic Reaction and Biological Oxidation For Treatment of Non-Biodegradabletextile EffluentDocument7 pagesChemical Catalytic Reaction and Biological Oxidation For Treatment of Non-Biodegradabletextile EffluentIrina Natalena OsantiNo ratings yet
- Physico-Chemical and Microbiological Analysis of Textile Dyeing EffluentsDocument5 pagesPhysico-Chemical and Microbiological Analysis of Textile Dyeing EffluentsIOSRjournalNo ratings yet
- Removal of A Basic Dye From Aqueous Solution by Adsorption UsingDocument6 pagesRemoval of A Basic Dye From Aqueous Solution by Adsorption Usingseranim22100% (1)
- Plantago Major L. (Greater Plantain) Is A Species of The Plan-Taginacea Family. It IsDocument4 pagesPlantago Major L. (Greater Plantain) Is A Species of The Plan-Taginacea Family. It IsabnerNo ratings yet
- Use of Palm Oil Fiber An Agricultural Waste For Removal of Methylene Blue From Aqueous Solution - FairusDocument8 pagesUse of Palm Oil Fiber An Agricultural Waste For Removal of Methylene Blue From Aqueous Solution - Fairusfairus100% (6)
- Treatment of Textile Wastewater by Coagulation Precipitation MethodDocument11 pagesTreatment of Textile Wastewater by Coagulation Precipitation MethodShehla RiazNo ratings yet
- Denim Wash.ADocument64 pagesDenim Wash.AMuhammad Mostafizur RahmanNo ratings yet
- Adsorption Characteristics, Diffusion and Isotherms of Modified Pig Waste For Removing Reactive Violet 5Document14 pagesAdsorption Characteristics, Diffusion and Isotherms of Modified Pig Waste For Removing Reactive Violet 5International Journal of Innovative Science and Research TechnologyNo ratings yet
- Performance Evaluation of A Common Effluent Treatment Plant For Tannery Industries PDFDocument4 pagesPerformance Evaluation of A Common Effluent Treatment Plant For Tannery Industries PDFJavier Sulivan Paredes MurNo ratings yet
- Decolorization of Mixture of Dyes: A Critical ReviewDocument25 pagesDecolorization of Mixture of Dyes: A Critical ReviewJuanPabloGuerreroNo ratings yet
- Thesis FinalDocument16 pagesThesis FinalabcdefNo ratings yet
- Thesis BookDocument33 pagesThesis BookSarwer Hussain FaisalNo ratings yet
- Decolorization of An Acidic Dye From Synthetic Wastewater by Sludge of Water Treatment PlantDocument6 pagesDecolorization of An Acidic Dye From Synthetic Wastewater by Sludge of Water Treatment PlantCarlos Kirela E. RaquelNo ratings yet
- Mam Report NewDocument47 pagesMam Report NewAlps AnaNo ratings yet
- Adsorption of Remazol Brilliant Blue R On AC From Anaerobically Digested Sewage SludgeDocument20 pagesAdsorption of Remazol Brilliant Blue R On AC From Anaerobically Digested Sewage Sludgeceo.didansiNo ratings yet
- Comparative Study of Adsorptive Removal of Congo Red and Brilliant Green Dyes From Water Using Peanut ShellDocument5 pagesComparative Study of Adsorptive Removal of Congo Red and Brilliant Green Dyes From Water Using Peanut ShellJonesHutaurukNo ratings yet
- Methylene Blue Dye Removal Using Ground NutDocument7 pagesMethylene Blue Dye Removal Using Ground NutMelroy CastalinoNo ratings yet
- Chaibakhsh2014.PDF BuenazoDocument7 pagesChaibakhsh2014.PDF BuenazoabnerNo ratings yet
- Adsorption Science and TechnologyDocument17 pagesAdsorption Science and Technologydeepika snehiNo ratings yet
- Biological Degradation of Reactive Dyes PDFDocument6 pagesBiological Degradation of Reactive Dyes PDFchemistryNo ratings yet
- Archive of SID: Removal of Surfactant From Industrial Wastewaters by Coagulation Flocculation ProcessDocument6 pagesArchive of SID: Removal of Surfactant From Industrial Wastewaters by Coagulation Flocculation ProcessrudymerchoNo ratings yet
- BATCH ADSORPTION STUDIES ON REMOVAL OF DYES FROM WASTE WATER USING MODIFIED SEASHELLS AS ADSORBENTS Ijariie5776Document7 pagesBATCH ADSORPTION STUDIES ON REMOVAL OF DYES FROM WASTE WATER USING MODIFIED SEASHELLS AS ADSORBENTS Ijariie5776Karthik Kalasipalya Vinod KumarNo ratings yet
- Journal of Environmental Chemical Engineering: SciencedirectDocument8 pagesJournal of Environmental Chemical Engineering: SciencedirectSushanta Kumar BeheraNo ratings yet
- Extraction of Methyl Red From Industrial Wastewater Using Xylene As An ExtractantDocument6 pagesExtraction of Methyl Red From Industrial Wastewater Using Xylene As An ExtractantUmi Dwi JayantiNo ratings yet
- Removing Reactive Dyes From Textile Effluent Using Banana FibreDocument7 pagesRemoving Reactive Dyes From Textile Effluent Using Banana FibreGarudaOzoNo ratings yet
- Mittal2007 PDFDocument6 pagesMittal2007 PDFFery Potter D DifkizNo ratings yet
- Neem Leaf 4Document12 pagesNeem Leaf 4shaggy hopkinsNo ratings yet
- Textile Effluent TreatmentDocument6 pagesTextile Effluent TreatmentvenkatharunNo ratings yet
- Kurade Et Al 2015 - J Biosci BioengDocument8 pagesKurade Et Al 2015 - J Biosci BioengRahul KhandareNo ratings yet
- TMP 9 ADBDocument6 pagesTMP 9 ADBFrontiersNo ratings yet
- RPM5Document4 pagesRPM5mmak946.lkiNo ratings yet
- Journal of Hazardous Materials: Sílvia C.R. Santos, Rui A.R. BoaventuraDocument9 pagesJournal of Hazardous Materials: Sílvia C.R. Santos, Rui A.R. BoaventuraJefersonCorreiaNo ratings yet
- Objective of The Environment LabDocument14 pagesObjective of The Environment LabAsma FayyazNo ratings yet
- Extraction, Optical Properties, and Aging Studies of Natural Pigments ofDocument13 pagesExtraction, Optical Properties, and Aging Studies of Natural Pigments ofBrian OsNo ratings yet
- Sample Format For Pilot TestingDocument9 pagesSample Format For Pilot TestingAziel VillapandoNo ratings yet
- Wastewater Treatment Using Non-Conventional Coagulants and OcculantsDocument2 pagesWastewater Treatment Using Non-Conventional Coagulants and OcculantsPedro Monteza ChamayaNo ratings yet
- Removal of Methyl Orange From Effluent Water by Silver/Copper Nanoparticles Deposited On Antigonon Leptopus Leaf Powder An AdsorbentDocument7 pagesRemoval of Methyl Orange From Effluent Water by Silver/Copper Nanoparticles Deposited On Antigonon Leptopus Leaf Powder An AdsorbentInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Efficient Encapsulation of Toxic Dye From Wastewater Using Biodegradable Polymeric AdsorbentDocument10 pagesEfficient Encapsulation of Toxic Dye From Wastewater Using Biodegradable Polymeric AdsorbentdivyaNo ratings yet
- Lab Report 4Document7 pagesLab Report 4Carol Wei100% (1)
- Dispersive Liquid-Liquid Microextraction Using The Freezed Floating Organic Drop For Rapid, Fast, and Sensitive Determination of LeadDocument12 pagesDispersive Liquid-Liquid Microextraction Using The Freezed Floating Organic Drop For Rapid, Fast, and Sensitive Determination of LeadrezacvNo ratings yet
- Isolation and Development of A Bacterial Consortium For Biodegradation of Textile Dyes Reactive Red 31 and Reactive Black 5Document9 pagesIsolation and Development of A Bacterial Consortium For Biodegradation of Textile Dyes Reactive Red 31 and Reactive Black 5IOSRjournalNo ratings yet
- CHJV04I04P0176Document16 pagesCHJV04I04P0176chemistryjournalNo ratings yet
- Synthesis and Characterization of Polyaniline Based Conducting PolymersDocument8 pagesSynthesis and Characterization of Polyaniline Based Conducting PolymerschemistryjournalNo ratings yet
- The Fundamental Characteristics of Pyrazinamide: A ReviewDocument4 pagesThe Fundamental Characteristics of Pyrazinamide: A ReviewchemistryjournalNo ratings yet
- CHJV04I02P0104Document6 pagesCHJV04I02P0104chemistryjournalNo ratings yet
- CHJV04I04P0164Document11 pagesCHJV04I04P0164chemistryjournalNo ratings yet
- CHJV04I02P0114Document4 pagesCHJV04I02P0114chemistryjournalNo ratings yet
- CHJV04I01P0065Document5 pagesCHJV04I01P0065chemistryjournalNo ratings yet
- Removal of Dyes From Aqueous Solution Using Low Cost AdsorbentDocument5 pagesRemoval of Dyes From Aqueous Solution Using Low Cost AdsorbentchemistryjournalNo ratings yet
- Synthesis and Study of Polypyrrole Thin Films by Silar MethodDocument6 pagesSynthesis and Study of Polypyrrole Thin Films by Silar MethodchemistryjournalNo ratings yet
- CHJV04I02P0084Document6 pagesCHJV04I02P0084chemistryjournalNo ratings yet
- CHJV03I04P0269Document8 pagesCHJV03I04P0269chemistryjournalNo ratings yet
- Monthly Variation of Physico-Chemical Characteristics of Wullar Lake, Srinagar, KashmirDocument8 pagesMonthly Variation of Physico-Chemical Characteristics of Wullar Lake, Srinagar, KashmirchemistryjournalNo ratings yet
- A Review: Development of New Anticancer Drugs Complementary To Transition Metal Complexes Such As Copper (II), Zinc (II), Nickel (II) and Cobalt (II)Document6 pagesA Review: Development of New Anticancer Drugs Complementary To Transition Metal Complexes Such As Copper (II), Zinc (II), Nickel (II) and Cobalt (II)chemistryjournalNo ratings yet
- Drug Dissolution and Drug Dissolution and It's EffectDocument6 pagesDrug Dissolution and Drug Dissolution and It's EffectchemistryjournalNo ratings yet
- CHJV04I03P0118Document7 pagesCHJV04I03P0118chemistryjournalNo ratings yet
- CHJV04I01P0051Document7 pagesCHJV04I01P0051chemistryjournalNo ratings yet
- CHJV04I01P0027Document7 pagesCHJV04I01P0027chemistryjournalNo ratings yet
- A Study of Energy, Ecology, Environment and Society Under Fuzzy Logic SpacesDocument12 pagesA Study of Energy, Ecology, Environment and Society Under Fuzzy Logic SpaceschemistryjournalNo ratings yet
- Synthesis and Characterization of Polypyrrole (PPY) Thin Film by Spin Coating TechniqueDocument6 pagesSynthesis and Characterization of Polypyrrole (PPY) Thin Film by Spin Coating TechniquechemistryjournalNo ratings yet
- Isolation of Stigmasterol From Alcoholic Extract of Bark of Calotropis ProceraDocument3 pagesIsolation of Stigmasterol From Alcoholic Extract of Bark of Calotropis ProcerachemistryjournalNo ratings yet
- CHJV04I01P0009Document11 pagesCHJV04I01P0009chemistryjournalNo ratings yet
- CHJV04I01P0043Document7 pagesCHJV04I01P0043chemistryjournalNo ratings yet
- CHJV03I04P0235Document5 pagesCHJV03I04P0235chemistryjournalNo ratings yet
- CHJV04I01P0059Document5 pagesCHJV04I01P0059chemistryjournalNo ratings yet
- CHJV04I01P0021Document6 pagesCHJV04I01P0021chemistryjournalNo ratings yet
- GC/MS Analysis of Essential Oil Isolated From The Roots of Cymbopogon Winterianus JowittDocument7 pagesGC/MS Analysis of Essential Oil Isolated From The Roots of Cymbopogon Winterianus JowittchemistryjournalNo ratings yet
- Electrochemical Preparation and Characterization of Fe-Cu Alloy On Anodized Alumina TemplatesDocument8 pagesElectrochemical Preparation and Characterization of Fe-Cu Alloy On Anodized Alumina TemplateschemistryjournalNo ratings yet
- Phytochemical Characterization of Feronia Limonia LeavesDocument4 pagesPhytochemical Characterization of Feronia Limonia LeaveschemistryjournalNo ratings yet
- CHJV04I01P0001Document7 pagesCHJV04I01P0001chemistryjournalNo ratings yet
- Phase Transfer Catalyzed Selective Reduction of Bifunctional MoietiesDocument6 pagesPhase Transfer Catalyzed Selective Reduction of Bifunctional MoietieschemistryjournalNo ratings yet
- Pescatarian Mediterranean Diet Cookbook 2 - Adele TylerDocument98 pagesPescatarian Mediterranean Diet Cookbook 2 - Adele Tylerrabino_rojoNo ratings yet
- Celitron ISS 25L - Product Spec Sheet V 2.1 enDocument9 pagesCelitron ISS 25L - Product Spec Sheet V 2.1 enyogadwiprasetyo8_161No ratings yet
- Epoxy Data - AF35LVE TDS - ED4 - 11.17Document8 pagesEpoxy Data - AF35LVE TDS - ED4 - 11.17HARESH REDDYNo ratings yet
- W4. Grade 10 Health - Q1 - M4 - v2Document22 pagesW4. Grade 10 Health - Q1 - M4 - v2Jesmael PantalunanNo ratings yet
- RIASEC Personality TestDocument2 pagesRIASEC Personality TestSarah Jane NomoNo ratings yet
- 1 PolarographyDocument20 pages1 PolarographyRiya Das100% (1)
- J. Bleger ArtDocument10 pagesJ. Bleger Artivancristina42No ratings yet
- Sepsis Management UCLA HealthDocument23 pagesSepsis Management UCLA HealthKomang_JananuragaNo ratings yet
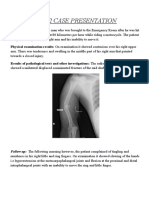
- PBL 2 Case PresentationDocument12 pagesPBL 2 Case PresentationRamish IrfanNo ratings yet
- Rate Break Up 15500Document1 pageRate Break Up 15500Yash Raj Bhardwaj100% (1)
- 1 A Finalexam FNH330 June 2015 Final Review QuestionsDocument6 pages1 A Finalexam FNH330 June 2015 Final Review QuestionsChinley HinacayNo ratings yet
- Speaking Topics For Final ReviewDocument3 pagesSpeaking Topics For Final ReviewNhư ÝNo ratings yet
- AUDCISE Unit 1 WorksheetsDocument2 pagesAUDCISE Unit 1 WorksheetsMarjet Cis QuintanaNo ratings yet
- Ethics, Privacy, and Security: Lesson 14Document16 pagesEthics, Privacy, and Security: Lesson 14Jennifer Ledesma-Pido100% (1)
- Upper Limb OrthosesDocument29 pagesUpper Limb OrthosesMaryam KhalidNo ratings yet
- Baxshin LABORATORY: Diagnostic Test and AnalysisDocument1 pageBaxshin LABORATORY: Diagnostic Test and AnalysisJabary HassanNo ratings yet
- Idioms and PharsesDocument0 pagesIdioms and PharsesPratik Ramesh Pappali100% (1)
- Factors That Contribute To Successful BakingDocument8 pagesFactors That Contribute To Successful BakingErrol San Juan100% (1)
- Occlusal Appliance TherapyDocument14 pagesOcclusal Appliance TherapyNam BuiNo ratings yet
- Food Safety PosterDocument1 pageFood Safety PosterMP CariappaNo ratings yet
- Krispy Kreme Doughnut Recipe - Immaculate BitesDocument2 pagesKrispy Kreme Doughnut Recipe - Immaculate Bitesdaisydrops6No ratings yet
- Exam Questions: Exam Title: Chapter MEK 8Document4 pagesExam Questions: Exam Title: Chapter MEK 8vishnu sharmaNo ratings yet
- Policy Schedule Personal Accident Insurance Policy: (Plan 5)Document2 pagesPolicy Schedule Personal Accident Insurance Policy: (Plan 5)Rana BiswasNo ratings yet
- Full Carrino Plaza Brochure and Application (General)Document8 pagesFull Carrino Plaza Brochure and Application (General)tanis581No ratings yet
- Geometry of N-BenzylideneanilineDocument5 pagesGeometry of N-BenzylideneanilineTheo DianiarikaNo ratings yet