Professional Documents
Culture Documents
Chapter 3 Summary
Uploaded by
KTINE08Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 3 Summary
Uploaded by
KTINE08Copyright:
Available Formats
TECHNOLOGICAL INSTITUTE OF THE PHILIPPINES
363 P. CASAL ST., QUIAPO, MANILA
CHEMICAL REACTION ENGINEERING
CHAPTER III SUMMARY
SUBMITTED BY:
KRISTINE ANN F. VILLANUEVA
BSChE/1110630
SUBMITTED TO:
ENGR. LORRAINE A. CARRILLO
PROFESSOR
NOVEMBER 31, 2014
CHAPTER III SUMMARY
RATE LAWS
Rate Laws are essential in Chemical Reaction Engineering. These will serve as the basic step in
solving problems in this course. But before proceeding to rate laws, it is vital that a student
understands what kind of reaction is happening so as to establish the proper equation. Rate Laws
are based on reactions. A chemical reaction involves transformation from one substance to
another and these different kinds of transformation also affect the equation to be used.
Reactions
Types of Reaction
1) Homogenous Reaction a reaction involving only one phase
2) Heterogenous Reaction a reaction involving two or more phases
3) Irreversible Reaction a reaction that proceeds in only one direction
4) Reversible Reaction a reaction that can proceed in the forward direction and backward
direction. We can determine that a reaction is reversible if it has two arrows with each
heading in opposite directions.
Molecularity of Reaction
Definition: The molecularity of a reaction depends on the atoms involved in a certain chemical
reaction.
1) Unimolecular only one atom is involved. (Ex. : Radioactive Decay)
2) Bimolecular two atoms are involved (Ex. : collision with free radicals)
3) Trimolecular three atoms are involved (Ex. : series of bimolecular reactions)
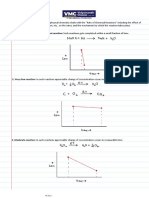
3.1 Relative Rates of Reaction
Stoichiometric coefficients of a reaction are related by:
A+ B C+ D
That for every mole of A consumed, moles of D are produced.
aA + bB cC+ dD
The species are related in that way for which
3.2 The Reaction Order and the Rate Law
Chemical Reaction Calculations need basis. Usually, species A which is considered as the
limiting reactant in a reaction is used for calculations. Since A is a reactant, the rate of
disappearance is calculated.
The rate of disappearance is described by the general equation:
-rA = {kA(T)}[fn(CA, CB,.)]
where kA is the reaction rate constant.
The rate of disappearance is the product of the reaction rate constant and the function of
concentrations. When the rate of disappearance, -rA, is related to the species concentrations in an
algebraic form, the equation becomes a kinetic expression also known as the Rate Law.
3.2.1 Power Law Models and Elementary Rate Laws
A power law model is one of the most common general forms used to confirm postulated forms.
The rate law is expressed as the product of concentrations of the individual reacting species with
each raised to a power.
-rA = kACACB
The sum of and is regarded as the reaction order. This is only valid for the reactions under
Elementary Rate Laws. Elementary Rate Laws apply for reactions that follow the molecularity of
the reaction.
The reaction order (n) is defined by the equation:
n = + .
There are four types of reaction order namely: Zero-Order, First-Order, Second-order and Thirdorder where n=0, 1, 2 and 3 respectively.
3.2.2 Non-elementary Rate Laws
Non-elementary rate laws are applicable for the reactions not following elementary rate laws.
Some of these include homogenous and heterogeneous reactions. Rate of disappearance of a
reactant per mass of catalyst in a heterogeneous reaction follow Langmuir-Hinshelwood kinetics.
These reactions cannot be deduced from the stoichiometry of the reaction involved. These
reactions are formed by a series of reactions wherein the reaction time is so fast that it could not
be considered as single reactions but as a whole overall reaction.
3.2.3 Reversible Reactions
Reversible reactions are the reactions that go forward and backward. The forward reaction is
where the reactants form products. The backward reaction, on the other hand, involves products
producing reactants. When equilibrium is reached, the rate of forward reaction becomes equal to
the rate of the backward reaction. An equilibrium constant, Kc, is considered wherein it is equal
to the concentration of the products at equilibrium all over the concentration of the reactants at
equilibrium with all concentrations raised to their own stoichiometric coefficient.
3.3 The Reaction Rate Constant
The reaction rate constant denoted as k is not truly a constant but rather a specific reaction rate
constant. This constant is independent of the concentrations but is strongly dependent on
temperature and also on pressure when gaseous reactions are involved.
Svante Arrhenius related the rate constant to temperature by the equation:
kA(T) = Ae-E/RT
where A = pre-exponential factor or frequency factor
E = activation energy, J/mol or cal/mol
R = gas constant= 8.314 J/mol K = 1.987 cal/mol K
T = absolute temperature, K
This relation is also known as the Arrhenius equation.
Arrehenius equation tells us that as the temperature increases, the rate constant also increases.
When connected to rate law, where the rate of formation is proportional to reaction rate constant
and the concentration of species present, the rate of formation/disappearance also increases.
The activation energy is a barrier to energy transfer between reacting molecules that in order for
a reaction to occur, this energy should be overcome. This energy is due to the requirements for
chemical bonding wherein energy must be provided to break and the eventually form new bonds
and to overcome the steric and electron repulsive forces.
Finally, the main purpose of knowing the way chemical reactions proceed is to create a design
for an equipment in which it will be contained. Volume and volumetric flowrate are just some of
the `important data that will be needed in designing effective equipment.
You might also like
- Heat Transfer Solved Problems: Thermal Resistance of Composite WallDocument2 pagesHeat Transfer Solved Problems: Thermal Resistance of Composite WallKTINE08100% (2)
- Chapter 2 Kinetics of Homogeneous Reaction EDITEDDocument19 pagesChapter 2 Kinetics of Homogeneous Reaction EDITEDJane TubongbanuaNo ratings yet
- Reaction rate determination and simulation of hydrogenation processDocument3 pagesReaction rate determination and simulation of hydrogenation processToMemNo ratings yet
- The Etteilla Tarot: Majors & Minors MeaningsDocument36 pagesThe Etteilla Tarot: Majors & Minors MeaningsRowan G100% (1)
- Gas Absorption Lecture NotesDocument11 pagesGas Absorption Lecture NotesMark Guevarra0% (1)
- Crystallizer SelectionDocument8 pagesCrystallizer SelectionKTINE08No ratings yet
- 2013 4M3 Liquid Liquid ExtractionDocument74 pages2013 4M3 Liquid Liquid ExtractionAndré Mendes PiolNo ratings yet
- CRYSTALLIZATION PROCESS OPTIMIZATIONDocument42 pagesCRYSTALLIZATION PROCESS OPTIMIZATIONKTINE0894% (16)
- Understand Azure Event HubsDocument12 pagesUnderstand Azure Event HubselisaNo ratings yet
- SelectionDocument1 pageSelectionKTINE08No ratings yet
- Reaction Kinetics: Reactions in SolutionFrom EverandReaction Kinetics: Reactions in SolutionRating: 3.5 out of 5 stars3.5/5 (4)
- TRK1 2013 Chapt 3 (Part 1)Document17 pagesTRK1 2013 Chapt 3 (Part 1)Yoel Dwi Putra GultomNo ratings yet
- Chemical Kinetics Rate Law OrderDocument23 pagesChemical Kinetics Rate Law OrderMontassar DridiNo ratings yet
- Understanding Chemical KineticsDocument96 pagesUnderstanding Chemical Kineticssalma khanNo ratings yet
- Chemical Kinetics 1234 FinalDocument22 pagesChemical Kinetics 1234 FinalJayesh SavaliyaNo ratings yet
- Chemical Kinetics 1Document114 pagesChemical Kinetics 1Ashish KrishnaNo ratings yet
- Approval SheetDocument25 pagesApproval SheetSelni Sandabunga'No ratings yet
- Chemical Kinetics: By:-Divyam Verma Ankur Kumar Deepak KumarDocument36 pagesChemical Kinetics: By:-Divyam Verma Ankur Kumar Deepak KumarAnindya BhattacharyaNo ratings yet
- Physical Chemistry Chemical KineticsDocument10 pagesPhysical Chemistry Chemical Kineticsبلسم محمود شاكرNo ratings yet
- CBSE Class 12 Chemistry Notes: Chemical Kinetics: HomepageDocument14 pagesCBSE Class 12 Chemistry Notes: Chemical Kinetics: HomepageBHAVYA BNo ratings yet
- Introduction To Reaction Kinetics, Hazle CoxDocument83 pagesIntroduction To Reaction Kinetics, Hazle CoxcachorroingenieroNo ratings yet
- Chemistry Notes For Class 12 Chapter 4 Chemical KineticsDocument11 pagesChemistry Notes For Class 12 Chapter 4 Chemical KineticsAyush singh PrinceNo ratings yet
- Chemical Kinetics & Reactor Design Course (3CHDocument91 pagesChemical Kinetics & Reactor Design Course (3CHMuhammad Ali Hashmi100% (1)
- 3 Kinetika Reaksi 2018 (Part 1)Document13 pages3 Kinetika Reaksi 2018 (Part 1)Iqbal Al FuadyNo ratings yet
- Chemical KineticsDocument8 pagesChemical KineticsHosam Hasan Abd ElhadyNo ratings yet
- Reactor EngineeringDocument19 pagesReactor EngineeringAlvine AyietaNo ratings yet
- Chemical Kinetics-1 NotesDocument14 pagesChemical Kinetics-1 NotesRachit rajputNo ratings yet
- Chemistry 4Document3 pagesChemistry 4Nothing is ImpossibleNo ratings yet
- Chemical KineticsDocument51 pagesChemical KineticsSrynnENo ratings yet
- Fair Use NoticeDocument15 pagesFair Use NoticeImran UnarNo ratings yet
- Addis Ababa University Chemical Reaction Engineering LectureDocument31 pagesAddis Ababa University Chemical Reaction Engineering LectureTalew TadesseNo ratings yet
- 3 Ley de Velocidad de ReacciónDocument44 pages3 Ley de Velocidad de ReacciónRonaldo Luis Guao BolañoNo ratings yet
- Reaction Kinetics ExplainedDocument31 pagesReaction Kinetics ExplainedchweetomahiNo ratings yet
- Chapter-04 Chemical KineticsDocument11 pagesChapter-04 Chemical Kineticsshrey4602No ratings yet
- Unit 1 Module 2 Rates Hand OutDocument7 pagesUnit 1 Module 2 Rates Hand OutLisa SawhNo ratings yet
- Chapter 6: Kinetics - Fast Facts: 6.1 Collision Theory and Rates of ReactionDocument2 pagesChapter 6: Kinetics - Fast Facts: 6.1 Collision Theory and Rates of ReactionChampbe Joo-LennoxNo ratings yet
- SS 2 Week 3Document71 pagesSS 2 Week 3Denzel MusaNo ratings yet
- Chemical Kinetics PresentationDocument13 pagesChemical Kinetics PresentationMohit TomarNo ratings yet
- Rates and Rate Laws: SpectrosDocument6 pagesRates and Rate Laws: Spectrosdharul khairNo ratings yet
- Introduction Chemical KineticsDocument4 pagesIntroduction Chemical KineticsDenis PrasetiaNo ratings yet
- Factors Affecting Rates of Chemical ReactionsDocument1 pageFactors Affecting Rates of Chemical ReactionsHoneylet Ü FerolNo ratings yet
- Research On Lab ReportDocument7 pagesResearch On Lab ReportCalleb OkelloNo ratings yet
- I. Title of Experiment: II. Date of Experiment: Iii. The End of Experiment: IV. Purpose of Experiment: V. Basic Theories A. Reaction RateDocument14 pagesI. Title of Experiment: II. Date of Experiment: Iii. The End of Experiment: IV. Purpose of Experiment: V. Basic Theories A. Reaction RateputriNo ratings yet
- Rangkuman TRK (Deva Punya)Document4 pagesRangkuman TRK (Deva Punya)gamalielNo ratings yet
- Acre MSC Part 3 20 Feb 2014Document16 pagesAcre MSC Part 3 20 Feb 2014Salman HaroonNo ratings yet
- XII - CHEMICAL KINETICS - Module 2Document5 pagesXII - CHEMICAL KINETICS - Module 2Rahul Joseph ThomasNo ratings yet
- Chapter 1Document30 pagesChapter 1Khalid SirajNo ratings yet
- PDF Revised Chemical Kinetics and CatalysisDocument68 pagesPDF Revised Chemical Kinetics and CatalysisTomateGreenNo ratings yet
- Rate Laws and Reaction KineticsDocument6 pagesRate Laws and Reaction KineticsThala SkNo ratings yet
- One Stop For Colleges Education Career: Minglebox Engineering PrepDocument14 pagesOne Stop For Colleges Education Career: Minglebox Engineering PrepVigneshwar DhamodharanNo ratings yet
- Kinetics and Mechanisms of Inorganic Reactions in SolutionDocument24 pagesKinetics and Mechanisms of Inorganic Reactions in SolutionMartyr LeoNo ratings yet
- Rate Equation: Zeroth-Order ReactionsDocument16 pagesRate Equation: Zeroth-Order ReactionsBastab DeyNo ratings yet
- Concentration Dependent Term v.2.0.Document22 pagesConcentration Dependent Term v.2.0.crystal macababbadNo ratings yet
- Chemical Kinetics NotesDocument11 pagesChemical Kinetics Notespala100% (1)
- PDF DocumentDocument9 pagesPDF DocumentpalesaNo ratings yet
- Chapter 3 PART1-Rate LawsDocument32 pagesChapter 3 PART1-Rate Laws林哲璋No ratings yet
- Reaction Kinetics and The Kinetics-Based Interpretation of EquilibriumDocument54 pagesReaction Kinetics and The Kinetics-Based Interpretation of EquilibriumwastequestNo ratings yet
- Chemical Kinetics Rate Laws and Reaction OrdersDocument30 pagesChemical Kinetics Rate Laws and Reaction OrdersBichitra GautamNo ratings yet
- Pharmaceutical Kinetics and Stability-Lec-2Document27 pagesPharmaceutical Kinetics and Stability-Lec-2husseinkamilhamid123456789No ratings yet
- Kinetics of Homogeneous ReactionsDocument13 pagesKinetics of Homogeneous ReactionsRahul ParmarNo ratings yet
- PCE Lecture 5 1 CRE IntroductionDocument20 pagesPCE Lecture 5 1 CRE IntroductionCH21B027 MEGAVARSHINI MNo ratings yet
- Chemical reaction rates and kineticsDocument12 pagesChemical reaction rates and kineticsadityaNo ratings yet
- Rate of ReactionDocument23 pagesRate of ReactionVirly vcNo ratings yet
- Chem 1108 Reflection No 4Document3 pagesChem 1108 Reflection No 4Renato BatumbakalNo ratings yet
- KineticsDocument26 pagesKineticsMelissa M. Abansi-BautistaNo ratings yet
- Rates and Equilibria of Organic Reactions: As Treated by Statistical, Thermodynamic and Extrathermodynamic MethodsFrom EverandRates and Equilibria of Organic Reactions: As Treated by Statistical, Thermodynamic and Extrathermodynamic MethodsNo ratings yet
- Problem Set - Settling and SedimentationDocument1 pageProblem Set - Settling and SedimentationKTINE08100% (1)
- HT 033 SolutionDocument6 pagesHT 033 SolutionKTINE08No ratings yet
- HT 030 SolutionDocument3 pagesHT 030 SolutionKTINE08No ratings yet
- Chemical Engineering Series: Heat Transfer Solved Problems: T t T t WC Mc K K θDocument1 pageChemical Engineering Series: Heat Transfer Solved Problems: T t T t WC Mc K K θKTINE08No ratings yet
- HT-029 SolutionDocument2 pagesHT-029 SolutionKTINE08No ratings yet
- HT 034 SolutionDocument2 pagesHT 034 SolutionKTINE08No ratings yet
- HT 036 SolutionDocument1 pageHT 036 SolutionKTINE08100% (2)
- Chemical Engineering Series: Heat Transfer Solved Problems: Q A H T TDocument2 pagesChemical Engineering Series: Heat Transfer Solved Problems: Q A H T TKTINE08No ratings yet
- HT 032 SolutionDocument2 pagesHT 032 SolutionKTINE08No ratings yet
- HT-026 SolutionDocument2 pagesHT-026 SolutionKTINE08No ratings yet
- Lenses Practice ProblemsDocument1 pageLenses Practice ProblemsKTINE08No ratings yet
- HT-027 SolutionDocument2 pagesHT-027 SolutionKTINE08No ratings yet
- Evaluation Form Pnri SeminarDocument2 pagesEvaluation Form Pnri SeminarKTINE08No ratings yet
- Manufacture of Sulfuric AcidDocument9 pagesManufacture of Sulfuric AcidDiajeng M.100% (1)
- Lecture 2 - Process Dynamic Models PDFDocument9 pagesLecture 2 - Process Dynamic Models PDFnoteasytobebooNo ratings yet
- Evaluation Form Pnri SeminarDocument2 pagesEvaluation Form Pnri SeminarKTINE08No ratings yet
- Process 3 For Soda AshDocument2 pagesProcess 3 For Soda AshKTINE08No ratings yet
- IEC Written ReportDocument9 pagesIEC Written ReportKTINE08No ratings yet
- How students evaluated their math tutorDocument5 pagesHow students evaluated their math tutorKTINE08No ratings yet
- Floor Plan (Testimonial Dinner)Document1 pageFloor Plan (Testimonial Dinner)KTINE08No ratings yet
- Case StudiesDocument46 pagesCase StudiesKTINE080% (1)
- Republic Act No. 318Document37 pagesRepublic Act No. 318KTINE08No ratings yet
- IE399 Summer Training ReportDocument17 pagesIE399 Summer Training ReportgokanayazNo ratings yet
- Nqs PLP E-Newsletter No68Document5 pagesNqs PLP E-Newsletter No68api-243291083No ratings yet
- Future Design of Accessibility in Games - A Design Vocabulary - ScienceDirectDocument16 pagesFuture Design of Accessibility in Games - A Design Vocabulary - ScienceDirectsulaNo ratings yet
- Donny UfoaksesDocument27 pagesDonny UfoaksesKang Bowo D'wizardNo ratings yet
- Research Paper On Organ DonationDocument8 pagesResearch Paper On Organ Donationsheeliya whiteNo ratings yet
- Obsolescence 2. Book Value 3. Depreciation 4. Depletion EtcDocument9 pagesObsolescence 2. Book Value 3. Depreciation 4. Depletion EtcKHAN AQSANo ratings yet
- Universal Robina Co. & Bdo Unibank Inc.: Research PaperDocument25 pagesUniversal Robina Co. & Bdo Unibank Inc.: Research PaperSariephine Grace ArasNo ratings yet
- BSC6900 UMTS V900R011C00SPC700 Parameter ReferenceDocument1,010 pagesBSC6900 UMTS V900R011C00SPC700 Parameter Referenceronnie_smgNo ratings yet
- Lesson 5 CMADocument10 pagesLesson 5 CMAAssma SabriNo ratings yet
- Learning Online: Veletsianos, GeorgeDocument11 pagesLearning Online: Veletsianos, GeorgePsico XavierNo ratings yet
- PowerhouseDocument10 pagesPowerhouseRanjan DhungelNo ratings yet
- All MeterialsDocument236 pagesAll MeterialsTamzid AhmedNo ratings yet
- Week 6Document7 pagesWeek 6Nguyễn HoàngNo ratings yet
- Fda PDFDocument2 pagesFda PDFVictorNo ratings yet
- National Products Classification Code For Services in IndiaDocument92 pagesNational Products Classification Code For Services in Indiakalanemi0% (2)
- W1inse6220 PDFDocument11 pagesW1inse6220 PDFpicalaNo ratings yet
- C11 RacloprideDocument5 pagesC11 RacloprideAvina 123No ratings yet
- Assessing Eyes NCM 103 ChecklistDocument7 pagesAssessing Eyes NCM 103 ChecklistNicole NipasNo ratings yet
- AP Euro Unit 2 Study GuideDocument11 pagesAP Euro Unit 2 Study GuideexmordisNo ratings yet
- Tupperware India's Perception StudyDocument10 pagesTupperware India's Perception StudyAnmol RahangdaleNo ratings yet
- Relay Coordination Using Digsilent PowerFactoryDocument12 pagesRelay Coordination Using Digsilent PowerFactoryutshab.ghosh2023No ratings yet
- EPF Passbook Details for Member ID RJRAJ19545850000014181Document3 pagesEPF Passbook Details for Member ID RJRAJ19545850000014181Parveen SainiNo ratings yet
- FINAL - Plastic Small Grants NOFO DocumentDocument23 pagesFINAL - Plastic Small Grants NOFO DocumentCarlos Del CastilloNo ratings yet
- Compare and Contrast High School and College EssayDocument6 pagesCompare and Contrast High School and College Essayafibkyielxfbab100% (1)
- April 3rd - Asynchronous Class - Questions-4Document3 pagesApril 3rd - Asynchronous Class - Questions-4alidrissiNo ratings yet
- Learn Square Roots & Plot on Number LineDocument11 pagesLearn Square Roots & Plot on Number LineADAM CRISOLOGONo ratings yet