Professional Documents
Culture Documents
Predicting The Azeotropic of Citronellal Enrichment Using Process Simulator PDF
Uploaded by
macleod230286Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Predicting The Azeotropic of Citronellal Enrichment Using Process Simulator PDF
Uploaded by
macleod230286Copyright:
Available Formats
International Conference On Chemical Sciences (ICCS-2007)
Innovation In Chemical Sciences For Better Life
Yogyakarta-Indonesia, 24-26 May, 2007
COMP/41-5
PREDICTING THE AZEOTROPIC OF CITRONELLAL ENRICHMENT USING
PROCESS SIMULATOR
Joddy Arya Laksmono*, Egi Agustian, Indri Badria Adilina
Process Technology and Synthesis of Essential Oil Research Group
Research Center for Chemistry Indonesian Institute of Sciences
Kawasan PUSPIPTEK Serpong, Tangerang, Banten, Indonesia 15314
ABSTRACT
In the laboratory, we have found three main components of citronella oil, citronellal, citronellol, and geraniol.
Citronellal enrichment is generally obtained by fractionation unit. Those three components are usually obtained in
azeotropic system. In the preliminary study, we have found that citronellal in the distillate with concentration of more
than 90% was mixed with other components, mainly citronellol and geraniol. Gas chromatography was used for
analysis of citronellal component. In this study, we have predicted the azeotropic system in fractionation unit for
citronellal enrichment as an illustration using ChemCAD process simulator. The result of citronellal enrichment
simulation will be discussed in this paper.
Keywords: Citronellal enrichment, azeotropic, simulation, ChemCAD.
INTRODUCTION
Citronella oil is one of the most interesting essential
oil, mainly it will be used in any industry of flavor and
fragrances, cosmetics, pharmaceutical, food, and so on.
Essential oil, usually has a vary components in their
mixture. Furthermore, separation and purification of the
essential oil mixtures will be difficult to separate.
Physically, many components in the essential oil have
same boiling point and chemical structured, or have a
boiling point near to the other components. We used
original Java citronella oil (Cymbopogon winterianus
Jowitt) as a raw material for separation simulation in the
laboratory. The smell of oil of citronella repels bloodfeeding mosquitoes, ticks, and fleas. As a non-toxic
substance, many people prefer citronella to other
chemical repellants such as DEET (N, N-diethyl-metatoluamide). Citronella oil appears in many products
designed to protect humans, pets, and open-air spaces
from the public health risks posed by mosquitoes and
ticks. A concerned person could use sprits on their
clothing, lotion or soap on their skin, treated collars on
their pets, and candles or pellet bags surrounding their
picnic [1].
Main component of citronella oil are citronellal,
citronellol, and geraniol. The physical properties of their
components shown that, citronella has a relative higher
different boiling point toward citronellol and geraniol.
Kindly this component has a 20 oC different boiling
temperature due to citronellol dan geraniol. Furthermore,
citronellol and geraniol has a relative nearly in their
boiling point, and then this both components are already
in mixture after separated from citronellal. The mixtures
of citronellol and geraniol could be named as rhodinol [2].
* Corresponding author. Tel: +62-21-7560929, Fax : +62-217560549; Email address : joddy_arya_laksmono@yahoo.com
Fractionation distillation is separation of liquid
mixture according to main compound vapor pressure
difference. Fractionation is process series evaporation
flash stages, which is arranged in series of vapor and
liquid, due to reversible flow from every stage to next
stage. In this condition, liquid will flow to the next
bottom stage and vapor will go to the next stage above.
In fractionation distillation system was completed with
reflux unit to increase fraction quality [3].
The Vapor-Liquid Equilibrium (VLE) is an
important role for design of fractionation unit. In this
paper, we are determining the VLE for separation of
citronella oil to produce citronellal, citronellol, and
geraniol. Design of columns for separating azeotropic
systems includes the analysis of feasible separations of
a mixture of a given composition xf,I, that is, the
determination of the feasible compositions of overhead
and bottom products. The answer to this key question
of rectification theory depends on three factors: the
features of the field of vaporliquid equilibrium ratios,
the mode of separation which is liquid essential oil, and
the scheme of the column (specifically, whether the
column has one feed) [4,5,6].
In this work, we are aim the equilibrium data for
separation of citronella oil using batch vacuum
fractionation at low pressure, and also a dimension
data for the column, such as outside and inside
diameter, height, theoretical tray number, and so on.
The data are consisted of Txy, xy graphics. The
equilibrium data was assessed using ChemCAD
simulator. We was simulated the separation process in
two step dynamics condition using Cubic State
Equation.
Proceeding of ICCS 2007, Yogyakarta-Indonesia, 24-25 May 2007
EXPERIMENTAL SECTION
Materials
Citronella oil which is original Java essential oil was
supplied from local commercial home industry with
concentrations of citronellal 32.15%, citronellol 12.95%,
and geraniol 20.54%. The physical properties of
citronella oil have shown in the table 1.
Apparatus and method
The unit operation was used a batch vacuum
fractionation with capacity 2 L (VTU High Temperature
Fractionator Model Pilodist 104 which is shown in figure
1. A simulator ChemCAD 5.0.2 version which is released
by ChemStation, Inc. Gas chromatography Shimadzu 14
A, column innowax, length 30 m capillary, column
temperature 50 oC 230 oC with heating rate 2 oC/min
and hold time at 230 oC in 30 min., injector temperature
280 oC, FID detector temperature 280 oC, and helium as
a gas carrier with rate 4 ml/min.
First step, we was determined the physical
properties of citronella oil raw material using
conventional method and GC. Separation of citronella oil
Table 1. Physical properties of citronella oil as raw
material.
No.
Parameter
Results
1
Density 20oC (gr/cm3)
0.8826
2
Viscosity (cP)
9.01
3
Refractive index (20oC)
1.4664
4
Ester value
31,01
5
Acid value
1,13
6
Optical rotation
-1.275
7
Appearance
Clearly dark yellow
8
Solubility in alcohol 95% 1:1 clear and so on
9
Fatty oil
Negative
10 Kruing oil
Negative
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
Agitation Motor
Magnetic stirrer
Heating mantle
Feed flask
Thermocouple in feed flask
Distillation column
Heating mantle
Cooler
Manometer U tube indicator
Thermocouple
Refluxs unit
Manometer U tube
Condensor
Vacuum sensor
Vacuum pipe
Distillate cooler
21. Control pan
Bottle products
22. Trap tube
Fraction flask
23. Controlled
Fraction separator
24. Vacuum pu
Figure 1. Pilot plant batch vacuum fractionation unit flow
diagram in the Research Center for Chemistry
Indonesian Institute of Sciences.
Joddy Arya Laksmono, et al.
to produced three main component was used a batch
vacuum fractionator in the vacuum pressure condition
40 80 mbar and temperature was 120 oC 150 oC.
We assumed that the separation has a binary system
citronellal-rhodinol. This process was determined the
physical properties of citronellal, citronellol, and
geraniol, and also to simulated the separation process
in order to appropriate data of higher purity and yield of
there three main components. Products were analyzed
by GC. Then, we have an optimum condition from the
experiment. We used the optimum condition and
components physical properties which was produced
from the process for simulation, and then we was
simulation using ChemCAD simulator to give a
predictive Vapor-Liquid Equilibrium (VLE) for citronellalrhodinol binary system.
RESULT AND DISCUSSION
The calculated values were obtained from the
equilibrium equations:
(1)
y i i P = xi i PiS Si exp iL P PiS / RT
Where P = 80 mbar, xi and yi are the mass fractions of
component i in the liquid and vapor phase,
respectively, i is its activity coefficient, iL , its liquid
molar volume, i and Si , its fugacity coefficients
under
unsaturated
and
saturated
conditions,
S
respectively for pure i and Pi , its vapor pressure at
saturation [7,8,9].
The simulation process separation of citronella oil
was conducted in two steps. These were according to
the numerical solution which was giving convergences
of the dynamic simulation. Figure 2 and 3 has shown
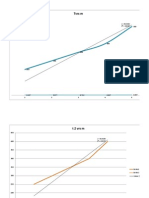
the dynamic simulation for this process. Figure 2 was
shown that mass fraction of three components;
citronellal, citronellol, and geraniol are respectively
constants in step 1 during the process until 4.5 hours
process time. This was indicating that equilibrium
condition could be reached out since the temperature
of process is constant; this condition would be giving a
constant mass fraction in the distillate during
equilibrium condition. Step two of simulation process
was shown that the separation has a dynamic section
and separation of citronellal, citronellol, and geraniol
has occurred, figure 3. Citronellal component was
separated to give a high purity and has not
contaminated with other components. However,
separation process of citronellol and geraniol has
occurred less than citronellal separation. Citronellol and
geraniol were giving a nearly concentration at the end
of process. This was caused that boiling point of both
components is nearly.
Proceeding of ICCS 2007, Yogyakarta-Indonesia, 24-25 May 2007
Figure 2. First step simulation processes in the batch
vacuum fractionation unit.
Figure 3. Step 2 simulation processes that indicating the
dynamic process.
The separation of citronella-citronellol system has
been in the miscible system. At the molecular level
appreciable negative deviations from Raoults law reflect
stronger intermolecular forces between unlike than
between like pairs of molecules. Conversely, appreciable
positive deviations result for solutions in which
intermolecular forces between like molecules are
stronger than between unlike. In this latter case the
forces between like molecules may be so strong in
comparison with those between unlike molecules as to
prevent complete miscibility. In this event the mixture
would form two separated liquid phases over some
composition range.
All data processing was performed using
ChemCAD simulator 5.0.2 version. Figure 4. shown a
Vapor-Liquid Equilibrium (T-xy) curve for binary
citronellal-citronellol system equilibrium. The equilibrium
of vapor-liquid for this system was occurred in the range
of temperature 121 oC 149 oC under the vacuum
condition. The curve gives information for scaling up
data.
Vapor-Liquid Equilibrium for a binary system, the
phase rule specifies that there are but two degrees of
freedom. Data for such system are invariably taken at
either constant pressure or at constant temperature.
Joddy Arya Laksmono, et al.
Figure 4. T-XY diagram for citronellal-citronellol binary
system at a constant vacuum pressure 80 mbar.
Table 2. xy data for citronellal-citronellol with K value
model: SRK.
Mass Fractions
T Deg C
P mbar
X1
Y1
148.53481 80.00000 0.00000 0.00000
146.65231 80.00000 0.04939 0.11889
144.85136 80.00000 0.09884 0.22398
143.12856 80.00000 0.14835 0.31707
141.48044 80.00000 0.19793 0.39970
139.90329 80.00000 0.24757 0.47320
138.39354 80.00000 0.29727 0.53875
136.94756 80.00000 0.34705 0.59735
135.56178 80.00000 0.39688 0.64986
134.23282 80.00000 0.44678 0.69704
132.95738 80.00000 0.49675 0.73953
131.73257 80.00000 0.54678 0.77790
130.55487 80.00000 0.59687 0.81262
129.42181 80.00000 0.64703 0.84412
128.33075 80.00000 0.69726 0.87277
127.27905 80.00000 0.74755 0.89888
126.26440 80.00000 0.79791 0.92273
125.28468 80.00000 0.84833 0.94457
124.33779 80.00000 0.89882 0.96461
123.42182 80.00000 0.94938 0.98303
122.53481 80.00000 1.00000 1.00000
Since only one degree of freedom remains, x1
may be regarded as the only independent variable in
either case. The equation for the liquid phase of a
binary system in this experiment at constant vacuum
pressure condition was seems in the below [11,12]:
d ln 1
d ln 2
H dT
(2)
= x1
+ x2
2 dx
dx1
dx1
RT 1
The temperature profile along the column height
was observed and simulated, figure 6. The temperature
range in each stage was observed relatively constant.
These were needed to reach a steady-state operation
of the column. This is important due to the possibly flat
temperature changes in spite of large composition
Proceeding of ICCS 2007, Yogyakarta-Indonesia, 24-25 May 2007
Figure 5. xy weigth fraction citronellal-citronellol system.
Figure 7. Residu curve map citronellal, citronellol, and
geraniol component by SRK at constant vacuum
pressure 80 mbar.
Table 3. Batch vacuum
calculation results.
fractionation
simulation
Operation Step 1:
Stream Name
Pot Charge Accumulator
Temp C
27.1525
73.3527
Pres mbar
80.0000
8.3332
Enth MJ
-30.199
-23.557
Vapor mole fraction 0.00000
0.00000
Total kmol
1.1102
0.5520
Total kg
171.5441
85.1462
Total std L m3
0.1995
0.0992
Flowrates in kmol
CITRONELLAL
0.8419
0.5520
CITRONELLOL
0.1490
0.0000
GERANIOL
0.1192
0.0000
Figure 6. Stage temperature profile in the batch vacuum
fractionation
during
citronellal-citronellol
system
separation.
variations. Before sampling and simulation the
temperature profile, the column top pressure and the
pressure drop were measured. After sampling the
volume streams at the top of the column and below the
packing were measured.
The measured streams were compared with those
obtained from the energy balances around the
condenser and the reboiler, so that the heat and mass
loss from the column could be calculated. The
compositions of samples were analyzed with gas
chromatography.
This simulation was used 36 stages in vacuum
fractionation column, and we tried to simulated
citronellal-citronellol-geraniol
separation
behaviors.
Binary system of citronellal-citronellol separation has
shown without azeotropic curves. However, figure 7. was
shown that citronellol-geraniol binary system has
indicating an azeotropic in (0.879, 0.121) coordinate at
148.476 oC. Citronellal, citronellol, and geraniol have
had a bubble point at 80 mbar vacuum pressure,
122.534 oC, 148.534 oC, and 150.804 oC respectively.
Joddy Arya Laksmono, et al.
Pot Residue
106.2513
24.6658
-18.680
0.00000
0.4928
76.3215
0.0886
0.2251
0.1487
0.1190
Distillate
73.3543
8.3332
-0.51210
0.00000
0.0120
1.8510
0.0022
0.0120
0.0000
0.0000
Operation Step 2:
Stream Name
Accumulator
Temp C
80.8966
Pres mbar
8.3332
Enth MJ
-22.234
Vapor mole fraction 0.00000
Total kmol
0.4920
Total kg
76.1443
Total std L m3
0.0885
Flowrates in kmol
CITRONELLAL
0.2899
CITRONELLOL
0.1254
GERANIOL
0.0767
Pot Residue
123.4819
24.6658
-0.083731
0.00000
0.0023
0.3493
0.0004
Distillate
101.1986
8.3332
-0.26895
0.00000
0.0060
0.9317
0.0011
0.0000
0.0003
0.0020
0.0000
0.0031
0.0029
CONCLUSION
The simulation of citronellal-citronellol-geraniol
was performed with ChemCAD. The essential content
of this paper is a listing of bundles of experimental to
found a physical properties which is useful for
simulation data, and simulation this separation
phenomenon obtained with binary in non-ideal system,
in bubble cap tray column and at vacuum pressure.
Also valuable technical information is provided for
construction of pilot scale fractionation column. The
results should be fitted with an experimental data to
Proceeding of ICCS 2007, Yogyakarta-Indonesia, 24-25 May 2007
find an accurate data for vapor-liquid equilibrium system.
6.
ACKNOWLEDGEMENT
This research was funded from Indonesian
government, and we should proudly thanks for this
opportunity. We are pleased to Mr. Tasrif for GC data
processing and to Mr. Yogi Hermawan with his fruitful
discussion.
7.
REFERENCES
1.
2.
3.
4.
5.
Egi Agustian, Asep Kadarohman, Anny Sulaswatty,
Fractionation of Citronellal from Citronella oil Using
Vacuum Distillation Technique, Prosiding Seminar
Fundamental dan Aplikasi Teknik Kimia 2004,
Surabaya, 7 8 Desember.
Sitorus, M., Esterifikasi Rodinol dari Minyak Sereh
dengan Anhidrida Asam Karboksilat, Thesis S2
Universitas Gajah Mada, Yogyakarta, 1995.
Haznan Abimanyu, Anny Sulaswatty, Wuryaningsih
dan Egi Agustian.,
Teknologi Distilasi Terfraksi
Dalam Pemurnian Komponen Minyak Atsiri,
Prosiding
Pemaparan
Hasil
Litbang
Ilmu
Pengetahuan Teknik 2003, Bandung.
Sami
Pelkonen,
Andrzej
Grak,
Andr
Ohligschlger, Ruth Kaesemann, Experimental
Study on Multicomponent Distillation in Packed
Columns, Chemical Engineering and Processing,
40, (2001), 235-243.
P. Pllmann, M.H. Bauer, and E. Bla,
Investigation of Vapor-Liquid Equilibrium of Nonideal Multicomponent Systems, Gas. Sep. Purif.,
Vol. 10, No. 4, pp. 225-241, 1996.
Joddy Arya Laksmono, et al.
8.
9.
10.
11.
12.
Alberto Arce, JoseMartnez-Ageitos, Eva Rodil,
Ana Soto, Phase Equilibria Involved in Extractive
Distillation
of
2-methoxy-2methylpropaneqmethanol Using 1-butanol as
Entrainer, Fluid Phase Equilibria, 171, 2000, 207
218.
F. B. Petlyuk, Simple Methods for Predicting
Feasible Sharp Separations of Azeotropic
Mixtures, Theoretical Foundations of Chemical
Engineering, Vol. 32, No. 3, 1998, pp. 245253.
Translated
from
Teoreticheskie
Osnovy
Khimicheskoi Tekhnologii, Vol. 32, No. 2, 1998,
pp. 279287. Original Russian Text Copyright
1998 by Petlyuk.
Arjun Vadapalli, J.D. Seader, A Generalized
Framework for Computing Bifurcation Diagrams
Using Process Simulation Programs, Computers
and Chemical Engineering, 25, (2001), 445464.
F. G. Smith III, R. A. Dimenna, Simulation of a
Batch Chemical Process Using Parallel
Computing with PVM and Speedup, Computers
and Chemical Engineering, 28, (2004), 1649
1659.
R. Schneider, F. Sander, A. Gorak, Dynamic
Simulation of Industrial Reactive Absorption
Processes,
Chemical
Engineering
and
Processing, 42, (2003), 955-964.
Perry, R.H & D. Green, 1984. Perrys Chemical
Engineering Handbook. Mc Graw-Hill Company,
New York.
J.M. Smith, H.C. Van Ness, Introduction to
Chemical Engineering Thermodynamics, 3rd Ed.
McGraw-Hill International book company, 290364,
1975,
Singapore.
You might also like
- COMP/41-5: Predicting The Azeotropic of Citronellal Enrichment Using Process SimulatorDocument5 pagesCOMP/41-5: Predicting The Azeotropic of Citronellal Enrichment Using Process SimulatorDicky GabrielNo ratings yet
- Kurniawan 2019 J. Phys. Conf. Ser. 1321 022038 PDFDocument6 pagesKurniawan 2019 J. Phys. Conf. Ser. 1321 022038 PDFAlpin HidayatullohNo ratings yet
- Aspen ModelDocument4 pagesAspen ModelAlex MashegoNo ratings yet
- Modelling The Kinetics of Steam Distillation of Essential Oils From Lemon Grass (Cymbopogon SPP.)Document9 pagesModelling The Kinetics of Steam Distillation of Essential Oils From Lemon Grass (Cymbopogon SPP.)saliomar2000No ratings yet
- ISIC19 Paper 220Document3 pagesISIC19 Paper 220Laura GreenNo ratings yet
- Isolation of Citral From Lemongrass Oil Using Steam Distillation Statistical Optimization by Response Surface MethodologDocument10 pagesIsolation of Citral From Lemongrass Oil Using Steam Distillation Statistical Optimization by Response Surface MethodologKinjarapu YamunaNo ratings yet
- Quim. Nova, Vol. 31, No. 3, 527-529, 2008Document3 pagesQuim. Nova, Vol. 31, No. 3, 527-529, 2008Tanato TartaroNo ratings yet
- Eden 2018 IOP Conf. Ser. Mater. Sci. Eng. 349 012067 PDFDocument9 pagesEden 2018 IOP Conf. Ser. Mater. Sci. Eng. 349 012067 PDFAisyah NurmafajahNo ratings yet
- Formal Report Distillation of ALcoholic BeveragesDocument12 pagesFormal Report Distillation of ALcoholic Beveragespatricia_moran_4No ratings yet
- Styrene ProductionDocument12 pagesStyrene ProductionMoni ValinschiNo ratings yet
- Distillation of Essential Oils From Mentha X PiperetaDocument8 pagesDistillation of Essential Oils From Mentha X PiperetageorgeNo ratings yet
- Hamzah 2013Document17 pagesHamzah 2013Theo MartinezNo ratings yet
- Chen 2008Document8 pagesChen 2008Yunita PujiastutiNo ratings yet
- Resr LabDocument12 pagesResr LabRenee GlodonNo ratings yet
- IOP Conf Series: Effect of Cosolvents & Surfactant in Aromatic ExtractionDocument8 pagesIOP Conf Series: Effect of Cosolvents & Surfactant in Aromatic Extractionعامر طايس سعيد عبد الجبارNo ratings yet
- Ansolin Et Al 2013Document9 pagesAnsolin Et Al 2013Francielly StechiNo ratings yet
- Transesterification of Neat and Used Frying Oil: Optimization For Biodiesel ProductionDocument8 pagesTransesterification of Neat and Used Frying Oil: Optimization For Biodiesel ProductionCristiNo ratings yet
- Benyahia Energy Evaluation of Ethanol Dehydration With Glycol Mixture As EntrainerDocument8 pagesBenyahia Energy Evaluation of Ethanol Dehydration With Glycol Mixture As EntrainerRajendraNo ratings yet
- D.I Engne ManuscriptDocument12 pagesD.I Engne ManuscriptChandramanikandanNo ratings yet
- Limonene PurificationDocument4 pagesLimonene PurificationTaviAlfonso100% (1)
- Catalytic and Non-Catalytic Esterification of Soybean Oil Deodorizer Distillate by Ethanol: Kinetic ModellingDocument6 pagesCatalytic and Non-Catalytic Esterification of Soybean Oil Deodorizer Distillate by Ethanol: Kinetic ModellingHugo VillardiNo ratings yet
- 1263 3507 1 PBDocument5 pages1263 3507 1 PBJohn TorrezNo ratings yet
- Biodiesel Production From Waste Frying Oil and Determination of Fuel PropertiesDocument5 pagesBiodiesel Production From Waste Frying Oil and Determination of Fuel PropertiesMáximo Décimo MeridioNo ratings yet
- Dissolved Gas Analysis of Overheating FaultDocument4 pagesDissolved Gas Analysis of Overheating FaultTedy MTNo ratings yet
- Batistella2002 Article MolecularDistillationProcessFo PDFDocument11 pagesBatistella2002 Article MolecularDistillationProcessFo PDFSrđan TufegdžićNo ratings yet
- Recycling of Used Lubricating Engine Oil by A Solvent Extraction ProcessDocument8 pagesRecycling of Used Lubricating Engine Oil by A Solvent Extraction ProcessTJPRC PublicationsNo ratings yet
- Storage Stability of α-tocopherol Extracted from Heated and Un-heated Palm Oil MesocarpDocument10 pagesStorage Stability of α-tocopherol Extracted from Heated and Un-heated Palm Oil MesocarpTanwarat ChaikaewNo ratings yet
- Adsorption Basics Part 1Document6 pagesAdsorption Basics Part 1Felix TsecoNo ratings yet
- 5990-9806EN AppNote 630-4500-5500 OilWaterDocument6 pages5990-9806EN AppNote 630-4500-5500 OilWaterJanardan KrishnanNo ratings yet
- Supercritical Extraction of Cocoa Butter From Cocoa Seed, Using Pure Carbon Dioxide, Carbon Dioxide With Ethanol As Co-Solvent and EthaneDocument6 pagesSupercritical Extraction of Cocoa Butter From Cocoa Seed, Using Pure Carbon Dioxide, Carbon Dioxide With Ethanol As Co-Solvent and Ethanejmrozo3No ratings yet
- Steam Distillation Essential OilsDocument7 pagesSteam Distillation Essential OilsRade NovakovicNo ratings yet
- Agus Aktawan SICEST2016 - Paper - 231 - CPB-035 BDocument3 pagesAgus Aktawan SICEST2016 - Paper - 231 - CPB-035 BJonathan SoeNo ratings yet
- Selective Catalytic Hydrogenation of Triglycerides: Activity and Selectivity Towards C18:1Document6 pagesSelective Catalytic Hydrogenation of Triglycerides: Activity and Selectivity Towards C18:1Al Musabbir LeeonNo ratings yet
- (Zeitschrift FR Naturforschung B) Supercritical CO2 Extraction of Essential Oil From Clove Bud Effect of Operation Conditions On The Selective Isolation of Eugenol and Eugenyl AcetateDocument5 pages(Zeitschrift FR Naturforschung B) Supercritical CO2 Extraction of Essential Oil From Clove Bud Effect of Operation Conditions On The Selective Isolation of Eugenol and Eugenyl AcetateZia Uzlifatul Fauzia Al HasanNo ratings yet
- Simulation of Ethanol Extractive Distillation With Mixed Glycols As Separating AgentDocument12 pagesSimulation of Ethanol Extractive Distillation With Mixed Glycols As Separating AgentViona WidyaNo ratings yet
- 4375-Article Text-17142-1-10-20140908Document8 pages4375-Article Text-17142-1-10-20140908nityaNo ratings yet
- Biodiesel from Rubber Seed Oil via EsterificationDocument7 pagesBiodiesel from Rubber Seed Oil via EsterificationThobroni AkbarNo ratings yet
- 5 Ijcms-V3i2-2012-02Document6 pages5 Ijcms-V3i2-2012-02saiNo ratings yet
- 5 Ijcms-V3i2-2012-02Document6 pages5 Ijcms-V3i2-2012-02saiNo ratings yet
- Analysis of VOCs in Perfume Samples by Gas ChromatographyDocument14 pagesAnalysis of VOCs in Perfume Samples by Gas ChromatographyAninaNo ratings yet
- 1 s2.0 S0378381205003651 Main PDFDocument5 pages1 s2.0 S0378381205003651 Main PDFأبى جزاك الله خيراNo ratings yet
- Physical Refining of Crude Palm OilDocument13 pagesPhysical Refining of Crude Palm Oilaffeena100% (1)
- 2862-Article Text-16501-1Document14 pages2862-Article Text-16501-1BUN SaretNo ratings yet
- Assignmen T 2 GCDocument9 pagesAssignmen T 2 GCMark SullivanNo ratings yet
- Imp 2Document8 pagesImp 2Shivam PandyaNo ratings yet
- Clove Oil Synthesis Organic ChemistryDocument8 pagesClove Oil Synthesis Organic ChemistrynewswagNo ratings yet
- Brazilian Journal of Chemical EngineeringDocument8 pagesBrazilian Journal of Chemical Engineeringcarol choquecallataNo ratings yet
- Extraction of Xylene and Naphthalene From Crude OilDocument8 pagesExtraction of Xylene and Naphthalene From Crude OilD'code SmibNo ratings yet
- Oliveira Et Al 2012Document10 pagesOliveira Et Al 2012Daniela De Araujo SampaioNo ratings yet
- 04 Saripah SobahDocument8 pages04 Saripah SobahArdifal JumaidiNo ratings yet
- Volatile Components of Clove Essential Oil Eugenia Caryophyllus Spreng Neutral FractionDocument8 pagesVolatile Components of Clove Essential Oil Eugenia Caryophyllus Spreng Neutral FractionTheo MartinezNo ratings yet
- SSRN Id3874836Document10 pagesSSRN Id3874836Anirban BhowalNo ratings yet
- Extraction and Refining of Essential Oil From Australian Tea Tree, Melaleuca Alterfornia, and The Antimicrobial Activity in Cosmetic ProductsDocument8 pagesExtraction and Refining of Essential Oil From Australian Tea Tree, Melaleuca Alterfornia, and The Antimicrobial Activity in Cosmetic ProductsHiền Đăng NguyễnNo ratings yet
- ST 11 PDFDocument8 pagesST 11 PDFAdriana StNo ratings yet
- Statistical Analysis of The Supercritical Fluid Oil Extraction From Grape SeedDocument8 pagesStatistical Analysis of The Supercritical Fluid Oil Extraction From Grape SeedALBA SOFIA PARRA CARVAJALNo ratings yet
- Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and PowerFrom EverandThermochemical Processing of Biomass: Conversion into Fuels, Chemicals and PowerNo ratings yet
- Essential Oils in Food Processing: Chemistry, Safety and ApplicationsFrom EverandEssential Oils in Food Processing: Chemistry, Safety and ApplicationsSeyed Mohammed Bagher HashemiNo ratings yet
- Bio-Based SolventsFrom EverandBio-Based SolventsFrançois JérômeNo ratings yet
- Air Pollution Particulates Ictineo I: Scrubber Scrubber Systems Are A Diverse Group ofDocument1 pageAir Pollution Particulates Ictineo I: Scrubber Scrubber Systems Are A Diverse Group ofmacleod230286No ratings yet
- Pa 60Document2 pagesPa 60macleod230286No ratings yet
- Para 100Document2 pagesPara 100macleod230286No ratings yet
- Calor Informe 8Document15 pagesCalor Informe 8macleod230286No ratings yet
- FM22 PDFDocument2 pagesFM22 PDFJesus Mac LeodNo ratings yet
- Exercise6 1Document11 pagesExercise6 1Teka KamNo ratings yet
- Manual Best Management PortsDocument156 pagesManual Best Management PortsAchraf DouiriNo ratings yet
- Azeotropic Distillation PDFDocument12 pagesAzeotropic Distillation PDFmacleod230286No ratings yet
- Tvrs MDocument3 pagesTvrs Mmacleod230286No ratings yet
- Chemcad TutorialDocument220 pagesChemcad Tutorialboun_dNo ratings yet
- Chemcad 6 User GuideDocument202 pagesChemcad 6 User Guideerhan ünal100% (1)
- Determination of Critical Micelle Concentrscion PDFDocument6 pagesDetermination of Critical Micelle Concentrscion PDFmacleod230286No ratings yet
- Chemcad Cc5 ExampleDocument37 pagesChemcad Cc5 ExampleBabulu BalarkanNo ratings yet
- Fractional batch distillation simulation in CHEMCADDocument10 pagesFractional batch distillation simulation in CHEMCADmacleod230286No ratings yet
- Determination of Critical Micelle Concentrscion PDFDocument6 pagesDetermination of Critical Micelle Concentrscion PDFmacleod230286No ratings yet
- Great Smog of 1952Document8 pagesGreat Smog of 1952macleod230286No ratings yet
- Application of Process Simulation Software METSIM in MetallurgyDocument7 pagesApplication of Process Simulation Software METSIM in Metallurgymacleod230286No ratings yet
- Chem Cad Binod Al PlotDocument9 pagesChem Cad Binod Al Plotmacleod230286No ratings yet
- Espectroscopia Uv VisibleDocument27 pagesEspectroscopia Uv Visiblemacleod230286No ratings yet
- Chemsep HelpDocument59 pagesChemsep Helpmacleod230286No ratings yet
- Kohler RRT ManualDocument40 pagesKohler RRT Manualjmh488100% (1)
- COMMUNITY HEALTH NURSING: PREVENTION, FAMILIES, PRIMARY CAREDocument4 pagesCOMMUNITY HEALTH NURSING: PREVENTION, FAMILIES, PRIMARY CAREJohn Vincent VasquezNo ratings yet
- Aqua Regia - WikipediaDocument5 pagesAqua Regia - WikipediaearthplightNo ratings yet
- Tactile Internet MSC V2Document28 pagesTactile Internet MSC V2hendNo ratings yet
- Modeling Relationships in Scatter PlotsDocument45 pagesModeling Relationships in Scatter PlotsSiddarth Kalyan100% (1)
- Pulp Digester FailuredDocument93 pagesPulp Digester FailuredTim Ku100% (1)
- C70Document3 pagesC70Jorge Luis Arevalo LopezNo ratings yet
- Meter Moving CoilDocument4 pagesMeter Moving Coilabecdf100% (1)
- Book ReviewDocument4 pagesBook ReviewṬhanuama BiateNo ratings yet
- Noon Fees StructureDocument8 pagesNoon Fees StructureNithin SreekumarNo ratings yet
- Analytical Positivism of Glanville Williams and Ludwig WittgensteinDocument9 pagesAnalytical Positivism of Glanville Williams and Ludwig WittgensteinPrabhakaran KarthikeyanNo ratings yet
- Sample Question Paper Class Ix Summative Assessment Ii English Code No. 101 (Communicative)Document15 pagesSample Question Paper Class Ix Summative Assessment Ii English Code No. 101 (Communicative)api-243565143No ratings yet
- Sandvik H8800 Crusher Parts ManualDocument3 pagesSandvik H8800 Crusher Parts ManualTomas Chien0% (1)
- Lake Lanao Policy StudyDocument30 pagesLake Lanao Policy StudyGodfrey MordenoNo ratings yet
- Chartering Terms ExplainedDocument49 pagesChartering Terms Explainedbrett1856No ratings yet
- Automated Home Rainwater Harvesting Earns MoneyDocument4 pagesAutomated Home Rainwater Harvesting Earns MoneysaravananNo ratings yet
- CS410 Series and CS417: User's GuideDocument209 pagesCS410 Series and CS417: User's Guident11No ratings yet
- Apple Environmental Responsibility Report 2014Document29 pagesApple Environmental Responsibility Report 2014fdgdfgdfgNo ratings yet
- Shears Cysts of The Oral and Maxillofacial Regions, 5th Edition (Paul Speight)Document382 pagesShears Cysts of The Oral and Maxillofacial Regions, 5th Edition (Paul Speight)Miriam Tovar OgazonNo ratings yet
- Metrology-Lab-Manual 3 Year 1semDocument41 pagesMetrology-Lab-Manual 3 Year 1semBHARATH Chandra100% (1)
- DMAE Powder Safety Data SheetDocument3 pagesDMAE Powder Safety Data SheetAInhoaNo ratings yet
- Belecobeauty Company ProfileDocument19 pagesBelecobeauty Company ProfileBisma BrawijayaNo ratings yet
- Power Mode Selection SystemDocument3 pagesPower Mode Selection SystemOficina FernandinhoNo ratings yet
- 10 1108 - JPBM 07 2022 4070Document19 pages10 1108 - JPBM 07 2022 4070erikNo ratings yet
- 63-2003 Local Water District Franchise and Income TaxDocument2 pages63-2003 Local Water District Franchise and Income Taxapi-247793055100% (1)
- Pages From 5054 - w15 - QP - 22-6 - Gas PressureDocument1 pagePages From 5054 - w15 - QP - 22-6 - Gas Pressurelelon ongNo ratings yet
- Computer PackagesDocument72 pagesComputer PackagesBildad JoashNo ratings yet
- Artists Budget TemplateDocument9 pagesArtists Budget TemplateMaia CelloNo ratings yet
- BOQ 2 BedroomDocument83 pagesBOQ 2 BedroomOgunfolaji Rasheed100% (1)
- Microbial Contamination Control in The Pharmaceutical IndustryDocument330 pagesMicrobial Contamination Control in The Pharmaceutical IndustryENRIQUE_POMALES683100% (5)