Professional Documents
Culture Documents
10 11648 J Ajpc 20130206 11
Uploaded by
conker4Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
10 11648 J Ajpc 20130206 11
Uploaded by
conker4Copyright:
Available Formats
American Journal of Physical Chemistry
2013; 2(6): 117-121
Published online November 30, 2013 (http://www.sciencepublishinggroup.com/j/ajpc)
doi: 10.11648/j.ajpc.20130206.11
Thermodynamic study of the liquid-liquid equilibrium
water-chloroform-acetic acid
Silio Lima de Moura, Jos Aroldo Viana dos Santos, Francisco Carlos Marques da Silva
Bioelectrochemistry Laboratory, Federal University of Piau, Teresina 64 049 550, Brazil
Email address:
siliosilicio@hotmail.com(S. L. Moura)
Tocite thisarticle:
Silio Lima de Moura, Jos Aroldo Viana dos Santos, Francisco Carlos Marques da Silva. Thermodynamic Study of the Liquid-Liquid
Equilibrium Water-Chloroform-Acetic Acid. American Journal of Physical Chemistry. Vol. 2, No. 6, 2013, pp. 117-121.
doi: 10.11648/j.ajpc.20130206.11
Abstract: Experimental liquid-liquid equilibria of the water-chloroform-acetic acid system were studied at temperature of
298.15 K. Complete phase diagrams were obtained by determining solubility and tie-line data. Data for the construction of
ties-lines were determined by preparing mixtures of known mole fraction of components in the region of formation of two
phases. Separation factors were evaluated for the immiscibility region.
Keywords: Liquid-Liquid Equilibrium, Microstructure, Ternary System
1. Introduction
Recovery of organic acids from dilute solutions resulting
from fermentation processes is important, and many
solvents have been tried in attempts to improve recovery
[1-19].
Pure liquids when mixed in appropriate proportions at
certain temperatures and pressures, do not form only one
homogeneous liquid phase, but two liquid phases with
different compositions. This fact is due to the two-phase
state to be more stable than the monophasic state. If these
phases are in equilibrium, then the phenomenon is called
liquid-liquid equilibrium (LLE) [20].
All systems seek thermodynamic equilibrium. The
thermodynamic stability criterion provides that must be
satisfied by providing that at a constant temperature and
pressure, a steady state is that in which present a minimum
of the Gibbs free energy (Equation 1) [21,22]:
,
(1)
When two or more substances are mixed, dGis defined as
the difference between the Gibbs free energy of the solution
and the pure compounds. If dG 0, forms a stable single
phase solution, but if dG 0, the homogeneous solution is
unstable and the system is forced to split into two or more
phases in order to minimize the Gibbs free energy. This way
or two-phase systems are formed by multiphasic.
System comprising two or more phases, thus a
heterogeneous system is a closed system and each phase
within this system is a open homogeneous system in the
closed total system. Such system, in which no chemical
reaction occurs, will be in equilibrium in relation to the
process of heat transfer, displacement of the border and mass
transfer.
The mechanical and thermal equilibrium, its necessary
that the pressure and temperature within the system are
uniform throughout all phases , to achieve the mechanical
and thermal equilibrium. If i is the chemical potential, it is
also expected to have a uniform value across all phases
composing the heterogeneous system. This was proved by
Gibbs in 1875, and the results for a closed heterogeneous
system in equilibrium without chemical reaction with
respect to the processes mentioned are [21, 22]:
.
.
(2)
(3)
.
.
(4)
where the superscripts represent the phases and the
subscripts represent the components.
The status of each phase of a steady state can be
characterized with n+2 variables: pressure, temperature, and
chemical potential of each of the n components in phase.
However, not all of these variables are independent, however,
the Gibbs-Duhem equation (Equation 5) shows how these
variables are related [22]:
118
Silio Lima de Moura et al.: Thermodynamic Study of the Liquid-Liquid Equilibrium Water-Chloroform-Acetic Acid
(5)
This equation introduces a restriction on simultaneous
variation of T, P and chemical potential i for a single phase.
Thus, the n+2 variables that can be used to characterize a
phase, only n+1 are independent. The limitation introduced
by the equation of Gibbs-Duhem causes one of the variables
is dependent. Therefore, it is said that a phase has n+1
degrees of freedom.
Thus, if each step of the system is in equilibrium, the total
number of independent variables is p(n+1). If the
heterogeneous system as a whole is in equilibrium, then
there are (p-1)(n +2) equilibrium relationships between the
p(n+1) variables given by the equations (2), (3) and (4).
Then the number of degrees of freedom, which is the
number of intensive variables minus the number of relations
or constraints are [22]:
1
2.2. Procedure
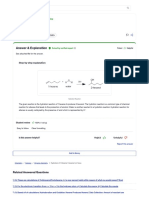
The binodal curve for the water-chloroform-acetic acid
ternary system was determined by the solubility method.
Binary mixtures of known compositions were shaken in a
glass erlenmeyer (Figure 1) equipped with a microburet with
an accuracy of 0.005 cm3. The third component (acetic
acid)was progressively added until the transition point was
reached. The end point was determined by observing the
transition from a heterogeneous to homogenous a mixture
until a permanent homogenous could be observed. Ternary
mixtures of known overall compositions lying within the
two phase region were prepared shaken thoroughly and then
allowed to reach equilibrium. Samples were carefully taken
from each phase and analyzed to obtain the tie lines. An
electronic balance, accurate to 0.1 mg, was used during the
experiments.
(6)
This equation is the Gibbs phase rule.
In this paper, we evaluate acetic acid as an agent for the
extraction of chloroform from dilute aqueous solutions, we
herein report liquid-liquid equilibrium results at
temperatures of 298.15 K for the ternary system of
water-chloroform-acetic acid.
2. Experimental
2.1. Materials
Acetic acid and butyl acetate with purities of 99.98 %
(W/W) and 99.50 % (W/W), respectively, were purchased
from Merck. Acetic acid and butyl acetate were used without
further purification. Deionized water was further distilled
before use. Densities were measured with an Anton
Paardensimeter (model 4500), along with some values from
the literature (Weast, 1990).
Figure1. Scheme of chloroform-water mixtures prepared for the
determination of the phase diagram.
3. Results and Discussion
Table 1 shows the mole fractions of all components
obtained from the experiments for he acetic acid solvent at
298.15 K. All points shown are the results of measurements
obtained in duplicates.
Table 1. Mole fraction of each substance.
Tube
Substance
H2O
CHCl3
H3CCOOH
0.12
0.54
0.37
0.26
0.36
0.39
0.36
0.26
0.37
0.43
0.20
0.36
0.49
0.14
0.37
0.53
0.11
0.36
0.59
0.08
0.34
0.62
0.05
0.32
0.74
0.02
0.24
Figure 2 illustrates the composition of each component of
Table 1. One observation is that there is a decrease of the
molar fractions of chloroform and acetic acid, with the
increase of the molar fraction of water. However, an
important data shows that for the composition
chloroform-water, the acetic acid addition a slight increase
and then immediately decrease more and more, meaning that
the system reaches equilibrium liquid-liquid with greater
ease with lower amount of solvent (acetic acid).The amount
of acetic acid is lower at the beginning in relation the amount
of chloroform, but this phenomenon is reversed immediately,
staying always above the composition of chloroform in the
system.
Figure 3 shows the increasing of chloroform composition
but a much larger statement of the composition of acetic acid,
confirmed Figure 2. This can be explained by the fact that
acetic acid having a different interaction with chloroform,
and this is more effective interaction with water,
remembering that this type of analysis same evaluating the
behavior of the two components in the system there is the
other component water.
American Journal of Physical Chemistry 2013; 2(6): 117-121
119
water and chloroform form microstructures partially
miscible, whereas acetic acid (white balls) forms a
microstructure completely miscible in each of them in any
proportion.
Figure2. Curves of the composition of chloroform and acetic acid vs. water
composition obtained for the system water-chloroform-acetic acid at
298.15 K.
Figure5. Ternary diagram with binodal curve and ties-lines (a, b, c) for the
system water-chloroform-acetic acid at 298.15K.
Figure3. Binodal curve of system chloroform vs. acetic acid (Mole fraction)
obtained for the system water-chloroform-acetic acid at 298.15 K.
Figure4. Liquid-Liquid equilibria (Mole fraction) for system
water-chloroform vs acetic acid at 298.15 K. () solubility (binodal curve)
data for water and () chloroform.
In the ternary diagram of Figure 5, the part that lies inside
the solubility curve is the region where two phases in
equilibrium has a microstructure rich in water (blue balls)
but also contains minor amounts of microstructures in other
dissolved components and other microstructure rich in
chloroform (green balls) microstructures which also
contains small amounts of other dissolved. This is because
The overall composition of the system with the addition
of acetic acid is shown in Figure 4. This graph depicts in a
partial diagram of the ternary system, because it can identify
nearby where the mole fraction of acetic acid tends to zero,
the composition of the other components (water and
chloroform) are distant or nearly pure as well as in vertices
of the ternary diagram.
Above the solubility line has become a single phase
region, where the three components form a microstructure
completely miscible. The point c'' is defined as the critical
point, ie, where the two curve segments finds itself. At this
point formed two liquid phases with the same composition
and density.
The points a and b represent the combined liquid layers in
the absence of acetic acid. The total composition of the
system is c, so that the lever rule, there is a greater amount
than the layer b of the layer. Adding a small amount of acetic
acid to the system, the composition varies along the line that
joins the vertex c corresponds to acetic acid, the new
composition is represented by c'.The addition of acetic acid
alters the composition of the two layers for the data values a'
and b'. Note that the acetic acid will preferably for the layer
b' rich in water, so that the correlation line between the two
solutions combined a' and b' does not remain parallel to
ab.The relative amounts of a' and b' are given by the lever
rule, that is, the ratio of the correlation line segments a'b',
this segment is referred ties-line or mooring line. With
addition of more acid, the composition varies along the
broken line cC; layer rich in water increases in quantity,
while the other rich in chloroform decreases.
As the correlation lines are not parallel , the point at which
the two solutions combined have the same composition is
not located on top of the binodal curve but is on one side at
the point c'', called lattice point. If the system have the
120
Silio Lima de Moura et al.: Thermodynamic Study of the Liquid-Liquid Equilibrium Water-Chloroform-Acetic Acid
composition initial C and will add acetic acid, the
composition will vary over cc'', immediately below of c'' the
two layers are present in comparable amounts; in c'' the
separation surface between the two layers disappears such as
the solution becomes homogeneous. If we increase the
temperature, the shape and size of the two-phase region will
change.
4. Conclusion
The study ternary system, water-chloroform-acetic acid at
temperature of 298.15 K, may be characterized by an
increase in solubility of a solvent in which was obtained a
microstructure of liquid-liquid equilibrium. In the study of
these microstructures, it can be concluded that the
performance of acetic acid specifically in the binodal curve
presented points of solubilizing effects which according to
the theory of Gibbs are thermodynamically favorable. In the
extreme case of ternary diagram, the substances are arranged
in a microstructure almost pure, that is, without the
formation of a microstructure with another liquid system.
These studies on the microstructures formed in liquid-liquid
equilibrium are important because through these, we can
obtained separation methods increasingly effective.
References
[1]
Upchurch, J.C. and Van Winkle, M., Liquid-Liquid
EquilibriaHeptadecanol-Water-Acetic
Acid
and
Heptadecanol-Water-Ethanol, Industrial and Engineering
Chemistry, 44, 618, 1952.
[2]
Correa, J.M., Blanco, A. and Arce, A., Liquid-Liquid
Equilibria of the System Water+AceticAcid+Methyl
Isopropyl Ketone Between 25 and 55 C, J. Chem. Eng. Data,
34, 4, 415, 1989.
[3]
Sayar, A.A., Tatl, B. and Dramur, U., Liquid-Liquid
Equilibria of the System Water +Acetic Acid+Cyclohexyl
Acetate Ternary, J. Chem. Eng. Data, 36, 378, 1991.
[4]
Kirk, R.E. and Othmer, D.F., Encyclopaedia of Chemical
Technology, 4th ed., Wiley-Interscience, Inc. New York, 1,
121, 1992.
[5]
Dramur, U. and Tatl, B., Liquid-Liquid Equilibria of
Water+AceticAcid+Phatalic Esters (Dimethyl Phatalate and
Diethyl Phatalate) Ternaries, J. Chem. Eng. Data, 38, 1, 23,
1993.
[6]
Briones, J.A., Mullins, J.C. and Thies, M.C., Liquid-Liquid
Equilibria for the Oleic Acid--Sitosterol-Water System at
Elevated Temperatures and Pressures. Ind. Eng. Chem. Res.,
33, 151, 1994.
[7]
Arce, A., Blanco, A., Souza, P. and Vidal, I., Liquid-Liquid
Equilibria of the Ternary Mixtures Water + Propionic Acid +
Methyl Ethyl Ketone and Water + Propionic Acid + Methyl
Propyl Ketone, J. Chem. Eng. Data, 40, 225, 1995.
[8]
Fahim, M.A., Al-Muhtaseb, S.A. and Alnashef, I.M.,
Liquid-Liquid Equilibria of the Ternary System
Water+Acetic Acid+1-Pentanol, J. Chem. Eng. Data, 41, 3,
562, 1996.
[9]
Fahim, M.A., Al-Muhtaseb, S.A. and Alnashef, I.M.,
Liquid-Liquid Equilibria of the Ternary System
Water+AceticAcid+Hexanol, J. Chem. Eng. Data, 42, 3, 183,
1997.
[10] Slimo, H.N., Bonatti, C.M., Zurita, J.L. and de Doz, M.B.G.,
Liquid-Liquid Equlibria for the System Water+Propionic
Acid+1-Butanol at 303.2 K. Effect of Addition of Sodium
Chloride, Fluid Phase Equilibria, 137, 1, 163, 1997.
[11] Weast, R.C., Handbook of Chemistry and Physics, 70th ed.,
CRC Press: Boca, Raton, Florida, 1990.
[12] Colombo, A., Battilana, P., Ragaini, V. and Bianchi, C.L.,
Liquid-Liquid Equilibria of the Ternary Systems
Water+AceticAcid+Ethyl
Acetate
and
Water+AceticAcid+Isophorone
(3,3,5-Trimethyl-cyclohexen-1one), J. Chem. Eng. Data, 44,
1, 35, 1999.
[13] Aljimaz, A.S., Fandary, M.S.H., Alkandary, J.A. and Fahim,
M.A., Liquid-Liquid Equilibria of the Ternary System
Water+Acetic Acid+1-Heptanol, J. Chem. Eng. Data, 45, 2,
301, 2000.
[14] Taghikhani, V., Vakili-Nezhaad, G.R., Khoshkbarchi, M.K.
and Shariaty-Niassar, M., Liquid-Liquid Equlibria of
Water+PropionicAcid+Metyl Butyl Ketone and of Water+
Propionic Acid+Metyl Isopropyl Ketone, J. Chem. Eng. Data,
46, 1107, 2001.
[15] Yousefi,L., RoayaeiE., TaghikhaniV., A. Safekordi,
ZahedzadehM. Experimental study and modelling of
saturation molality of NaCl in quaternary aqueous electrolyte
solutions at various temperatures. Desalination, 267, 3,
228-232, 2011.
[16] HashemiSh., GhotbiC., TaghikhaniV.,BehzadiB. Application
of quasi-chemical models to liquidliquid equilibrium
calculations for ternary systems containing water, propionic
acid and organic solvents. Fluid Phase Equilibria, 226,
251-259, 2004.
[17] ShahriariSh., TaghikhaniV., VossoughiM., Safe kordiA.A.,
AlemzadehI., PazukiG. R. Measurement of partition
coefficients of -amylase and amyloglucosidase enzymes in
aqueous two-phase systems containing poly(ethylene glycol)
and Na2SO4/KH2PO4 at different temperatures. Fluid Phase
Equilibria, 292, 2, 80-86, 2010.
[18] SafaviM., GhotbiC., TaghikhaniV., JaliliA. H., MehdizadehA.
Study of the solubility of CO2, H2S and their mixture in the
ionic
liquid1-octyl-3-methylimidazolium
hexafluorophosphate: Experimental and modelling. The
Journal of Chemical Thermodynamics, 65, 220-232, 2013.
[19] JaliliA. H., MehdizadehA., ShokouhiM., SakhaeiniaH.,
TaghikhaniV.Solubility
of
CO2
in
1-(2-hydroxyethyl)-3-methylimidazolium ionic liquids with
different anions. The Journal of Chemical Thermodynamics,
42, 6, 787-791, 2010.
[20] Smith, J.M., Van Ness, H. C.,Abbott, M.M. Introduo
Termodinmica da Engenharia Qumica. 5 ed. Rio de Janeiro:
LTC, 2000.
[21] Atkins, P.W. Fsico-Qumica Fundamentos. 3 ed. Rio de
Janeiro: LTC, 2003.
American Journal of Physical Chemistry 2013; 2(6): 117-121
121
[22] Castellan, G. W. Fundamentos de Fsico-Qumica. 1 ed. Rio
de Janeiro: LTC, 2008.
[24] Treybal, R. E.
McGraw-Hill,1951.
Liquid
Extraction.
New
York:
[23] Senol, A., Liquid-liquid equilibria for the system (water +
carboxylic acid + chloroform): Thermodynamic modeling.
Fluid Phase Equilibria, 243, p. 51-56, 2006.
[25] Treybal, R. E. Extraccionen Fase Liquida. Mxico:
McGraw-Hill,1968.
You might also like
- Pal 2005Document5 pagesPal 2005conker4No ratings yet
- American Association For The Advancement of Science ScienceDocument6 pagesAmerican Association For The Advancement of Science Scienceconker4No ratings yet
- Lesson15 9 PDFDocument5 pagesLesson15 9 PDFconker4No ratings yet
- Introduccion Esta ChidaDocument12 pagesIntroduccion Esta Chidaconker4No ratings yet
- Chemical Reactions ChernobylDocument5 pagesChemical Reactions Chernobylconker4No ratings yet
- Saiki A 2010Document2 pagesSaiki A 2010conker4No ratings yet
- Colbert2006 PDFDocument13 pagesColbert2006 PDFconker4No ratings yet
- Oishi 2001Document4 pagesOishi 2001conker4100% (1)
- ADocument3 pagesAconker4No ratings yet
- Dittmer 2001Document2 pagesDittmer 2001conker4No ratings yet
- Patel 2001Document2 pagesPatel 2001conker4No ratings yet
- Gane San 2003Document3 pagesGane San 2003conker4No ratings yet
- Reductive Amination With Sodium TriacetoxyborohydrideReductive Amination With Sodium TriacetoxyborohydrideDocument14 pagesReductive Amination With Sodium TriacetoxyborohydrideReductive Amination With Sodium Triacetoxyborohydrideiramshagufta100% (1)
- MC Garvey 2001Document4 pagesMC Garvey 2001conker4No ratings yet
- Zinc Carbonate: Claisen RearrangementsDocument1 pageZinc Carbonate: Claisen Rearrangementsconker4No ratings yet
- Synthesis of 2C-B From Anise OilDocument1 pageSynthesis of 2C-B From Anise OilKrazYNinjA2010100% (2)
- Psychedelic ChemistryDocument79 pagesPsychedelic ChemistryMuzakfiles100% (4)
- Brahmadeo Dewprashad - The Chemistry of CocaineDocument8 pagesBrahmadeo Dewprashad - The Chemistry of CocainePoloGreenNo ratings yet
- 1135396612Document49 pages1135396612trunNo ratings yet
- 0910 0273Document8 pages0910 0273conker4No ratings yet
- Brahmadeo Dewprashad - The Chemistry of CocaineDocument8 pagesBrahmadeo Dewprashad - The Chemistry of CocainePoloGreenNo ratings yet
- Acs Joc 6b00647Document8 pagesAcs Joc 6b00647conker4No ratings yet
- Scerri Trouble PT EiC January2012 Tcm18-212413Document5 pagesScerri Trouble PT EiC January2012 Tcm18-212413conker4No ratings yet
- Small-Molecule Organic SemiconductorsDocument8 pagesSmall-Molecule Organic Semiconductorsconker4No ratings yet
- 4Document6 pages4conker4No ratings yet
- Organic & Biomolecular Chemistry Volume 4 Issue 12 2006 (Doi 10.1039/b602413k) Carey, John S. Laffan, David Thomson, Colin Williams, Mike T. - Analysis of The Reactions Used For The PreparationDocument11 pagesOrganic & Biomolecular Chemistry Volume 4 Issue 12 2006 (Doi 10.1039/b602413k) Carey, John S. Laffan, David Thomson, Colin Williams, Mike T. - Analysis of The Reactions Used For The Preparationconker4No ratings yet
- Je 990257 GDocument4 pagesJe 990257 Gconker4No ratings yet
- 1 s2.0 S0040403901011856 MainDocument4 pages1 s2.0 S0040403901011856 Mainconker4No ratings yet
- Furylacrylic AcidDocument4 pagesFurylacrylic Acidconker4No ratings yet
- 03a-GP Organomet CatDocument40 pages03a-GP Organomet Catconker4No ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Midterm Exam Schedule-Summer 2022 Weekdays and WeekendDocument14 pagesMidterm Exam Schedule-Summer 2022 Weekdays and Weekendmansoor malikNo ratings yet
- Rapid Prototyping PPT SeminarDocument32 pagesRapid Prototyping PPT SeminarShantha Kumar G C0% (1)
- Fiber Series CTCDocument31 pagesFiber Series CTCJorge GaitanNo ratings yet
- Case Analysis SampleDocument22 pagesCase Analysis SampleMicaela EncinasNo ratings yet
- DSP Lab 6Document7 pagesDSP Lab 6Ali MohsinNo ratings yet
- Discovery of A New Energy VortexDocument4 pagesDiscovery of A New Energy Vortexnblack3335140No ratings yet
- PPT11 12 Ic 2.3 PPT1112 Id 2.4 Realized That The Methods of Philosophy LeadDocument47 pagesPPT11 12 Ic 2.3 PPT1112 Id 2.4 Realized That The Methods of Philosophy LeadShayne Pagwagan100% (1)
- MX60 Manual Rev6 2Document48 pagesMX60 Manual Rev6 2wgenNo ratings yet
- Itp582b-515-01-Ib 2Document12 pagesItp582b-515-01-Ib 2Cara & Drei Amazing JourneyNo ratings yet
- VPLS Practice GuideDocument7 pagesVPLS Practice Guidedelgado08No ratings yet
- Comparative Analysis of Public and Private Educational Institutions: A Case Study of District Vehari-PakistanDocument10 pagesComparative Analysis of Public and Private Educational Institutions: A Case Study of District Vehari-PakistannithyaNo ratings yet
- Magnetic Resonant Coupling Based Wireless PowerDocument7 pagesMagnetic Resonant Coupling Based Wireless PowerHartantoNo ratings yet
- W 9540Document6 pagesW 9540imharveNo ratings yet
- (Solved) Hydration of 1-Hexene 1-Hexene To 2 Hexanol Equation Write The Equation of The Reaction - Course HeroDocument2 pages(Solved) Hydration of 1-Hexene 1-Hexene To 2 Hexanol Equation Write The Equation of The Reaction - Course HeroJdkrkejNo ratings yet
- Homework - Cardinal NumbersDocument2 pagesHomework - Cardinal NumbersMAELSASANo ratings yet
- Final Lab Report-20bci7108Document22 pagesFinal Lab Report-20bci7108rupa sreeNo ratings yet
- Catalog Produk Arduino Rajacell Ver10.4 - End - UserDocument192 pagesCatalog Produk Arduino Rajacell Ver10.4 - End - UserSanto SetiawanNo ratings yet
- Schwarzer Schmarsow - The Emergence of Architectural Space August Schmarsows Theory of RaumgestaltungDocument15 pagesSchwarzer Schmarsow - The Emergence of Architectural Space August Schmarsows Theory of RaumgestaltungDimitra BilliaNo ratings yet
- LG FlatRon RepairDocument55 pagesLG FlatRon Repairdany89roNo ratings yet
- Adverb. Pronoun. Numeral: Historical DevelopmentDocument22 pagesAdverb. Pronoun. Numeral: Historical DevelopmentКристина ГураNo ratings yet
- Neil - Bernardo@eee - Upd.edu - PH Bernalyn - Decena@eee - Upd.edu - PH Ephraim - Lizardo@eee - Upd.edu - PHDocument3 pagesNeil - Bernardo@eee - Upd.edu - PH Bernalyn - Decena@eee - Upd.edu - PH Ephraim - Lizardo@eee - Upd.edu - PHbdec95No ratings yet
- Unit 3 Study Guide and ExercisesDocument2 pagesUnit 3 Study Guide and ExercisesTuan NguyenNo ratings yet
- Maintenance of 132 KV SwitchgearDocument34 pagesMaintenance of 132 KV SwitchgearMuhammad Abdul Rauf100% (1)
- Strategik Sains THN 4Document9 pagesStrategik Sains THN 4fletchertchrNo ratings yet
- Polymer-Plastics Technology and EngineeringDocument6 pagesPolymer-Plastics Technology and Engineeringsamuelben87No ratings yet
- 5.4 Hypergeometric DistributionDocument5 pages5.4 Hypergeometric DistributionFahad IqbalNo ratings yet
- MAT3700-MayJune ExamDocument3 pagesMAT3700-MayJune ExamNhlanhla NdebeleNo ratings yet
- 42re TechDocument7 pages42re Techmontrosepatriot100% (2)
- REvision Test - 1Document2 pagesREvision Test - 1JagendraNo ratings yet