Professional Documents
Culture Documents
JF 403722 Q
Uploaded by
conker4Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
JF 403722 Q
Uploaded by
conker4Copyright:
Available Formats
Article
pubs.acs.org/JAFC
Preparation and Physicochemical Characteristics of Polylactide
Microspheres of Emamectin Benzoate by Modied Solvent
Evaporation/Extraction Method
Shao Fei Zhang, Peng Hao Chen, Fei Zhang, Yan Fang Yang, De Kun Liu, and Gang Wu*
Key Laboratory of Biopesticide and Chemical Biology (Ministry of Education), Fujian Agriculture and Forestry University, Fuzhou,
China 350002
S Supporting Information
*
ABSTRACT: Emamectin benzoate is highly eective against insect pests and widely used in the world. However, its biological
activity is limited because of high resistance of target insects and rapid degradation speed in elds. Preparation and
physicochemical characterization of degradable microcapsules of emamectin benzoate were studied by modied solvent
evaporation/extraction method using polylactide (PLA) as wall material. The inuence of dierent compositions of the solvent in
internal organic phase and external aqueous phase on diameter, span, pesticide loading, and entrapment rate of the microspheres
was investigated. The results indicated that the process of solvent extraction and the formation of the microcapsules would be
accelerated by adding water-miscible organic solvents such as ethyl ether, acetone, ethyl acetate, or n-butanol into internal organic
phase and external aqueous phase. Accelerated formation of the microcapsules would result in entrapment rates of emamectin
benzoate increased to as high as 97%. In addition, by adding ethanol into the external aqueous phase, diameters would reduce to
6.28 m, whereas the loading eciency of emamectin benzoate did not increase. The PLA microspheres prepared under
optimum conditions were smoother and more spherical. The degradation rate in PLA microspheres of emamectin benzoate on
the 10th day was 4.29 0.74%, whereas the degradation rates of emamectin benzoate in methanol solution and solid technical
material were 46.3 2.11 and 22.7 1.51%, respectively. The PLA skeleton had combined with emamectin benzoate in an
amorphous or molecular state by using dierential scanning calorimetry (DSC) determination. The results indicated that PLA
microspheres of emamectin benzoate with high entrapment rate, loading eciency, and physicochemical characteristics could be
obtained by adding water-miscible organic solvents into the internal organic phase and external aqueous phase.
KEYWORDS: solvent evaporation/extraction method, emamectin benzoate, polylactide microspheres, physicochemical characteristics,
dierential scanning calorimetry
greatly in elds.4 The problems could be solved by application
of a microencapsulation formulation of emamectin benzoate.
Microencapsulation of pesticides is a method used to obtain
products with controlled release properties, and it is a process
that allows an active product to be isolated from the external
medium by forming microspheres or microcapsules.5 Biodegradable microsphere-based controlled release systems have
been widely studied for pesticides and drug-delivery devices.69
A wide range of pesticides have been processed into
microspheres to prolong their eectiveness using a variety of
materials such as ethyl cellulose, lignin, and chitosan.1014
Biodegradable poly(D,L-lactic acid) (PLA) and poly(D,L-lacticco-glycolic acid) (PLGA) were the most useful among the
microparticles and implants, which promised platforms to
provide long-acting systemic.9,15
There were many methods to prepare microspheres of
pesticides including emulsion solvent evaporation, spray-drying,
and phase separation. Emulsion solvent evaporation is the most
widely investigated with the advantages that it required neither
INTRODUCTION
Insect pests are the main limiting factor for vegetable
production in tropical Asia. Farmers often use synthetic
insecticides indiscriminately, and heavy insect resistance to
insecticides is very common in the elds. To control pests
eciently, some new pesticides, with novel modes of action,
have been developed recently. Among them, emamectin
benzoate is a novel insecticide deriving from the natural
product avermectin family with improved thermal stability,
greater water solubility, and a broader spectrum of insecticidal
activity than those of avermectin.1 Emamectin benzoate, with
novel modes of action similar to that of avermectin (GABAand glutamate-gated chloride channel agonist), high selectivity,
and greater safety to nontarget organisms, is highly eective
against lepidopteran pests and widely used in the world. It is
1880400-fold more potent against diamondback moth,
Plutella xylostella, cabbage looper, Trichoplusia ni (Hubner),
and beet armyworm, Spodoptera exigua (Hubner), than other
traditional insecticides, such as pronil, chlorfenapyr, and
tebufenozide.2 However, signicant resistance to emamectin
benzoate in target pests was found because of long-term heavy
usage of this insecticide.3 On the other hand, emamectin
benzoate was sensitive to light and had a rapid degradation rate
in natural conditions so that its biological activity was limited
2013 American Chemical Society
Received:
Revised:
Accepted:
Published:
12219
August 30, 2013
November 25, 2013
November 27, 2013
November 27, 2013
dx.doi.org/10.1021/jf403722q | J. Agric. Food Chem. 2013, 61, 1221912225
Journal of Agricultural and Food Chemistry
Article
on to obtain PLA microspheres of emamectin benzoate with
high entrapment rate and smooth and rounded surface by using
the modied solvent evaporation/extraction method in this
study.
elevated temperatures nor phase separation inducing
agent.1618 In the application process of the traditional
emulsion solvent evaporation method, the pesticide and the
polymer were dissolved into an organic solvent to form the
organic phase. Then the organic phase was dispersed into a
large volume of aqueous phase in the presence of an emulsier.
Then organic solvent was removed by evaporation, and solid
microspheres were formed with the pesticide encapsulated.19,20
In our previous study, PLA microencapsulation of spinosad
and chlorpyrifos was prepared by using an emulsion solvent
evaporation method, respectively,21,22 and chlorpyrifos microencapsulated by PLA had a slower degradation rate than that in
traditional pesticide formulations in natural condition.23 The
microencapsulation of emamectin benzoate should be studied
because of its high toxicity and wide use and its high
degradation rate in the eld. However, on the basis of our
previous study, the entrapment rate of emamectin benzoate was
lower when the traditional emulsion solvent evaporation
method was used because emamectin benzoate was slightly
soluble in water and easily diused into the external aqueous
phase. In addition, microspheres prepared from the traditional
solvent evaporation method usually had a rough surface as
emamectin benzoate diused into the external aqueous phase
was adsorbed in the surface of the microspheres.17 Therefore,
how to prevent emamectin benzoate from diusing into the
external aqueous solution became an urgent problem. To solve
the problems, modied emulsion solvent evaporation/extraction method was used to prepare PLA microspheres containing
emamectin benzoate.
Dierent from the traditional emulsion solvent evaporation
method, in a modied emulsion solvent evaporation/extraction
method, the water-miscible organic solvent such as acetone,
methanol, or ethanol was added into the organic phase as
extracting solvent, and the mixed organic solvent could be
extracted more quickly into the external aqueous phase after the
formation of organic liquid droplets. Therefore, the mixed
organic solvent containing water-miscible organic solvent
would accelerate the formation of microspheres.24,25 Quick
formation of microspheres would result in an increase of the
entrapment rate of pesticide because the loss of pesticide
mainly occurred in a short time before the formation of
microspheres.26 Furthermore, the adsorption of pesticide on
the surface of microspheres could be decreased by quick
formation of microspheres at the same time. It was suggested
that microspheres with a smooth and rounded surface could be
obtained by using the solvent evaporation/extraction method.27
To study the optimized conditions for preparation of
emamectin benzoate microspheres, some factors inuencing
the characteristics of microspheres were optimized in our
previous work (see the Supporting Information). However, the
entrapment rate of emamectin benzoate microspheres was
<80%. Because emamectin benzoate was slightly soluble in
water, emamectin benzoate was easily diused into the external
aqueous solution, which resulted in the decline of the
entrapment rate. In this study, we changed the composition
of internal organic phase and external aqueous phase to
accelerate the formation process of microspheres. The diameter
of microspheres would change not only with the composition
of the internal organic phase or external aqueous phase but also
with the emulsifying speed. The inuence of the composition of
the internal organic phase or external aqueous phase
emulsifying speed and that of the emulsifying speed on the
physicochemical characteristics of microspheres were focused
MATERIALS AND METHODS
Materials. The chemicals were provided by the manufacturers:
technical grade emamectin benzoate (95.8% pure) from Jiamusi
Xingyu Biotechnique Development Co., Ltd., Heilongjiang, China;
polylactide (PLA) (technical grade, Mw = 80000) from Esun Industrial
Co., Ltd., Shenzhen, China; gelatin (chemically pure) from Sinopharm
Chemical Reagent Limited Corp., Co., Ltd., Beijing, China. Methanol
and acetonitrile used for high-performance liquid chromatography
(HPLC) were of chromatograpy grade. Others were of analytical
reagent grade.
Preparation of Microspheres. Briey, 0.3132 g of emamectin
benzoate and 0.6 g of PLA were dissolved in 10 mL of
dichloromethane or the mixed organic solvents of dichloromethane
and one other organic solvent (ethyl ether, acetone, ethyl acetate, or nbutanol) in an icewater bath. In this study, dichloromethane (or the
mixed organic solvents) containing emamectin benzoate and PLA was
hereafter dened as the internal organic phase. The blends of 0.3132 g
of emamectin benzoate, 0.6 g of PLA, and 10 mL of organic solvent(s)
were emulsied in a previously prepared aqueous solution of gelatin
(250 mL) at 7000 rpm for 30 s to form an oil/water (O/W) emulsion
using a high-speed disperser. The aqueous solution of gelatin 1.5% (w/
v) was hereafter dened as the external aqueous phase. The 250 mL
solution of O/W emulsion was immediately added to 1000 mL of
distilled water solution after 0.3 mL of organic silicon defoamer was
added into the O/W emulsion. The organic solvent components in the
water solution of the O/W emulsion were extracted and evaporated by
stirring the solution overnight at 700 rpm and 30 C, and the solid
microspheres were formed gradually. After extraction and evaporation
of organic solvent components for 4 h, the solid microspheres were
collected by centrifugation and drying. In addition, blank PLA
microspheres were prepared using the same method without the
addition of emamectin benzoate.
Eects of Dierent Factors on the Characteristics of
Microspheres. A single-factor exploration method was used to
study the eects of dierent factors (including composition of internal
organic solvents and external aqueous phase) on the diameter, span,
pesticide loading, and entrapment rate of microspheres. The eects of
dierent kinds and contents of extracting solvent in the internal
organic phase or in the external aqueous phase on the characteristics of
microspheres were studied, respectively. For instance, dierent kinds
of water-miscible organic solvent such as ethyl ether, acetone, ethyl
acetate, and n-butanol in the internal organic phase, dierent contents
of extracting solvent such as 25, 37.5, 50, and 62.5% acetone in the
internal organic phase, and dierent contents of extracting solvent
such as 0, 0.4,0.8, 1.2, and 1.6% ethanol in the external aqueous phase
were used. In addition, the eects of dierent emulsifying speeds
(6000, 7000, 8000, and 9000 rpm) were studied.
Microsphere Characteristics. Morphology of Microspheres. In
the preparation process of microspheres, the morphology of
microspheres was observed by light microscope. Dry microsphere
samples were sputter-coated with gold by using a JFC-1200 Sputter
Coater 9 (Japan Electron), and the morphology of microspheres was
analyzed by scanning electron microscopy (SEM) (JSM-5310LV,
Japan Electron).
Entrapment Rate and Pesticide Loading. The entrapment rate was
dened as the ratio of actual-to-theoretical pesticide content. The
pesticide loading was dened as the actual content of emamectin
benzoate in microspheres. The 0.02 g microsphere solid was dissolved
with 2 mL of dichloromethane. After dichloromethane had evaporated
completely, the sample was washed by using 1.5 mL of methanol
under ultrasonic condition ve times, and the methanol solution was
collected and diluted to 10 mL. Emamectin benzoate concentration in
the methanol solution was determined by HPLC using an Amemyst
c18-H column at 25 C. The mobile phase was composed of
12220
dx.doi.org/10.1021/jf403722q | J. Agric. Food Chem. 2013, 61, 1221912225
Journal of Agricultural and Food Chemistry
Article
Table 1. Characteristic of Microspheres of Dierent Conditions
emulsifying speed
(rpm)
external aqueous phase
6000
7000
8000
9000
7000
7000
7000
7000
7000
7000
1.5%
1.5%
1.5%
1.5%
1.5%
1.5%
1.5%
1.5%
1.5%
1.5%
gelatin
gelatin
gelatin
gelatin
gelatin
gelatin
gelatin
gelatin
gelatin
gelatin
7000
1.5% gelatin
7000
7000
7000
7000
1.5% gelatin + 0.4% EtOH
1.5% gelatin + 0.8% EtOH
1.5% gelatin + 1.2% EtOH
1.5% gelatin + 1.6% EtOH
internal organic phase
diameter (m)
DCM
DCM
DCM
DCM
DCM/ether (1:1)
DCM/n-butanol (1:1)
DCM/acetic ether(1:1)
DCM/acetone(1:1)
DCM/acetone (1.5:0.5)
DCM/acetone
(1.25:0.75)
DCM/acetone
(0.75:1.25)
DCM
DCM
DCM
DCM
14.4
11.6
9.02
6.02
17.2
9.77
13.4
16.5
12.2
14.8
1.09
0.51
0.59
0.57
0.64
0.98
1.10
1.41
0.74
0.44
fg
e
cd
a
i
d
f
hi
e
fg
15.4 0.47 gh
10.2
9.04
7.99
6.82
0.38
0.36
0.36
0.36
d
cd
bc
ab
pesticide loading
(%)
span
1.50
1.45
1.44
1.40
1.47
1.55
1.53
1.49
1.24
1.44
0.04
0.03
0.03
0.04
0.05
0.03
0.08
0.04
0.09
0.05
cd
cd
cd
bc
cd
d
d
cd
a
bcd
1.72 0.04 e
1.40
1.33
1.42
1.43
0.02
0.02
0.03
0.03
bc
b
bcd
bcd
26.0
24.4
22.4
19.7
24.2
23.5
28.0
31.8
25.9
29.4
0.34
0.78
0.58
0.36
0.82
0.90
1.00
0.73
0.75
1.16
fh
ef
de
c
ef
ed
ig
k
fh
g
27.7 0.73 hi
23.2
21.4
17.1
13.2
0.44
0.44
1.25
0.70
def
d
b
a
entrapment rate
(%)
79.2
74.3
68.2
60.1
73.8
71.6
85.3
97.0
79.0
89.4
1.02
2.37
1.76
1.11
2.50
2.75
3.05
2.22
2.30
3.54
fh
ef
de
c
ef
ed
ig
k
fh
g
84.3 2.22 hi
70.5
65.1
52.1
40.3
1.34
1.34
3.82
2.12
def
d
b
a
DCM, dichloromethane; EtOH, ethanol. Dierent lower case letters indicate signicant dierence (P < 0.05).
Figure 1. Eects of emulsifying speed on the quality of microcapsules.
Figure 2. Eects of internal organic phase on the quality of microcapsules. In control, dichloromethane alone was used. In other treatments, the
dichloromethane was mixed with ether, n-butynol, ethyl acetate, or acetone at the ratio of 1:1, respectively.
methanol/acetonitrile/triethylamine solution (0.1%, 50:40:10), with a
ow rate of 1.0 mL/min. The detection wavelength was 245 nm, and
the injection amount was 20 L. From the concentration, the
entrapment rate and content of emamectin benzoate in the prepared
microspheres were calculated.
Mean Diameter and Span. Amounts of 0.25 g of microspheres and
0.2 g of sodium phosphate were dissolved with 30 mL of distilled
water under ultrasonic condition. The mean diameter was measured
using an LS-POP(7) laser particle size analyzer (OMEC Instrument
Co., Ltd., Zhuhai, China) after high-speed sample introduction. Span =
(D90 D10)/D50. D50 represents the mean diameter of microspheres,
D90 represents the diameter of 90% of the microspheres, and D10
represents the diameter of 10% of the microspheres.
Dierential Scanning Calorimetry (DSC). Ten milligrams of four
samples, including emamectin benzoate, PLA microspheres of
emamectin benzoate, a physical mixture of emamectin benzoate and
blank PLA microspheres, and blank PLA microspheres without
emamectin benzoate, was used for analysis by DSC. Thermal analyses
were performed using a DSC instrument (Netzsce, Co., Ltd.,
Germany) with a heating rate of 5 C/min and the atmosphere was air.
Photolysis Stability. The samples, that is, emamectin benzoate
microsphere solid (0.03g) and the mixture of blank PLA microspheres
and emamectin benzoate (0.03g), were weighed and put in 10 mL
small beakers, respectively. In addition, emamectin benzoate technical
(0.03g) was mixed with 10 mL of methanol in a 10 mL colorless
volumetric ask. The three samples were sealed and kept under natural
sunlight condition. The degradation rate of the three samples, that is,
emamectin benzoate microspheres, mixture of blank PLA microspheres and emamectin benzoate, and emamectin benzoate technical,
was determined, respectively, by HPLC after the samples were kept
under natural sunlight condition for 0, 1, 4, 7, or 10 days. At least three
replicates were performed for each assay.
RESULTS
Eects of Emulsifying Speed on Microsphere Characteristics. The mean diameter and the entrapment rate
12221
dx.doi.org/10.1021/jf403722q | J. Agric. Food Chem. 2013, 61, 1221912225
Journal of Agricultural and Food Chemistry
Article
Figure 3. Scanning electron micrographs of microspheres prepared using dierent internal organic phases: (A, C) internal organic phase was the
mixture of dichloromethane with acetone (1:1); (B, D) internal organic phase was dichloromethane alone.
Figure 4. Eects of acetone content in internal organic phase on the quality of microcapsules.
reduced from 14.4 to 6.02 m and from 79.2 to 60.1%,
respectively, when the emulsifying speed was increased from
6000 to 9000 rpm. In addition, the span of microspheres
showed a decreased tendency with the increase of emulsifying
speed (Table 1; Figure 1).
Inuences of Internal Organic Phase on Microsphere
Characteristics. Internal Organic Phase Species. Several
kinds of organic solvents with greater polarity than dichloromethane were selected as extraction solvent to be mixed with
dichloromethane at a certain proportion. To improve the
diusion speed of organic solvents to the external water phase,
accelerate the formation process of microspheres, and improve
the entrapment rate nally, solvents of n-butyl alcohol, acetone,
ethyl acetate, and ether with dierent polarities were used.
Among the extraction solvents, n-butyl had the strongest
polarity and ether had the weakest.
Microspheres with a smooth and round surface could be
obtained after mixing dichloromethane with the extraction
solvents. The mean diameter of microspheres prepared from
dierent solvents decreased from diethyl ether, acetone, ethyl
acetate, and n-butyl alcohol in turn, whereas the span of
microspheres displayed an opposite tendency (Table 1; Figure
2). The particle size of microspheres increased when the
microspheres were prepared from the mixtures of dichloromethane with ether, acetone, or ethyl acetate, respectively, as
compared to the microspheres prepared from dichloromethane
alone. However, the entrapment rate of microspheres increased
signicantly, and the highest entrapment rate could be found
when acetone was used as organic solvent composition (Table
1; Figure 2). The diameter of microspheres increased but
entrapment rate reduced when ether was used as organic
solvent composition (Table 1; Figure 2). However, when nbutyl alcohol was used as organic solvent composition, the
12222
dx.doi.org/10.1021/jf403722q | J. Agric. Food Chem. 2013, 61, 1221912225
Journal of Agricultural and Food Chemistry
Article
Figure 5. Eects of ethyl alcohol content in external aqueous phase on the quality of microcapsules.
Figure 6. DSC spectrogram diagram (left) and thermal weight loss (right): a, emamectin benzoate; b, PLA microspheres of emamectin benzoate; c,
physical mixture of emamectin benzoate and blank PLA microspheres; d, blank PLA microspheres without emamectin benzoate.
polymeric microspheres.28,29 As shown in Figure 6, the melting
peaks of emamectin benzoate and blank PLA microspheres
appeared at 210 C (thermogram a) and 370 C (thermogram
d), respectively. The result of thermal weight loss indicated that
emamectin benzoate and blank PLA microspheres lost the most
weight at 180220 C (thermogram a) and 340360 C
(thermogram d), respectively, corresponding to the DSC
results. When emamectin benzoate and blank PLA microspheres were mixed as a physical mixture (thermogram c), the
melting peak appeared at both 210 and 370 C approximately.
However, after emamectin benzoate was encapsulated in PLA
microspheres (thermogram b), its melting peak appeared at 360
C and the melting peak of emamectin benzoate at 210 C
disappeared.
Photolysis Stability. Among the three samples, that is,
emamectin benzoate in PLA microspheres solid, the mixture of
blank PLA microspheres and emamectin benzoate, and the
methanol solution of emamectin benzoate, the methanol
solution of emamectin benzoate had the highest degradation
rate, whereas emamectin benzoate in PLA microspheres had
the lowest degradation rate. The degradation rates of
emamectin benzoate in methanol solution and emamectin
benzoate solid technical material were 46.3 2.11 and 22.7
1.51%, respectively, under natural sunlight condition on the
10th day. However, the degradation rate of emamectin
benzoate in PLA microspheres on the 10th day was 4.29
0.74%. Signicant dierences in the degradation rates of
emamectin benzoate could be found on the rst day between
emamectin benzoate in methanol solution and in PLA
microspheres or on the third day between solid emamectin
benzoate and PLA microspheres of emamectin benzoate after
the three samples had been kept under natural light condition
(Figure 7). The results indicated that microencapsulation of
emamectin benzoate displayed a signicantly lower degradation
rate of emamectin benzoate under natural light condition, as
diameter of microspheres decreased signicantly because
alcohol could reduce the surface tension of the oilwater
interface. At the same time, the entrapment rate of microspheres decreased signicantly as the specic surface area of the
emulsion droplets increased (Table 1; Figure 2).
Content of Acetone in Internal Organic Phase. Dichloromethane and acetone were mixed (at the ratio of dichloromethane/acetone = 1.5:0.5, 1.25:0.75, 1:1, or 0.75:1.25,
respectively) to form the internal organic phase to investigate
the inuence of dierent contents of acetone in the internal
organic phase on the characteristics of microspheres. Microspheres prepared from dierent contents of acetone could be
formed quickly, and the solid shell of microspheres could be
observed within a few minutes under a light microscope.
Compared with the surface of microspheres prepared using the
single solvent dichloromethane, the surface of microspheres
prepared using a mixture of dichloromethane and acetone was
smoother and more rounded, without adsorption of emamectin
benzoate on the surface (Figure 3).
With the increasing content of acetone, the entrapment rate
of microspheres gradually increased from 79.02 to 97.02% and
the diameter and span of microcapsules increased slightly.
However, when the amount of acetone in the internal organic
phase was >50%, the entrapment rate of microspheres
decreased substantially (Table 1; Figure 4). Therefore, the
entrapment rate of microspheres could be improved by adding
a suitable content of acetone in the internal organic phase.
Eect of Extracting Solvent in the External Aqueous
Phase on Microsphere Characteristics. The diameter and
entrapment rate of emamectin benzoate microspheres
decreased with increasing ethanol content in the external
aqueous phase, and the trend of span variation was not
pronounced (Table 1; Figure 5).
Analysis on Dierential Scanning Calorimetry. DSC is
often used to analyze the physical state of drugs encapsulated in
12223
dx.doi.org/10.1021/jf403722q | J. Agric. Food Chem. 2013, 61, 1221912225
Journal of Agricultural and Food Chemistry
Article
obtained by changing the emusifying speed. In conclusion,
microspheres of emamectin benzoate with high entrapment
rate and nice diameter could be obtained by adding a suitable
amount of polar solvent and using appropriate emusifying
speed.
The inuence of dierent contents of ethanol in the external
aqueous phase was investigated in this paper. It was reported
that the entrapment rate of microspheres increased when
methanol was added into external aqueous phase as extracting
solvent in the preparation of egg albumin microspheres.31 On
the contrary, Yao et al. reported that diameter and entrapment
rate of microspheres decreased when ethanol was used as
extracting solvent in the preparation of Tranilast poly(D,Llactide) microspheres.32 In the present study, the entrapment
rate of emamectin benzoate did not increase after the addition
of organic solvent in the external aqueous phase, but the
diameters of microspheres decreased. Our results were similar
to the results of Yao et al.32 Our results provided evidence that
the entrapment rate of microspheres would decline if ethanol
was added into the external aqueous phase, because ethanol
would result in reducing the surface tension of the emulsion
and in smaller diameter, larger specic surface area, and lower
entrapment rate. In addition, emamectin benzoate might diuse
into the external aqueous phase with ethanol as it was soluble in
methanol, even though emamectin benzoate microspheres
could be formed quickly.
The DSC results show that the combination of PLA and
emamectin benzoate presented a synergistic eect in reducing
the Tm of PLA. The facts indicated some anity between the
polymers and emamectin benzoate. The DSC results revealed
that PLA had combined with the active ingredient of the
pesticide in the amorphous or molecular state, but not in a
simple physical mixing or adsorption on the surface of the
microspheres. Our result indicated that DSC was an available
method for the analysis of the combination of microspheres
and pesticide. Similar analysis for characteristics of microspheres by using DSC determination was also used in the
analysis of chlorpyrifos microspheres.
Photolysis stability was an important indicator to measure
characteristics of microspheres. The results from the study of
photolysis stability also indicated that emamectin benzoate in
microspheres could be protected from light degradation by wall
material PLA. In addition, the results obtained by both DSC
and photolysis stability indicated that emamectin benzoate and
PLA had combined with each other well.
Release characteristic of pesticide was also an important
indicator of microspheres characteristics. It was proved that
microsphere formulations of medicines or pesticides prepared
using the solvent evaporation method or the solvent
evaporation/extraction method had an obviously sustained
eect and could meet the needs of slow release.1014,24,25
Emamectin benzoate, a slightly soluble chemical, was
prepared as a microsphere formulation of emamectin benzoate
with high entrapment rate and great photolysis stability by
using the solvent evaporation/extraction method. It was
suggested that the solvent evaporation/extraction method,
which has been widely used in the medical eld, would be a
good method in the microencapsulation of pesticides, in
particular, for pesticides with slight water solubility. Our study
broadened the scope of application of pesticide microcapsules
and provided a reference for the microencapsulation of
pesticides.
Figure 7. Light degradation curve of emamectin benzoate in PLA
microspheres, solid emamectin benzoate, and methanol solution of
emamectin benzoate.
compared to emamectin benzoate in methanol solution or solid
emamectin benzoate.
DISCUSSION
It was known that emulsifying speed was the main parameter
for controlling the dispersions droplet size and directly aected
the diameter of microspheres under the existence of sucient
emulsier condition.16 Our results indicated dierent diameters
of microspheres could be obtained by changing the emulsifying
speed according to actual needs in experiments. With the
increase of emulsifying speed, the dispersion and the specic
surface area of organic emulsion droplets in continuous phase
increased, but the organic emulsion droplet sizes decreased.
The facts resulted in the diusion probability of the emamectin
benzoate to external aqueous phase increased and the
entrapment rate decreased. Generally speaking, higher
emulsifying speed and stronger shear force would result in
better dispersion eect of dispersed organic phase in external
aqueous phase and smaller particle size of microspheres.30
Therefore, in the preparation process of emamectin benzoate
microspheres by using the emulsion solvent evaporation/
extraction method, the emulsifying speed was the critical factor
that aected the emulsion droplet size and nally inuenced the
size and entrapment rate of microspheres.
Bodmeier et al. reported that the entrapment rate of drug
increased when dichloromethane was mixed with organic
solvents such as acetone, methanol, ethanol, dimethyl sulfoxide,
and ethyl acetate in the preparation of poly(D,L-lactide)
microspheres containing quinidine or quinidine sulfate
prepared by the solvent evaporation method.26 Niwa et al.
reported that nanospheres with higher entrapment rate and
lower size could be obtained when a mixture of acetone and
dichloromethane (or acetone and chloroform) was used as
organic solvent in the preparation of PLGA nanospheres
containing indomethacin and 5-uorouracil, and the diameter
of nanospheres decreased when the amount of acetone in
internal organic phase increased.25 In this paper, the entrapment rate of emamectin benzoate increased when a suitable
amount of acetone or ethyl acetate was added into the internal
organic phase. However, dierent from the results above,25,26
the diameters of microspheres increased when the amount of
acetone in internal organic phase increased. The increase of
microphere diameter might be related to the fact that the
formation speed of microspheres was so fast that microspheres
gathered together easily. On the other hand, microspheres with
high entrapment rate and suitable diameter could be also
12224
dx.doi.org/10.1021/jf403722q | J. Agric. Food Chem. 2013, 61, 1221912225
Journal of Agricultural and Food Chemistry
Article
(14) Fernandez-Perez, M.; Gonzalez-Pradas, E.; Urena-Amate, M. D.
Controlled release of Imidacloprid from alignin matrix: water release
and mobility study. J. Agric. Food Chem. 1998, 46, 38263834.
(15) Ying, Z.; Schwendeman, S. P. Minimizing acylation of peptides
in PLGA microspheres. J. Controlled Release 2012, 162, 119126.
(16) Sergio, F.; Merkle, H. P.; Gander, B. Microencapsulation by
solvent extraction/ evaporation: reviewing the state of the art of
microsphere preparation process technology. J. Controlled Release
2005, 102, 313332.
(17) Alcock, R.; Bibby, D. C.; Gard, T. G. Encapsulation of
recombinant hepatitis B surface antigen in oligosaccharide ester
derivatives by spray drying. J. Microencapsul. 2003, 20, 759.
(18) Benita, S. Microencapsulation: Method and Industrial Applications;
Dekker: New York, USA, 1996; p 51.
(19) Ruan, G.; Feng, S. S. Preparation and characterization of
poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) (PLA-PEGPLA) microspheres for controlled release of paclitaxel. Biomaterials
2003, 24, 50375044.
(20) Zhou, Z.; Xu, J.; Liu, X.; Li, X. M.; Li, S. Y.; Yang, K.; Wang, X.
F.; Liu, M.; Zhang, Q. Q. Non-spherical racemic polylactide
microarchitectures formation via solvent evaporation method. Polymer
2009, 50, 38413850.
(21) Huang, B. B.; Huang, R.; Cai, X. J.; Shan, S. B.; Wu, Z. J.; Wu,
G. Study on key process of preparation of spinosad microsphere: I.
Chin. J. Pestic. Sci. 2011, 13, 314318.
(22) Guo, R. F.; Huang, B. B.; Yang, X. W.; Wu, Z. J.; Wu, G.
Preparation and characteristics analysis of microspheres of chlorpyrifos
and polylactic acid. Chin. J. Pestic. Sci. 2011, 13, 409414.
(23) Yang, S. Y.; Liu, D. K.; Zhang, C.; Zhang, S. F.; Wu, Z. J.; Wu,
G. Decline dynamics of chlorpyrifos microspheres and emulsifiable
concentrate in water and its toxicity to the larvae of Aedes albopictus.
Chin. J. Pestic. Sci. 2013, 15, 464468.
(24) Cowsar, D. R.; Tice, T. R.; Gilley, R. M.; English, J. P.
Poly(lactide-co-glycolide) microcapsules for controlled release of
steroids. Methods Enzymol. 1985, 112, 343.
(25) Niwa, T.; Takeuchi, H.; Hino, T.; Kunou, N.; Kawashima, Y.
Preparation of biodegradable nanospheres of water-soluble and
insoluble drugs with D,L-lactide/glycolide copolymer by a novel
spontaneous emulsification diffusion method, and the drug release
behavior. J. Controlled Release 1993, 25, 8998.
(26) Bodmeier, R.; Mcginity, J. W. Solvent selection in the
preparation of poly(D,L-lactide) microspheres prepared by the solvent
evaporation method. Int. J. Pharm. 1988, 43, 1791.
(27) Lu, B.; Wu, W. Optimization of preparation of dexamethasone
acetate-loaded poly(D,L-lactide) microspheres by central composite
design. Acta Pharm. Sinica 1999, 34, 387391.
(28) Dubernet, C. Thermoanalysis of microspheres. Thermochim.
Acta 1995, 248, 259269.
(29) Izumikawa, S.; Yoshioka, S.; Aso, Y.; Takeda, Y. Preparation of
poly(L-lactide) microspheres of different crystalline morphology and
effect of crystalline morphology on drug release rate. J. Controlled
Release 1991, 15, 133140.
(30) Lu, B. New Techniques and New Dosage Forms of Drugs; Peoples
Medical Publishing House: Beijing, China, 2005; pp 187190.
(31) Yeh, M. K.; Coomaes, A. G.; Jenkins, P. G.; Davis, S. S. A novel
emulsifyication solvent extraction technique for production of protein
loaded biodegradable microparticles for vaccine and drug delivery. J.
Controlled Release 1995, 33, 4371.
(32) Yao, B. X.; Zou, Y.; Que, L.; Wu, W. Study on preparation and
in vitro characterization of Tranilast poly(D,L-lactide) microspheres by
a modified solvent evaporation/extraction method. Chin. Pharm. J.
2006, 41, 608612.
ASSOCIATED CONTENT
S Supporting Information
*
Additional experimental details. This material is available free of
charge via the Internet at http://pubs.acs.org.
AUTHOR INFORMATION
Funding
This study was supported by SRFDP of China (No.
20103515110010) and the Natural Science Foundation of
Fujian Province (No. 2012J01085).
Notes
The authors declare no competing nancial interest.
REFERENCES
(1) Zhu, J.; He, Y. P.; Gao, M. X.; Zhou, W. J.; Hua, J.; Shen, J. L.;
Zhu, Y. C. Photodegradation of emamectin benzoate and its influence
on efficacy against the rice stem borer, Chilo suppressalis. Crop Prot.
2011, 30, 13561362.
(2) Jansson, R. K.; Brown, R.; Cartwright, B.; Cox, D.; Dunbar, D.
M.; Dybas, R. A.; Eckel, C.; Lasota, J. A.; Mookerjee, P. K.; Norton, J.
A.; Peterson, R. F.; Starner, V. R.; White, S. Emamectin benzoate: a
novel avermectin derivative for control of lepidopterous pests. In
Proceedings of the Third International Workshop, Kuala Lumpur,
Malaysia, The management of diamondback moth and other crucifer
pests; Cornell University: Ithaca, NY, USA, 1996; pp 171177.
(3) Zhao, J. Z.; Collins, H. L.; Li, Y. X.; Mau, R. F.; Thompson, G.
D.; Hertlein, M.; Andaloro, J. T.; Boykin, R.; Shelton, A. M.
Monitoring of diamondback moth (Lepidoptera: Plutellidae) resistance to spinosad, indoxacarb, and emamectin benzoate. J. Econ.
Entomol. 2006, 99, 176181.
(4) Miller, G. C.; Zepp, R. G. Extrapolating photolysis rate from the
laboratory to the environment. Residue Rev. 1983, 85, 89110.
(5) Hirech, K.; Payan, S.; Carnelle, G.; Brujes, L.; Legrand, J.
Microencapsulation of an insecticide by interfacial polymerisation.
Powder Technol. 2003, 130, 324330.
(6) Berkland, C.; Kipper, J. M.; Narasimhan, B.; Narasimhan, B.;
Kim, K. K.; Pack, D. W. Microsphere size, precipitation kinetics and
drug distribution control drug release from biodegradable polyanhydride microspheres. J. Controlled Release 2004, 94, 129.
(7) Peng, D. M.; Huang, K. L.; Liu, Y. F.; Liu, S. Q. Preparation of
novel polymeric microspheres for controlled release of finasteride. Int.
J. Pharm. 2007, 342, 8286.
(8) Thompson, W. W.; Anderson, D. B.; Heiman, M. L.
Biodegradable microspheres as a delivery system for rismorelin
porcine, a porcine-growth-hormone-releasing-hormone. J. Controlled
Release 1997, 43, 922.
(9) Mi, F. L.; Shyu, S. S.; Lin, Y. M.; Wu, Y. B.; Peng, C. K.; Tsai, Y.
H. Chitin/PLGA blend microspheres as a biodegradable drug delivery
system: a new delivery system for protein. Biomaterials 2003, 24,
50235036.
(10) Takayuki, T.; Masahiro, Y.; Yasuo, H.; Kouichiro, S.; Shiro, K.
Preparation of polylactide/poly(-caprolactone) microspheres enclosing acetamiprid and evaluation of release behavior. Polym. Bull. 2008,
61, 391397.
(11) Fernandez-Urrusuno, R.; Gines, J. M.; Morillo, E. Development
of controlled release formulations of alachlor in ethylcellulose. J.
Microencapsul. 2000, 17, 331342.
(12) Koek, F. N.; Wilkins, R. M.; Cain, R. B.; Arica, M. Y.;
Alaeddinoglu, G.; Hasirci, V. Controlled release of aldicarb from lignin
loaded ionotropic hydrogel microspheres. J. Microencapsul. 1999, 16,
613623.
(13) Suave, J.; DallAgnol, E. C.; Pezzin, A. P. T.; Meier, M. M.; Silva,
D. A. K. Biodegradable microspheres of poly(3-hydroxybutyrate)/
poly(-caprolactone) loaded with malathion pesticide: preparation,
characterization, and in vitro controlled release testing. J. Appl. Polym.
Sci. 2010, 117, 34193427.
12225
dx.doi.org/10.1021/jf403722q | J. Agric. Food Chem. 2013, 61, 1221912225
You might also like
- Chapter 6-Altimetry PPL January 2015Document13 pagesChapter 6-Altimetry PPL January 2015MustaphaNo ratings yet
- Introduction To Seismic Interpretation El AmalDocument33 pagesIntroduction To Seismic Interpretation El AmalFredrick Oshogbunu Ovakporaye100% (1)
- Purlin DesignDocument9 pagesPurlin DesignMungkorn SattNo ratings yet
- GEA Cassette Geko DF GB PDFDocument40 pagesGEA Cassette Geko DF GB PDFghenceaNo ratings yet
- 03 Ball Mill EDMDocument47 pages03 Ball Mill EDMrecai100% (1)
- Manual Liofilizador BiobaseDocument14 pagesManual Liofilizador BiobaseAlejandro Palomino100% (3)
- Submitted By: Varun Singh (2017A1PS0851G)Document17 pagesSubmitted By: Varun Singh (2017A1PS0851G)Varun SinghNo ratings yet
- Cano Odena Et Al CA Membranes Rev August 2010-PreprintDocument21 pagesCano Odena Et Al CA Membranes Rev August 2010-PreprintIma LismawatyNo ratings yet
- 10097-Article Text-65916-1-10-20210104Document16 pages10097-Article Text-65916-1-10-20210104soumaya kheroufNo ratings yet
- Removal of Nitrogen in Wastewater by Polyvinyl Alcohol (PVA) - Immobilization of Effective MicroorganismsDocument5 pagesRemoval of Nitrogen in Wastewater by Polyvinyl Alcohol (PVA) - Immobilization of Effective MicroorganismsfiorellaNo ratings yet
- Bijps, 77-92Document16 pagesBijps, 77-92ashfaq ahmedNo ratings yet
- PAPER-heliyon Trabajo PDFDocument7 pagesPAPER-heliyon Trabajo PDFSilNo ratings yet
- Towards Coupling Dispersive FinalDocument9 pagesTowards Coupling Dispersive FinalKadesh Hanah McCarthyNo ratings yet
- Floculantes de AlmidonDocument81 pagesFloculantes de AlmidonqamhNo ratings yet
- Zakaria 2015Document12 pagesZakaria 2015Jesús BarónNo ratings yet
- An Enzymatic Approach To The Cleaning of Ultrafiltration PDFDocument8 pagesAn Enzymatic Approach To The Cleaning of Ultrafiltration PDFsamrickyNo ratings yet
- Koumanova Et Al., 2000Document7 pagesKoumanova Et Al., 2000jebuzinNo ratings yet
- Xing 2013Document8 pagesXing 2013Vijaya GosuNo ratings yet
- Single Emulsion-Solvent Evaporation Technique and Modifications For The Preparation of Pharmaceutical Polymeric NanoparticlesDocument15 pagesSingle Emulsion-Solvent Evaporation Technique and Modifications For The Preparation of Pharmaceutical Polymeric NanoparticlesChâu Trần Lê TuyếtNo ratings yet
- Nanoparticulas EmulsionesDocument6 pagesNanoparticulas EmulsionesDavid Leonardo CañasNo ratings yet
- Journal of Industrial and Engineering Chemistry: Sarah Chaouchi, Oualid HamdaouiDocument10 pagesJournal of Industrial and Engineering Chemistry: Sarah Chaouchi, Oualid HamdaouiAnonymous 5NXc6NuNo ratings yet
- Research Article: Silica Sol-Gel Entrapment of The Enzyme ChloroperoxidaseDocument11 pagesResearch Article: Silica Sol-Gel Entrapment of The Enzyme ChloroperoxidaseShalwa 29No ratings yet
- ATPS2Document8 pagesATPS2Nicoly SilvaNo ratings yet
- Migration From Polycarbonate Packaging To Food Simulants During Microwave HeatingDocument9 pagesMigration From Polycarbonate Packaging To Food Simulants During Microwave Heatinggs67570No ratings yet
- Processes 08 00648 v2Document12 pagesProcesses 08 00648 v2Lidiane LimaNo ratings yet
- Foods 12 01415Document13 pagesFoods 12 01415saprifarmasiNo ratings yet
- Tran2009 Microalga PDFDocument6 pagesTran2009 Microalga PDFAdriana PerezNo ratings yet
- Ece 6Document11 pagesEce 6Ellie satrianiNo ratings yet
- Preparation and Performance of Cellulose Acetate-Polyurethane Blend Membranes and Their Applications - IIDocument14 pagesPreparation and Performance of Cellulose Acetate-Polyurethane Blend Membranes and Their Applications - IIBambang Purnama HadiNo ratings yet
- Micro Metformine 2020Document11 pagesMicro Metformine 2020boulfissaneNo ratings yet
- 730 Hydrotesting EngineeringDocument10 pages730 Hydrotesting EngineeringDien Bien NhamNo ratings yet
- 2011 Basheer Et Al Aspergillus Awamori Lipase ProductionDocument12 pages2011 Basheer Et Al Aspergillus Awamori Lipase ProductiontigapeptindonesiaNo ratings yet
- 10 1016@j Cogsc 2017 03 010Document5 pages10 1016@j Cogsc 2017 03 010rahmanNo ratings yet
- Materials: Multi-Functional Laccase Immobilized Hydrogel Microparticles For Efficient Removal of Bisphenol ADocument14 pagesMaterials: Multi-Functional Laccase Immobilized Hydrogel Microparticles For Efficient Removal of Bisphenol AcarolasbdNo ratings yet
- Preparation and Characterization of Melittin-Loaded Poly (Dl-Lactic Acid) or Poly (Dl-Lactic-Co-Glycolic Acid) Microspheres Made by The Double Emulsion MethodDocument10 pagesPreparation and Characterization of Melittin-Loaded Poly (Dl-Lactic Acid) or Poly (Dl-Lactic-Co-Glycolic Acid) Microspheres Made by The Double Emulsion MethoddetesNo ratings yet
- Study of Surfactant Combinations and Development of A Novel Nanoemulsion For Minimising Variations in Bioavailab..Document12 pagesStudy of Surfactant Combinations and Development of A Novel Nanoemulsion For Minimising Variations in Bioavailab..Vikas BaliNo ratings yet
- Water 15 00202 v3Document16 pagesWater 15 00202 v3Hanaa ZERGUINo ratings yet
- 2023materialstoday PaperDocument5 pages2023materialstoday PaperDr. JyotiNo ratings yet
- Preparation of Cation Exchange Resin Filled EVAL Hollow Fiber Membrane AdsorbentDocument8 pagesPreparation of Cation Exchange Resin Filled EVAL Hollow Fiber Membrane AdsorbentThu HươngNo ratings yet
- Conway PolyDocument10 pagesConway PolyGaleri DesilNo ratings yet
- Lab 3 Enzyme PurificationDocument7 pagesLab 3 Enzyme PurificationNanashiNo ratings yet
- Tongco2020 Article EnhancementOfHydrolysisAndBiogDocument9 pagesTongco2020 Article EnhancementOfHydrolysisAndBiogJim TsikasNo ratings yet
- Preparation of Chitosan Particles Suitable For Enzyme ImmobilizationDocument7 pagesPreparation of Chitosan Particles Suitable For Enzyme ImmobilizationMUHAMMAD HANIF ROSYIDI 2041201010No ratings yet
- Recovery and Purification of Surfactin From Fermentation Broth by A Two-Step Ultrafiltration ProcessDocument7 pagesRecovery and Purification of Surfactin From Fermentation Broth by A Two-Step Ultrafiltration Processmhafez1979No ratings yet
- 2019Document22 pages2019Karolina GawlakNo ratings yet
- Lorichika Gustinda Larasati - 142114153005 - Bioremediasi - Pak AminDocument25 pagesLorichika Gustinda Larasati - 142114153005 - Bioremediasi - Pak AminLorichika Gustinda LarasatiNo ratings yet
- Dematteis2016 PDFDocument15 pagesDematteis2016 PDFSukma SidhiNo ratings yet
- Based On Poly (Vinyl Alcohol) (PVA) Crosslinked With Malic Acid (MA) in Different Proportions of PVA:MADocument1 pageBased On Poly (Vinyl Alcohol) (PVA) Crosslinked With Malic Acid (MA) in Different Proportions of PVA:MAsusanarutaNo ratings yet
- Biochemical Engineering JournalDocument8 pagesBiochemical Engineering JournalbrendaNo ratings yet
- Dye RemovalDocument12 pagesDye RemovalaisyahNo ratings yet
- AJGC Volume 4 Issue 3 Pages 327-339Document13 pagesAJGC Volume 4 Issue 3 Pages 327-339Karen HernándezNo ratings yet
- Encapsulation of Bioactive Compounds in Nanoemulsion-Based Delivery SystemsDocument6 pagesEncapsulation of Bioactive Compounds in Nanoemulsion-Based Delivery SystemsjapoleonaNo ratings yet
- 25 Iajps25102017 PDFDocument7 pages25 Iajps25102017 PDFBaru Chandrasekhar RaoNo ratings yet
- Can e Guner 2004Document12 pagesCan e Guner 2004Vithória Carolyna Trindade Dos SantosNo ratings yet
- Colloid and Interface Science Communications: SciencedirectDocument8 pagesColloid and Interface Science Communications: SciencedirectOsneider Peña CuetoNo ratings yet
- Paper QadriDocument6 pagesPaper Qadrimhafez1979No ratings yet
- Modelling and Optimisation of Ultrasound Assisted Extraction of Roselle Phenolic Compounds Using The Surface Response MethodDocument9 pagesModelling and Optimisation of Ultrasound Assisted Extraction of Roselle Phenolic Compounds Using The Surface Response Methodjonaiit1234No ratings yet
- 1 s2.0 S266682112100034X MainDocument10 pages1 s2.0 S266682112100034X MainsarahmiramaiNo ratings yet
- 1984 05 30 - Cleaning Strategy For Biofilm Removal On RO MembranesDocument9 pages1984 05 30 - Cleaning Strategy For Biofilm Removal On RO MembranesbouteloupNo ratings yet
- Bioreactor Systems Using The White Rot Fungus Trametes For Bioremediation of Industrial WastewaterDocument158 pagesBioreactor Systems Using The White Rot Fungus Trametes For Bioremediation of Industrial Wastewaterali abdulrahman al-ezziNo ratings yet
- Application Potential of Grapefruit Peel As Dye Sorbent Kinetics, Equilibrium and PDFDocument9 pagesApplication Potential of Grapefruit Peel As Dye Sorbent Kinetics, Equilibrium and PDFSaadi BadisNo ratings yet
- Practical Research 2Document10 pagesPractical Research 2Jazzle TanNo ratings yet
- Synthesis of Macroporous Poly (Glycidyl Methacrylate) Microspheres by Surfactant Reverse Micelles Swelling MethodDocument10 pagesSynthesis of Macroporous Poly (Glycidyl Methacrylate) Microspheres by Surfactant Reverse Micelles Swelling MethodBos KayuNo ratings yet
- PR 15003Document6 pagesPR 15003Satvika AdhiNo ratings yet
- Lesson15 9 PDFDocument5 pagesLesson15 9 PDFconker4No ratings yet
- Introduccion Esta ChidaDocument12 pagesIntroduccion Esta Chidaconker4No ratings yet
- American Association For The Advancement of Science ScienceDocument6 pagesAmerican Association For The Advancement of Science Scienceconker4No ratings yet
- Pal 2005Document5 pagesPal 2005conker4No ratings yet
- Colbert2006 PDFDocument13 pagesColbert2006 PDFconker4No ratings yet
- Chemical Reactions ChernobylDocument5 pagesChemical Reactions Chernobylconker4No ratings yet
- Zinc Carbonate: Claisen RearrangementsDocument1 pageZinc Carbonate: Claisen Rearrangementsconker4No ratings yet
- Ed 400711 RDocument4 pagesEd 400711 Rconker4No ratings yet
- Perspectives: Development Trends For Human Monoclonal Antibody TherapeuticsDocument8 pagesPerspectives: Development Trends For Human Monoclonal Antibody Therapeuticsconker4No ratings yet
- Small-Molecule Organic SemiconductorsDocument8 pagesSmall-Molecule Organic Semiconductorsconker4No ratings yet
- Crystallography Reviews: To Cite This Article: John R. Helliwell (2013) Honouring The Two Braggs: The First X-Ray CrystalDocument10 pagesCrystallography Reviews: To Cite This Article: John R. Helliwell (2013) Honouring The Two Braggs: The First X-Ray Crystalconker4No ratings yet
- 5.3bTFMPPpre ReviewDocument23 pages5.3bTFMPPpre Reviewconker4No ratings yet
- Operation and Calibration of UV-VIS SpectrophotometerDocument8 pagesOperation and Calibration of UV-VIS SpectrophotometerMaruthi K100% (1)
- Optical Communications4Document19 pagesOptical Communications4keane1No ratings yet
- 4D Rods 3D Structures Via Programmable 1D Composite R 2018 Materials DesiDocument10 pages4D Rods 3D Structures Via Programmable 1D Composite R 2018 Materials DesiJorge Luis Garcia ZuñigaNo ratings yet
- Gear Drives Vs Belt DrivesDocument17 pagesGear Drives Vs Belt DrivesAaryan MahakalkarNo ratings yet
- Desalination: P. Palomar, J.L. Lara, I.J. LosadaDocument15 pagesDesalination: P. Palomar, J.L. Lara, I.J. Losadajean miguel oscorima celisNo ratings yet
- Origins of Ferromagnetism in Transition Metal Doped DiamondDocument4 pagesOrigins of Ferromagnetism in Transition Metal Doped DiamondAlcidess R QuispeNo ratings yet
- AS NotesDocument222 pagesAS NotesRayyan HashmiNo ratings yet
- Surfadone LP: Specialty SurfactantsDocument12 pagesSurfadone LP: Specialty SurfactantsSohaib BraziNo ratings yet
- Rubber Products in Automotive Applications: Standard Classification System ForDocument53 pagesRubber Products in Automotive Applications: Standard Classification System ForKhimeshNo ratings yet
- Class X LP LightDocument1 pageClass X LP LightNiklesh SelvaNo ratings yet
- Use of Ambient Dose Equivalent, Directional DoseDocument15 pagesUse of Ambient Dose Equivalent, Directional DoseMuhammad NaveedNo ratings yet
- General Welding Procedures (0679, 7000) (REHS1841-23)Document73 pagesGeneral Welding Procedures (0679, 7000) (REHS1841-23)Anderson Oliveira SilvaNo ratings yet
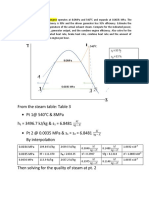
- From The Steam Table: Table 3 PT 1@ 540 C & 8mpa H 3496.7 KJ/KG & S 6.8481 PT 2 at 0.0035 Mpa & S S 6.8481 by InterpolationDocument5 pagesFrom The Steam Table: Table 3 PT 1@ 540 C & 8mpa H 3496.7 KJ/KG & S 6.8481 PT 2 at 0.0035 Mpa & S S 6.8481 by InterpolationMark AgusNo ratings yet
- BallisticDocument17 pagesBallisticMary Clair BarbadilloNo ratings yet
- MGS Capacity CalculatorDocument13 pagesMGS Capacity CalculatorRodolfo MendezNo ratings yet
- List of NDT EquipmentsDocument1 pageList of NDT EquipmentsAnandNo ratings yet
- 30 Electrochemistry WSDocument81 pages30 Electrochemistry WSVayaNo ratings yet
- January 2017 (IAL) MS - Unit 1 Edexcel Physics A-LevelDocument16 pagesJanuary 2017 (IAL) MS - Unit 1 Edexcel Physics A-LevelNyraStardollNo ratings yet
- WORKSHOPDocument9 pagesWORKSHOPManjunatha EikilaNo ratings yet
- Dielectric Properties of KDP-type Ferroelectric Crystals in The Presence of External Electric FieldDocument6 pagesDielectric Properties of KDP-type Ferroelectric Crystals in The Presence of External Electric Fieldبكر القرعانيNo ratings yet
- Physics Jeopardy Game - Forces & MotionDocument52 pagesPhysics Jeopardy Game - Forces & MotionHolly Maria CassonNo ratings yet
- Unit One Material and Geometry of Cutting Tools 2015Document46 pagesUnit One Material and Geometry of Cutting Tools 2015elnat feyisa100% (1)
- MEC-307 Design of Machine Element 6 Semester (3 Year) : Name: Chirag Shetty Urn No: 2020-B-26101996Document18 pagesMEC-307 Design of Machine Element 6 Semester (3 Year) : Name: Chirag Shetty Urn No: 2020-B-26101996Siddharth SinghNo ratings yet
- M.S. Abd-ElhadyDocument8 pagesM.S. Abd-ElhadyNasser93No ratings yet