Professional Documents
Culture Documents
IPROFCHenC01T01ST01 st01 1P
Uploaded by
Anand SwarnkarOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
IPROFCHenC01T01ST01 st01 1P
Uploaded by
Anand SwarnkarCopyright:
Available Formats
CHEMICAL EQUILIBRIUM
When a chemical reaction takes place in a closed container which prevents the entry or
escape of any of the substances involved in the reaction, the quantities of these
components change as some are consumed and others are formed. Eventually this change
will come to an end, after which the composition will remain unchanged as long as the
system remains undisturbed. The system is then said to be in its equilibrium state, or more
simply, at equilibrium
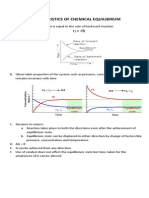
i.e. A state of a chemical reaction in which rate of forward reaction ( rf ) becomes equal to
the rate of backward reaction ( rb ).
The direction in which we write a chemical reaction (and thus which components are
considered reactants and which are products) is arbitrary. Thus the equations
H2 + I2
2HI
synthesis of hydrogen iodide
and
2HI
H2 + I2 dissociation of hydrogen iodide
represent the same chemical reaction system in which the roles of the components are
reversed, and both yield the same mixture of components when the change is completed.
NOTE: A chemical reaction is in equilibrium when there is no tendency for the quantities of
reactants and products to change.
TYPES OF REACTIONS
a) Reversible reactions attain equilibrium state
b) Irreversible reactions do not attain equilibrium state
A chemical equation of the form A
B represents the transformation of A into B, but it
does not imply that all of the reactants will be converted into products, or that the
reverse reaction B
A cannot occurs. In general, both processes can be expected to
occur, resulting in an equilibrium mixture containing all of the components of the reaction
system. (We use the word components when we do not wish to distinguish between
reactants and products.) If the equilibrium state is one in which significant quantities of
both reactants and products are present (as in the hydrogen iodide example given
above), then the reaction is said to incomplete or reversible.
If it is desired to emphasize the reversibility of a reaction, the single arrow in the equation is
replaced with a pair of hooked lines pointing in opposite directions, as in A
B. There
is no fundamental difference between the meanings of A
B and in A
B,
however some older text-books even use A = B.
A reaction is said to be complete when the equilibrium composition contains no significant
amount of the reactants. However, a reaction that is complete when written in one
direction is said not to occur when written in the reverse direction.
In principle, all chemical reactions are reversible, but this reversibility may not be
observable,
if the fraction of products in the equilibrium mixture is very small,
Or if the reverse reaction is kinetically inhibited.
EXAMPLES OF REVERSIBLE HOMOGENEOUS REACTIONS:
A. Examples of Gaseous reactions in a closed vessel
a)
b)
c)
d)
e)
H2 + I2
N2 + 3H2
2SO2 + O2
PCl5
N2 + O2
2HI
2NH3
2SO3
PCl3 + Cl2
2NO
B. Liquid phase reaction
CH3COOH (l) + C2H5OH (l)
CH3COOC2H5 (l)
C. Solid phase reaction
C (diamond)
C (graphite)
EXAMPLES OF REVERSIBLE HETEROGENEOUS REACTIONS:
A. Heterogeneous reactions in a closed vessel.
a)
b)
c)
d)
CaCO3 (s)
C (s) + H2O (g)
3Fe (s) + 4H2O (g)
NH4HS (s)
CaO (s) + CO2 (g)
H2 (g) + CO (g)
Fe3O4(s) + H2S (g)
NH3 (g) + H2S (g)
EXAMPLES OF IRREVERSIBLE REACTIONS
a) Decomposition reaction
2KClO3 (s)
2KCl (s) + 3O2 (g)
b) Precipitation reaction
NaCl (aq) + AgCl (aq)
AgCl (s) + NaNO3 (aq)
c) Neutralisation reaction
NaCl (aq) + HCl (aq)
NaCl (aq) + H2O (aq)
d) Combustion of a gas
CH4 (g) + 2O2 (g)
CO2 (g) + 2H2O (l)
You might also like
- Past 15 Years-Essay Question Papers Ias - Civil Services Exam For 2015Document8 pagesPast 15 Years-Essay Question Papers Ias - Civil Services Exam For 2015Anand SwarnkarNo ratings yet
- Hydrogenation: Heterogeneous CatalysisDocument10 pagesHydrogenation: Heterogeneous CatalysisAnand SwarnkarNo ratings yet
- Iterview QuestionDocument5 pagesIterview QuestionAnand SwarnkarNo ratings yet
- SamajDocument21 pagesSamajAnand SwarnkarNo ratings yet
- Le Chatelier's Principle: Example Involving Change of ConcentrationDocument2 pagesLe Chatelier's Principle: Example Involving Change of ConcentrationAnand SwarnkarNo ratings yet
- Mol or 2calk Mol Depending On The Limit of HDocument2 pagesMol or 2calk Mol Depending On The Limit of HAnand SwarnkarNo ratings yet
- Characteristics of Chemical Equilibirum: H + I 2HI 2hi H + IDocument1 pageCharacteristics of Chemical Equilibirum: H + I 2HI 2hi H + IAnand SwarnkarNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- MGPSDocument35 pagesMGPSraghuNo ratings yet
- Product Information: Crawler Excavator: Generation EngineDocument16 pagesProduct Information: Crawler Excavator: Generation EngineTeguh J. AlkausarNo ratings yet
- Phy Sci PartIDocument318 pagesPhy Sci PartIsollu786_889163149No ratings yet
- Capacitors: Publishing As Pearson (Imprint) BoylestadDocument71 pagesCapacitors: Publishing As Pearson (Imprint) BoylestadFaisal UddinNo ratings yet
- E20 Heat Load Calculation SheetDocument1 pageE20 Heat Load Calculation SheetȘtefan C. Petre50% (2)
- FC45X-X Cummins Leroy GRDocument7 pagesFC45X-X Cummins Leroy GRIsrael GomezNo ratings yet
- R11 Segment 12Document49 pagesR11 Segment 12Dæmon PhobosNo ratings yet
- Daikin - Air Conditioning Training MaterialDocument92 pagesDaikin - Air Conditioning Training MaterialJM ArcillaNo ratings yet
- Electrical Safety - EEE1021 Experiment - 6: Line Charging Current in AC More Over Poses Serious Problems in CablesDocument5 pagesElectrical Safety - EEE1021 Experiment - 6: Line Charging Current in AC More Over Poses Serious Problems in CablesRobert JrNo ratings yet
- CBC-DH For Wheisman-2022.11-DH2209-05Document1 pageCBC-DH For Wheisman-2022.11-DH2209-05Martín OsorioNo ratings yet
- Electric PV PPT New3Document32 pagesElectric PV PPT New3Anushka PagalNo ratings yet
- HT Xlpe CableDocument21 pagesHT Xlpe CablePritam SinghNo ratings yet
- Date Tehnice 436 ZXDocument6 pagesDate Tehnice 436 ZXMB ViorelNo ratings yet
- Bank Soal KD 3.9Document6 pagesBank Soal KD 3.9Rakhmady AdamiNo ratings yet
- Data Sheet Tcg2032 DeutzDocument3 pagesData Sheet Tcg2032 DeutzMaximiliano SanchezNo ratings yet
- 3tf ContactorDocument25 pages3tf Contactorpadminitt100% (1)
- HYDX-4 Operation Manual 201103Document29 pagesHYDX-4 Operation Manual 201103jorge squellaNo ratings yet
- API Standards ListDocument9 pagesAPI Standards ListSohail Aziz Ahmad MalikNo ratings yet
- T-MEET324LA Experiment No.12 Shell Tube Heat Exchanger MEE31Document15 pagesT-MEET324LA Experiment No.12 Shell Tube Heat Exchanger MEE31Paul Ryan GeneralNo ratings yet
- Traction Drive HES880 - Flyer - 3AUA0000161471 - RevF - EN PDFDocument2 pagesTraction Drive HES880 - Flyer - 3AUA0000161471 - RevF - EN PDFjalilemadiNo ratings yet
- Ali 2018Document6 pagesAli 2018rosendo rojas barraganNo ratings yet
- Basic Electronic For Marine EngineersDocument45 pagesBasic Electronic For Marine EngineersŞansal DikmenerNo ratings yet
- List Three Examples of Matter Changing From One State To AnotherDocument31 pagesList Three Examples of Matter Changing From One State To AnotherygjyoNo ratings yet
- Project EvsDocument8 pagesProject Evsnaveenkmr04550% (2)
- Lecture 5. Neutron LoggingDocument72 pagesLecture 5. Neutron LoggingDimash AbdrakhmanovNo ratings yet
- Desigo SystemDocument9 pagesDesigo Systemcindy .(00000050761)No ratings yet
- Chapter 13 - Chemical EquilibriumDocument52 pagesChapter 13 - Chemical EquilibriummukhlishNo ratings yet
- GROHE Pricelist-2020 en GB PDFDocument1,020 pagesGROHE Pricelist-2020 en GB PDFNikhil AggarwalNo ratings yet
- Unit - 3 Solar Photovoltaic SystemDocument15 pagesUnit - 3 Solar Photovoltaic SystemSudhir MallampatiNo ratings yet
- Aermec PXA E INSTALLATION MANUAL EngDocument8 pagesAermec PXA E INSTALLATION MANUAL EngMauricio Andres Vidal ColomaNo ratings yet