Professional Documents
Culture Documents
Design of Heat Treatment Cycles
Uploaded by
Anonymous s6xbqCpvSWCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Design of Heat Treatment Cycles
Uploaded by
Anonymous s6xbqCpvSWCopyright:
Available Formats
Design of Heat Treatment Cycles: A Case Study for Salt Bath Har
Although heat treatment operations have strong bearing on the final product quality, H
trial and error methods. The present work describes an engineering approach to the de
Industry accepted heat treatment processing cycles might sometimes lead to longer pr
efficiency, and higher energy consumption. This article elaborates on important engine
austenitising temperature and soaking time, selection of appropriate salt, rectification o
was successfully applied to an industrial scale heat treating operation for the productio
Accurate Engineering Company Ltd. is leading Indian manufacturer of precision measur
facilities in Pune, India, the company offers a comprehensive range of measuring equip
measuring fixtures, and three coordinate measuring instruments.
The heat treatment operation is an important step in the manufacturing of these precis
product with stringent dimensional control as well as high wear-resistance. The commo
equipment are:
salt bath hardening operation, where the components are heated in a salt bath fu
cryogenic treatment for stabilization of martensitic microstructure, and
tempering operation for obtaining a desirable combination of strength, hardness
Accurate Engineering recently procured a large amount of steel from Germany at a ver
used by one of the leading German slip gauge manufacturers. Although, the compositio
At the Accurate heat treatment shop, attempts were made to develop the heat treatme
from 30 to 45 HRC were observed during these trials, well below the required hardness
During the past two decades, the process engineering group of Tata Research Developm
projects on model based optimization of various metallurgical operations. The Frank Gr
this steel to obtain uniform hardness of HRC 60 after quenching and tempering operati
Approach to Design of the Heat Treatment Cycle
Hardening of steel is achieved by transforming the ferrite+pearlite p
austenite phase to martensite phase by cooling. Under standard pro
There will always be a minimal amount of retained austenite in the s
complete hardness, improve toughness and minimize distortion duri
As depicted in Fig. 1(a), the transformation to austenite requires he
hypereutectoid steel (with %C > 0.8). During the subsequent quenc
Fig. 1 Schematic of
transformation to softer phases like pearlite and bainite. This is sche
hardening operation.
(Left) Heating to the
austenitizing
temperature shown
as shaded regime,
and (Right) high
cooling rate for
hardening.
The important metallurgical issues in designing a hardening cycle for tool steels are:
Selection of austenising temperature,
Adequate soaking time for thermal homogenization of the component,
Selection of appropriate quenching media to obtain required cooling rate,
Cooling the component to the room temperature,
Tempering temperature and time.
However, several other practical aspects, such as selection of salt and its neutrality ma
These issues are elaborated in the following section.
Steel Grade
Identification of the steel grade is the most important parameter for
Frank grade steel are tabulated in Table I. The composition falls in th
is a shallow hardening tool steel and in rods above 0.5 inch in diame
soft and tough core. These characteristics make it desirable for man
and is readily formable by forging. W2 grade responds uniformly to
Selection of Salt for Process
When selecting a salt for a given application, the following issues m
The required heating temperature of the steel part must lie wi
The melting point should be low to avoid prolonged heat-up ti
The salt must be compatible with quenching media; and
The ease with which the salt is washed from the workpiece after heat treatment
At present, Accurate uses a proprietary MNC-661 heat treatment salt, supplied by Mata
that its melting point is 1220 F (660 C) and recommended working range is 1508 to 15
as high as 1832 F (1000 C). Using a salt above its recommended working temperature
oxidation and decarburization in the workpiece. Therefore, a suitable alternative had to
Barium chloride-based salts are widely used for salt bath heat treatment of tool steels.
salts are given in Table II. For the Frank Grade steel, Salt #2 with 70% BaCl2 and 20%
Salt Bath Temperature & Soaking Time
Proper control of salt bath temperature in the austenitizing range is
lower temperatures will prevent the complete transformation of pea
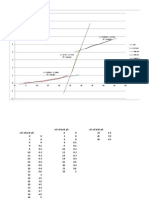
The time-temperature-transformation diagram for this grade is give
(732 to 743 C), so the recommended austenitizing temperature (ba
1555 F (775 to 845 C). For complex shapes and larger parts, it is re
prior to hardening.
enable the
recommended
time of
As a best practice, uniformity surveys should be c
treatment temperature. These surveys are usuall
as illustrated in Fig. 3. The soaking time in a salt
complete phase transformation to austenite. Long
soaking time in the salt bath furnace is 20 to 25
approximately 10 to 30 minutes for the parts in t
Salt
Neutral salts used
oxides and
the case of salt
based salts (Salt#
graphite rod for
Rectification
for austenitizing steel become contaminated with
dissolved metals renders the bath oxidizing and d
bath furnaces with immersed electrodes, daily re
1,2,3 in Table II) rectification should be done by
one hour for every 4 hours of operation.
Fig. 2 TTT diagram of
AISI W2 grade steel.
Fig. 3 Suggested
locations for
uniformity survey.
Tempering
Tempering modifies the properties of quenched h
and toughness. In general, two or more shorter t
retained austenite and for tempering the freshly
suggested double tempering process for the Fran
hardened tool steels like this steel should be tem
temperature, to prevent or minimize cracking.
EXPERIMENTATION AND RESULTS
Based on the items discussed above, the followin
Fig. 5 Schematic of
hardness test
Fig. 4 Typical cooling locations.
curve for a
Fig. 6 Average and
quenchant with three standard deviation of
different stages of
central points and
cooling.
corner for as-heat
treated, 0.1 mm and
0.3 mm ground
samples.
Salt Bath Temperature of 1508
Holding Time of 15 minutes
Quenching in Water
Tempering at 392 F (200 C) for 45 minutes
Fifteen samples measuring 55 mm x 35 mm x 10 mm were heat treated. The Rockwell
as at depths of 0.1 mm and 0.3 mm. The location where each hardness measurement
measurements on the fifteen samples are summarized in Fig. 6.
The mean hardness and standard deviation of the center point and the corners are plot
the as-heat treated sample had an average hardness value of 44.8 HRC with a standar
variation is not acceptable in the final product. In the 0.1 mm depth sample, the avera
deviation to 9.8. The hardness value further improved to an average of 60.6 with a sta
hardness value is highly desirable for the slip gauges. It must be noted that the hardne
company.
The low hardness and wide variability of the as-heat treated samples was primarily due
mm to 0.3 mm suggests decarburization in the product. It is believed that the decarbu
used at Accurate Engineering.
It is interesting to note that in all the three cases, the center points showed a lower ha
be the result of a higher tendency of vapor blanket formation in the sample center as c
vapor blanket during the first state of quenching and could improve hardness uniformit
RECOMMENDATIONS
The summary of recommendations as determined by the process analysis and the expe
Salt mixture of 70% BaCl2 + 30% NaCl should be used at an austenitizing tempe
Rectification of the above salt must be done every four hours of operation with 1
in the bath for 1 hour.
Uniformity survey of the bath temperature should be conducted before loading th
The workpiece should be cleaned from scale before heating in the salt bath furna
The workpiece should be quickly transferred from the salt bath to the quench wa
Quenching water should be agitated in order to achieve hardness uniformity.
When the part can be held by hand, it should be quickly transferred to the tempe
The tempering cycle should be repeated to achieve better dimensional stability.
The parts should be cleaned in a solution of 10% NaOH by weight in water follow
SUMMARY
By using the suggested heat treatment cycle determined for the Frank Grade steel, the
was observed and attributed to the use of salt bath at higher than recommended temp
You might also like
- Manual PDFDocument200 pagesManual PDFEddie grassgunter100% (1)
- Preventing Scale Loss During Heat Treatment & Hot Forging - With ImagesDocument17 pagesPreventing Scale Loss During Heat Treatment & Hot Forging - With ImagesSrikar Shenoy100% (1)
- Lecture 3 Titanium and Its AlloysDocument13 pagesLecture 3 Titanium and Its AlloysMarisa RobertsNo ratings yet
- SIS-S 501 50 AG Hot Rolled Steel Rounds (Up To Dia 180mm)Document5 pagesSIS-S 501 50 AG Hot Rolled Steel Rounds (Up To Dia 180mm)Rajoo PrajapatiNo ratings yet
- Epoxidation of Vegetable Oils - A ReviewDocument8 pagesEpoxidation of Vegetable Oils - A ReviewNorberto SchischoffNo ratings yet
- Edexcel IAL Physics Unit 3 NotesDocument46 pagesEdexcel IAL Physics Unit 3 Notessshyam3100% (2)
- Road Construction NotesDocument19 pagesRoad Construction NotesVanessa Apolinario100% (7)
- ModuleDocument22 pagesModulesugiartoNo ratings yet
- Heat TreatmentDocument34 pagesHeat Treatmentrahul72No ratings yet
- Introduction of Heat and Surface Treatment PDFDocument45 pagesIntroduction of Heat and Surface Treatment PDFScott BakerNo ratings yet
- Use of The Niyama Criterion To Predict Shrinkage-Related Leaks in High-Nickel Steel and Nickel-Based Alloy CastingsDocument18 pagesUse of The Niyama Criterion To Predict Shrinkage-Related Leaks in High-Nickel Steel and Nickel-Based Alloy CastingsCarlos Ortega Jones100% (1)
- Cast Iron Welding ProcedureDocument2 pagesCast Iron Welding ProcedureAnand Kesarkar100% (1)
- Titanium FormingDocument5 pagesTitanium FormingSiavash IraniNo ratings yet
- Normalizing Temperature and Time Effect On Micro Structure and Change in Mechanical Properties of Forged Steel Billet For Axle ProductionDocument3 pagesNormalizing Temperature and Time Effect On Micro Structure and Change in Mechanical Properties of Forged Steel Billet For Axle ProductionAnonymous izrFWiQNo ratings yet
- Design Considerations of CastingsDocument8 pagesDesign Considerations of Castingshaqjmi100% (2)
- TM23 - Heat Treatment of Metals PDFDocument32 pagesTM23 - Heat Treatment of Metals PDFAlessio NotariNo ratings yet
- Preheat Calculation 1 PDFDocument3 pagesPreheat Calculation 1 PDFravi00098No ratings yet
- Alloy Steel ASTM A217 GRADE WC6Document2 pagesAlloy Steel ASTM A217 GRADE WC6Dika Wahyu WijayaNo ratings yet
- CR StandardsDocument6 pagesCR Standardsalpha_beta48No ratings yet
- Closed Die Forging Reading MaterialDocument27 pagesClosed Die Forging Reading MaterialSaravanakumar Rajagopal100% (1)
- Feeding Risering For Steel Casting Design PDFDocument10 pagesFeeding Risering For Steel Casting Design PDFHusen TaufiqNo ratings yet
- Application Manual Chapter 6 - Feeding & GatingDocument148 pagesApplication Manual Chapter 6 - Feeding & GatingVishal MaliNo ratings yet
- Is Phosphorus Bad For SteelDocument19 pagesIs Phosphorus Bad For SteelKarun Dev100% (1)
- Review of Type IV Cracking of Weldments in 9Document64 pagesReview of Type IV Cracking of Weldments in 9Krishnan SanthanarajNo ratings yet
- Tolerance Chart For Casting For Intolerable Dimensional As Per ISO 8062, IS 2102Document2 pagesTolerance Chart For Casting For Intolerable Dimensional As Per ISO 8062, IS 2102Umesh Sakhareliya100% (1)
- Continuous Cooling Transforming DiagramDocument26 pagesContinuous Cooling Transforming DiagramaunginternetNo ratings yet
- Isothermal Heat Treatment PDFDocument6 pagesIsothermal Heat Treatment PDFsmani170No ratings yet
- LIBRO3Document43 pagesLIBRO3Camilo LacoutureNo ratings yet
- Riview On Cold Drawing Process PDFDocument7 pagesRiview On Cold Drawing Process PDFAmandeep Singh GujralNo ratings yet
- A 488A 488M 01 Welding Qualifications of Procedures and Personnel PDFDocument16 pagesA 488A 488M 01 Welding Qualifications of Procedures and Personnel PDFshakeelahmadjsrNo ratings yet
- Forging - The Process: Metal Forging Is A Metal Forming Process That Involves ApplyingDocument25 pagesForging - The Process: Metal Forging Is A Metal Forming Process That Involves ApplyingAdityasinh DesaiNo ratings yet
- Alloy Steel Castings Table 1Document16 pagesAlloy Steel Castings Table 1takumi_zNo ratings yet
- AnyCasting Software Intro SAND PrintDocument45 pagesAnyCasting Software Intro SAND PrintChuah Chun KitNo ratings yet
- STD-INSP-0125 IGC Phases Practice - A - (ASTM A923) DUPLEXDocument2 pagesSTD-INSP-0125 IGC Phases Practice - A - (ASTM A923) DUPLEXAkshay KalraNo ratings yet
- MSI DC 01 FosecoDocument4 pagesMSI DC 01 FosecoVivek Shrivastava100% (1)
- Challenges in Fabrication of 2.15Cr 1mo 0.25V ReactorsDocument56 pagesChallenges in Fabrication of 2.15Cr 1mo 0.25V Reactorsnikhileshkumar_mishr100% (1)
- Thermanit 17-06 (Boehler)Document1 pageThermanit 17-06 (Boehler)queno1No ratings yet
- Hot Tap Welding ParametersDocument7 pagesHot Tap Welding ParametersSiva RamNo ratings yet
- Aisi 4140 Alloy Steel (Uns g41400)Document4 pagesAisi 4140 Alloy Steel (Uns g41400)Deepak ChaurasiaNo ratings yet
- Heat Treatment of Steels: (I) (Ii) (Iii)Document32 pagesHeat Treatment of Steels: (I) (Ii) (Iii)Srushti MNo ratings yet
- Welding Database CCT DiagramDocument1 pageWelding Database CCT DiagramcostycgNo ratings yet
- 5-Hot Tear PDFDocument9 pages5-Hot Tear PDFPeeka Prabhakara RaoNo ratings yet
- The Oolitization Rate Determination of Bentonite Moulding Mixture PDFDocument4 pagesThe Oolitization Rate Determination of Bentonite Moulding Mixture PDFj33689 mvrht comNo ratings yet
- Forgings PDFDocument23 pagesForgings PDFrrameshsmitNo ratings yet
- Ascometal Grades en PDFDocument8 pagesAscometal Grades en PDFJuan LeonNo ratings yet
- Materials Failure AnalysisDocument53 pagesMaterials Failure Analysisulsan busanNo ratings yet
- Typical Heat Treatment Defects of GearsDocument8 pagesTypical Heat Treatment Defects of GearsRamon BrownNo ratings yet
- Forgingforging ProcessDocument13 pagesForgingforging Processpatel ketanNo ratings yet
- Die Casting Heat Treatment Process To Increase Strength Part 2Document6 pagesDie Casting Heat Treatment Process To Increase Strength Part 2itslowNo ratings yet
- Design - Part 4 - Job Knowledge 93Document4 pagesDesign - Part 4 - Job Knowledge 93Billy TanNo ratings yet
- 16.0 Grade Wise Chemical CompositionDocument29 pages16.0 Grade Wise Chemical CompositionrohitNo ratings yet
- Forging Process: Prof. P. P. Date Department of Mechanical Engineering, IIT BombayDocument27 pagesForging Process: Prof. P. P. Date Department of Mechanical Engineering, IIT BombayThomas StanlyNo ratings yet
- Distortion Reduction in Gear by Simple Heat Treatment Process by Simple FixtureDocument7 pagesDistortion Reduction in Gear by Simple Heat Treatment Process by Simple FixtureVireshVerma100% (1)
- Plate Material SpecificationDocument8 pagesPlate Material SpecificationKarthic KeyanNo ratings yet
- Gears HoningDocument7 pagesGears Honingsav33No ratings yet
- Metal Structure and Metallography TechniqueDocument11 pagesMetal Structure and Metallography TechniqueLilian RoseNo ratings yet
- The Welding Metallurgy of HASTELLOY Alloys C-4, C-22, and C-276Document2 pagesThe Welding Metallurgy of HASTELLOY Alloys C-4, C-22, and C-276Ivan GarzonNo ratings yet
- 254smo (Uns 31254)Document8 pages254smo (Uns 31254)Yang Gul LeeNo ratings yet
- Feeding Steel and Ductile Iron CastingDocument22 pagesFeeding Steel and Ductile Iron Castingjosemiguelzu100% (1)
- Weldclad WLDC 3Document1 pageWeldclad WLDC 3furiousgaulNo ratings yet
- CMT Welding Research PaperDocument23 pagesCMT Welding Research PaperUpendra93No ratings yet
- Handbook On Electroplating With Manufacture of ElectrochemicalsDocument13 pagesHandbook On Electroplating With Manufacture of ElectrochemicalsAbeerNo ratings yet
- TPM TrainingDocument26 pagesTPM TrainingAnonymous s6xbqCpvSWNo ratings yet
- Lecture On Circular CurvesDocument43 pagesLecture On Circular CurvesAnonymous s6xbqCpvSW100% (2)
- AISI A2 SteelDocument2 pagesAISI A2 SteelAnonymous s6xbqCpvSWNo ratings yet
- Lecture On Circular CurvesDocument43 pagesLecture On Circular CurvesAnonymous s6xbqCpvSW100% (2)
- Lecture On Circular CurvesDocument43 pagesLecture On Circular CurvesAnonymous s6xbqCpvSW100% (2)
- Spot WeldingDocument3 pagesSpot WeldingAnonymous s6xbqCpvSWNo ratings yet
- Copper Forging AlloysDocument2 pagesCopper Forging AlloysAnonymous s6xbqCpvSWNo ratings yet
- Polymers Questions With AnswerDocument2 pagesPolymers Questions With AnswerAnonymous s6xbqCpvSWNo ratings yet
- In High Power CO2 Laser WeldingDocument1 pageIn High Power CO2 Laser WeldingAnonymous s6xbqCpvSWNo ratings yet
- Gases For Laser Cutting and WeldingDocument2 pagesGases For Laser Cutting and WeldingAnonymous s6xbqCpvSWNo ratings yet
- Design of Heat Treatment CyclesDocument9 pagesDesign of Heat Treatment CyclesAnonymous s6xbqCpvSWNo ratings yet
- Phase DiagramsDocument3 pagesPhase DiagramsOmoshola BrightNo ratings yet
- Gases For Laser Cutting and WeldingDocument2 pagesGases For Laser Cutting and WeldingAnonymous s6xbqCpvSWNo ratings yet
- SLVCDocument6 pagesSLVCAnonymous s6xbqCpvSWNo ratings yet
- Potentio PH VolDocument3 pagesPotentio PH VolAnonymous s6xbqCpvSWNo ratings yet
- Linear Variable Differential TransducerDocument2 pagesLinear Variable Differential TransducerAnonymous s6xbqCpvSWNo ratings yet
- SteelDocument3 pagesSteelAnonymous s6xbqCpvSWNo ratings yet
- Iit Jee BooksDocument1 pageIit Jee BooksAnonymous s6xbqCpvSWNo ratings yet
- Simplified Model For Evaluating Soil Liquefaction Potential Using CPTUDocument8 pagesSimplified Model For Evaluating Soil Liquefaction Potential Using CPTUIbrahim SuryaNo ratings yet
- F3 00 EngDocument15 pagesF3 00 Engobiwan2009No ratings yet
- Report On SoftenerDocument72 pagesReport On SoftenerNipu Sen100% (4)
- Steady Incompressible Flow in Pressure Conduits (PartB)Document21 pagesSteady Incompressible Flow in Pressure Conduits (PartB)naefmubarakNo ratings yet
- Whole Numbers and Basic OperationsDocument6 pagesWhole Numbers and Basic Operationsapi-389461198No ratings yet
- Pythagorean TheoremDocument8 pagesPythagorean Theoremakiyama_ma83No ratings yet
- CS 2742 (Logic in Computer Science) - Fall 2011: Antonina KolokolovaDocument3 pagesCS 2742 (Logic in Computer Science) - Fall 2011: Antonina KolokolovaZhichaoWangNo ratings yet
- AxaxxDocument19 pagesAxaxxkara_25No ratings yet
- Enthalpy 2Document11 pagesEnthalpy 2SB KPNo ratings yet
- Experiment 3 - 1st and 2nd Law of ThermodynamicsDocument4 pagesExperiment 3 - 1st and 2nd Law of ThermodynamicsWee Chuan YeanNo ratings yet
- Paper 2 2001Document20 pagesPaper 2 2001DisturbedPotatoNo ratings yet
- Corresponds To The Correct Answer. Write You Answers On The Space Before Each NumberDocument2 pagesCorresponds To The Correct Answer. Write You Answers On The Space Before Each NumberJESSA SUMAYANGNo ratings yet
- EarthingDocument4 pagesEarthingShirishNo ratings yet
- ErrtDocument101 pagesErrtMadhav KumarNo ratings yet
- Exercise 3 Rice Combine HarvesterDocument6 pagesExercise 3 Rice Combine Harvesterangelo lorenzo tamayoNo ratings yet
- 1 s2.0 S2352484722007922 MainDocument21 pages1 s2.0 S2352484722007922 MainEspinoza Payano Blas RussNo ratings yet
- Electronique - Audio - Microphone Valve PreampDocument7 pagesElectronique - Audio - Microphone Valve PreampkoukihamedNo ratings yet
- Phase Locked Loop PLLDocument35 pagesPhase Locked Loop PLLenzuekNo ratings yet
- 24-Fracturing Horizontal Wells PDFDocument37 pages24-Fracturing Horizontal Wells PDFmorcaronte08No ratings yet
- Rubik's Revenge (4x4x4)Document5 pagesRubik's Revenge (4x4x4)Febbi Abdul MuktiNo ratings yet
- Ben Vanderloo Qualifications Resume PDFDocument1 pageBen Vanderloo Qualifications Resume PDFASHVINI GAUTAMNo ratings yet
- Visual Design Elements and PrinciplesDocument9 pagesVisual Design Elements and PrinciplesgamerootNo ratings yet
- New MCQ EMD An DME 2020 F1 - FinalDocument4 pagesNew MCQ EMD An DME 2020 F1 - FinalSandipkumar Vhanakade100% (1)
- NES 838 Part2Document42 pagesNES 838 Part2QTESNo ratings yet
- 2 Manual de Proteccin Catdica Cathodic Protection HandbooDocument59 pages2 Manual de Proteccin Catdica Cathodic Protection Handboogoyote100% (1)