Professional Documents
Culture Documents
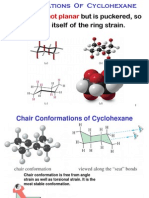
4 Cyclohexane: Axial Bond Equatorial Bond
Uploaded by
AminahNatashaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
4 Cyclohexane: Axial Bond Equatorial Bond
Uploaded by
AminahNatashaCopyright:
Available Formats
Ch 4 Cyclohexane
Cyclohexane
Aims
In this chapter you will first learn the conformational analysis of cycloalkanes with smaller
numbers of carbon atoms, from cyclopropane (C3) to cyclopentane (C5). Compared to alkanes with
equivalent numbers of carbon atoms, rotations about the single bond in smaller cycloalkanes are
much less flexible due to their cyclic structure.
The most important feature of cyclohexane is that all C-C-C have the tetrahedral angle
(10928). It has two chair and two boat conformers, and the two chair forms interconvert with each
other via boat forms. Many natural products contain cyclohexane-like structures as their components,
and this fact makes the study of cyclohexane particularly important.
In addition, you will learn how the stereochemistry of cyclohexane is widened by substituted
cyclohexanes. The conformational analysis of methyl- and dimethyl-cyclohexanes will be very good
examples to promote your study of this point.
New terms and concepts
cycloalkane
ring inversion
axial bond
equatorial bond
chair form
boat form
population of conformer
cis-trans isomerism (cyclic compounds)
1,3-diaxial interaction
Goals of this chapter
1
2
3
After you master this chapter successfully, you will be able to do the following:
to build up molecular models of cyclohexane in a chair or in a boat form. To compare the
relative stability of these two conformers based on the butane-gauche interaction.
to understand the ring inversion of cyclohexane and axial-equatorial exchange that
accompanies this inversion.
to explain the relative stability of conformers of substituted cyclohexanes in terms of the larger
stability of the equatorial conformer and the 1,3-diaxial interaction.
4.1 Cyclohexane; chair form and boat form
In the past, chemists believed that cycloalkanes, including cyclohexane, were planar like
benzene (see below). Since the C-C-C bond angle of the sp3 hybridized carbon chain is 10928,
cycloalkanes will suffer some strain if they are planar.
60o
90o
cyclopropane
cyclobutane
108o
cyclopentane
120o
cyclohexane
All cycloalkanes, except cyclopentane, become unstable because of this strain (Baeyers strain
theory). According to this theory, the most stable cycloalkane is cyclopentane, and cyclohexane is
1(13)
Ch 4 Cyclohexane
the next.
Although this theory could explain the high reactivity of cyclopropane, it gradually became
clear that cyclohexane would be more stable if a nonplanar structure is assumed. Under this
assumption, the C-C-C bond angle of cyclohexane could maintain the tetrahedral angle. Because
of the cyclic nature, the number of structures cyclohexane can take is much fewer than its acyclic
analog hexane. There is, however, a flip-flop of the ring as the rotation about the C-C bond of
cyclohexane takes place. The movement of the ring can most conveniently be defined by the torsion
angle made by the four successive carbon atoms (a butane unit). If we choose C1~C4 (1) as the
butane unit, the torsion angle can be defined as shown in 2. You should confirm the discussion above
by using a molecular model.
C1
C6
C5
C1
C4
C2
C3
C3
C2
C4
Q4.1
Are there any restrictions on the angle made by the four successive carbon atoms of
cyclohexane?
Q4.2
In order to understand the stereochemistry of cyclohexane, how many butane units should be
taken into consideration?
A4.1
In the case of butane, can be any value from 0 to 360 (or 180 to +180 ). In the case of
cyclohexane, there is a severe restriction on . The allowed value is ca. 90 as a maximum. Such a
conformation as ap is impossible.
If the torsion angle-energy diagram for C1~C4, butane unit of cyclohexane, is essentially
identical with that of butane (Fig. 2.4), then the staggered form 3 (which is referred to as butane
gauche unit) should be more stable than the eclipsed form 4.
H
H
C3
H
H
C4
C2
C3
C1
C1
C4
C2
HH
If a given cyclohexane contains six butane-gauche units, it should be the most stable conformer
when all bond angles are tetrahedral.
Q4.3
Draw a perspective view of cyclohexane with six butane-gauche units. Confirm, with the aid of
a molecular model, that all bond angles are tetrahedral.
A4.2
6 units; C1~C4, C2~C5, C3~C6, C4~C1, C5~C2 and C6~C3. With these all torsion angles in
2(13)
Ch 4 Cyclohexane
cyclohexane can be examined.
The conformer of cyclohexane 5 or 5 is called the chair form, which is the most stable one.
There is another conformer of cyclohexane 6 or 6 in which all bond angles are tetrahedral.
This conformer is called the boat form.
C1
C4
C2 C3
C5
C6
6'
Q4.4
Carry out the conformational analysis of 6 with reference to six butane units. Compare its
stability with the chair form.
A4.3
C4
C3
C2
C6
C5
C1
5
5'
Q4.5
Draw the Newman projections of 5 and 6 viewed along the direction of the C2-C3 and C6-C5
bonds
A4.4
In 6, there are two butane-eclipsed units (C1-C2-C3-C4 and C1-C6-C5-C4). The other butane units
are all staggered. Hence the energy difference between 5 and 6 can be approximated as the energy
difference between the butane in the gauche form and the butane in the eclipsed form, i.e., 21 x 2 =
42 kJ mol-1.
A4.5
H H
C4
H
H
C2
H
H
C2
H
H
C6
C1
H
H
H
C6
H H
C1
C4
HH
HH
Boat form 8
Chair form 7
It is rather tricky to portray two hydrogen atoms bonded to C1 and C4, which are beyond the
scope of the rules for drawing Newman projections. Here the flying-wedge drawing has been partly
employed.
S4.1 Cyclohexane: chair form and boat form.
chair form: consists of six butane-gauche units
boat form: consists of four butane-gauche units and two butane-eclipsed units
Now let us consider twelve hydrogen atoms of cyclohexane. Of the two C-H bonds for each
3(13)
Ch 4 Cyclohexane
carbon atom, one is perpendicular to the pseudo-plane made by the six carbon atoms, and the other
parallel to it. If we asume that cyclohexane resembles the earth, the chain of six carbon atoms may
be accepted as the equator. Based on this analogy, the C-H bond perpendicular to the equator (hence
parallel to the axis of the earth) is called an axial bond, and that parallel to the equator an
equatorial bond. An atom or a group of atoms bonded to the these bonds are referred to as axial
atoms (groups) or equatorial atoms (groups), respectively.
H
H
H
H
H
H
equatorial-Hydrogen 10
axial-Hydrogen 9
There are two ways to portray a cyclohexane in the perspective view. With one way, the C2-C3C5-C6 plane is horizontal (as in 5). This way is advantageous when making a comparison with a boat
form. With the other way, the C2-C3-C5-C6 plane is slightly tilted so that the axial bonds are
perpendicular (as in 9). This method is convenient when it is important to differentiate between the
axial and the equatorial.
4.2 Ring inversion of cyclohexane
The relation between a chair cyclohexane and a boat cyclohexane can be most conveniently
examined with the aid of a molecular model (Fig. 4.1). If you lift up the leg of 11 (i.e., C1) gently,
you will obtain a boat cyclohexane 12 (process i). Notice that rotation about the C-C bonds takes
place at the same time. This chair-boat interconversion is referred to as the ring inversion of
cyclohexane. This phenomenon is a type of the chemical exchange.
4
(i)
4
2
( viii )
11
(v)
12
( iv )
( vii )
( ii )
( vi )
5
4
( iii )
2
6
3
5
4
14
13
Figure 4.1 Inversion of a cyclohexane ring
In addition to process (i) (11 12), there is another process (v) having equal probability to
lead to the other boat form (11 14). The boat form 12 can be reconverted to 11 by pulling down
the bow (or the stern) (C1) (process viii) or converted to another chair 13 by pulling down the stern
4(13)
Ch 4 Cyclohexane
(or the bow) (C4) (process ii).
The second chair 13 can be converted to boat 14 by pulling down the support C1 (process iii) or
to boat 12 by lifting up the leg C4 (process vii). The boat form 14 can similarly be converted to two
chairs 11 or 13 (processes iv and vi). Since the energy barrier of the boat chair interconversion is
low, the process 11 12 13 14 takes place very easily at ambient temperature.
Q4.6
With the help of a molecular model of the carbon skeleton (no hydrogen atoms attached),
confirm that chair forms 11 and 13, and boat forms 12 and 14 are identical.
In the case of cyclohexane, the two chair forms 11 and 13, obtained by the ring inversion, were
proved identical as far as the shape of the carbon skeleton is concerned. Can you say, however, that
these two are strictly identical? Your belief will waver if you make a chair form with six axial
hydrogen atoms 15. By inversion notice that the second chair form 16 has six equatorial hydrogen
atoms rather than six axial hydrogen atoms. Similarly, a chair form with six equatorial hydrogen
atoms 17 is converted to the second chair form with six axial hydrogen atoms, 18.
H
H
H
H
H
H
H
15
16
H
H
H
H
H
H
17
18
Figure 4.2 Axial-equatorial exchange by ring inversion
Inversion of a chair form of cyclohexane will not reproduce the exactly identical chair form. If
we neglect hydrogen atoms, 11 and 13 are identical. However, 15 or 17 are not identical with 16 or
18 if hydrogen atoms are taken into consideration. If we can differentiate axial hydrogen atoms from
equatorial hydrogen atoms, cyclohexane 19 is not identical with cyclohexane 20 that is formed from
19 by ring inversion. This fact will be greatly clarified after you have studied substituted
cyclohexanes.
H
H
H
H
H
H
H
19
20
5(13)
Ch 4 Cyclohexane
S4.2 Ring inversion of cyclohexane
Ring inversion: a chair cyclohexane is converted to another chair form via a boat form.
S4.3 Axial, equatorial
axial: the direction perpendicular to the molecular plane: to be converted to equatorial by ring
inversion
equatorial; the direction parallel to the molecular plane: to be converted to axial by ring inversion
O
O
axial
equatorial
Q4.7
Which of the following three statements is acceptable as a description of cyclohexane?
(a) A 50:50 mixture of chair cyclohexane and boat cyclohexane
(b) Only as one of the chair forms
(c) A 50:50 mixture of two chair forms.
A4.6
You had better make two of 11, and convert these to 12 and 14 to compare.
A4.7
(c). Strictly speaking, however, a small amount of boat forms may coexist. The answer is
correct if we can assume that the population of the boat form is negligibly small.
There is another point to be considered. It is difficult to differentiate between (b) and (c). What
we can observe experimentally is that there is only one type of cyclohexane, that is, a chair
cyclohexane. At room temperature, the chair-chair interconversion takes place ca. 100,000 times s-1.
Even at a very low temperature (-67C), the frequency is ca. 100 times s-1.
4.3 Ring inversion of substituted cyclohexanes
Methylcyclohexane, which is obtained by replacing one of the hydrogen atoms of cyclohexane
with a methyl group, is a very intriguing compound. The two chair forms of methylcyclohexane are
not identical as is the case with cyclohexane itself. The equatorial methyl derivative 21 and the axial
methyl derivative 22 form a pair of stereoisomers (Fig. 4.3)
CH3
CH3
CH3
equatorial isomer 21
axial isomer 22
6(13)
Ch 4 Cyclohexane
Figure 4.3 Ring inversion of methylcyclohexane
The two chair forms of cyclohexane 11 and 13 are identical, but the two chair forms of
methylcyclohexane 21 and 22 are stereoisomers. Then, how would you characterize the difference
between 21 and 22? Return to cyclohexane and examine the twelve hydrogen atoms, preferably with
the aid of a molecular model. The interatomic distances among the three axial hydrogen atoms
bonded to C1, C3 and C5 are relatively short. If one of those three hydrogen atoms, e.g., the hydrogen
atom bonded to C1, is substituted by a large group such as methyl, the distance between the methyl
group and the axial hydrogen atoms bonded to C3 or C5 become much shorter and generate a large
steric interaction. This interaction is called the 1,3-diaxial interaction. This will make the energy of
the axial methylcyclohexane higher than that of the equatorial one.
S4.4 1,3-diaxial interaction
Steric hindrance (repulsion) between two axial substituents at C1 and C3 (or equivalent relation) of
chair cyclohexane.
c1
c5
c3
Q4.8
Explain the reason why axial methylcyclohexane 22 is unstable as compared with equatorial
methylcyclohexane 21 in terms of gauche and butane-eclipsed units. Estimate the energy difference
between 21and 22.
Q4.9
It was found by experiments that the free energy difference G between 21 and 22 is
7.5 kJ mol-1. Calculate the population of each isomer at (a) 27C and (b) -100C.
A4.8
The most significant difference is that the butane unit C3-C2-C1-CH3 is gauche for equatorial
methylcyclohexane 23 while it is antiperiplanar (trans) for 24. In other word, 23 has more energy
than 24 has by twice the difference between butane-gauche and butane- antiperiplanar units, i.e., 2 x
4 = 8 kJ mol-1.
Q4.10
t-Butylcyclohexane is obtained by substituting one of the hydrogen atoms of cyclohexane with
a bulky substituent t-butyl group (CH3)3C-. It is known that this compound exists solely as an
equatorial isomer, and that no ring inversion takes place. Why?
A4.9
The population of the axial isomer is (a) 4.8 %, (b) 0.55%. The lower the temperature, the less
the population of the unstable isomer is.
A4.10
Because of its bulkiness, the 1,3-diaxial interaction between the axial t-butyl group and the
atom (or group of atoms) in their relevant position is extremely large if the t-butyl group becomes
axial. In fact the axial t-butyl group at C1 will collides with the axial hydrogen atoms at C3 or C5.
7(13)
Ch 4 Cyclohexane
4.4 Substituted cyclohexanes
If there is no ring inversion of cyclohexane, or if the conformers of cyclohexane are averaged
by ring inversion, there is only one isomer for methylcyclohexane. In fact, ring inversion does exist,
and there are two isomers for methylcyclohexane, axial and equatorial isomers. Thus, the number of
isomers of substituted cyclohexanes will vary whether or not the averaging by the ring inversion is
taken into consideration.
If we admit averaging of cyclohexane ring by inversion, we need not worry about the chair and
boat forms of cyclohexane. Cyclohexane may be regarded as a planar molecule. Methylcyclohexane
can be written as 25 or 26. We may differentiate the upper and lower sides of the plane, but adopting
this procedure is to admit that one side is the axial side and the other the equatorial side.
Me
Me
25
26
If we admit averaging, there is one isomer 27 for 1,1-dimethylcyclohexane, and there are two
isomers 28 and 29 for 1,2-dimethylcyclohexane. 28 is the cis isomer and 29 is the trans isomer.
1,1-dimethyl-
1,2-dimethylMe
Me
Me
Me
Me
27
Me
cis
trans
28
29
Q4.11
When averaging by ring inversion is considered, how many isomers are there for 1,3- and
1,4-dimethylcyclohexane?
However, if averaging by ring inversion is not accepted, or if 21 and 22 of methylcyclohexane
are regarded as isomers, the situation will be different. The case of 1,1-dimethylcyclohexane is
rather special. For this compound, the number of isomers is one whether or not averaging is
accepted. By ring inversion, 27ae is converted to the identical molecule 27ea .
Me
Me
Me
Me
27ae
27ea
The situation is different for 1,2-dimethylcyclohexane. There are two trans isomers; one with
two equatorial methyl groups (34ee), which, by ring inversion, turn into the other isomer with two
axial methyl groups (34aa). 34ee and 34aa are different compounds in the same sense that 21 and 22
are different. Hence the population should not be 50:50, and it is expected that 34aa has a much
larger energy due to the double 1,3-diaxial interaction of two axial methyl groups, and the
equilibrium indicated below should largely be shifted to the left side.
8(13)
Ch 4 Cyclohexane
Me
Me
Me
Me
34ee
34aa
How about the cis isomer? How is 35ea related to 35ae? As for the conformation of methyl
groups is concerned, the two compounds have the same stereochemistry and hence the same energy.
The equilibrium below should be 50:50. Detailed analysis of the structure of two compounds with
the aid of a molecular model will reveal an important point, which will be discussed later.
Me
Me
Me
Me
35ae
35ea
Q4.12
Following the example of 34 and 35, develop a conformational analysis of cis- and trans-1,3dimethylcyclohexane 36 and 37.
A4.11
The results are similar with 1,2-dimethylcyclohexane. There are cis and trans isomers for each.
Me
Me
Me
Me
Me
Me
Me
Me
cis
trans
cis
trans
30
31
32
33
Q4.13
Develop a conformational analysis of cis- and trans-1,4-dimethylcyclohexane 38 and 39 in a
similar manner.
A4.12
In the case of the cis isomer 36, the equilibrium should be shifted to the 36ee. Wih the trans
isomer 37, conformation of the two methyl groups is equal for 37ea and 37ae. Hence the equilibrium
remains 50:50.
9(13)
Ch 4 Cyclohexane
Me
Me
Me
Me
36ee
36aa
Me
Me
Me
Me
37ae
37ea
Q4.14
Estimate the number of butane-gauche unit in each conformer of dimethylcyclohexane, and
discuss the relative stability using these data.
A4.13
In the case of the cis isomer 38, the equilibrium ea ae should be 50:50 while for the trans
isomer 39, the equilibrium 39ee 39aa should shift to the left.
Me
Me
Me
Me
38ea
38ae
Me
Me
Me
Me
39ee
39aa
The larger the number of butane-gauche units, the more unstable the conformer. Especially in
cis-1,3-isomer 36aa, an 1,3-diaxial interaction between two methyl groups is involved., and
expected to be the least stable among these nine stereoisomers.
Q4.15
Decalin C10H18 is the perhydro derivative of naphthalene, and consists in two cyclohexanes that
share one edge. If the cyclohexanes in decallin remain in a chair conformation, how many
stereoisomers are possible for decalin?
A4.14
trans-1,2 aa
ee
cis-1,2
ae = ea
4
1
3
trans-1,3 ae = ea
cis-1,3
aa
ee
2 trans-1,4 aa
4
ee
0 cis-1,1
ae = ea
4
0
2
Q4.16
There is some resemblance between the structure of decalin and that of 1,210(13)
Ch 4 Cyclohexane
dimethylcyclohexane. Confirm that the cis isomer 41 is susceptible to a ring inversion like that of
cis-1,2-dimethylcyclohexane, but that such an inversion is impossible for the trans isomer 40.
Explain.
A4.15
2. One is trans-decalin 40 and the other cis-decalin 41.It will be very instructive if you
carefully study, preferably with the aid of a molecular model, the stereochemistry of the fused part
(i.e., the shared edge), and compare the stereochemistry with that of 1,2-dimethylcyclohexane.
H
H
40
41
A4.16
The reason why the ring inversion is impossible for the trans isomer 40 is that it is an ee aa
inversion. The inversion of the cis isomer is an ea ae inversion. You should confirm these point
with a molecular model.
H
H
H
H
41a
41b
4.5 Cyclopropane, cyclobutane, cyclopentane
Among cycloalkanes CnH2n, all of these with C = 3~5, that is, cyclopropane 42, cyclobutane 43
and cyclopentane 44, have C-C-C bond angles that deviated from the tetrahedral angle.
Cyclopropane 43 has a high chemical reactivity, but its ring is rigid and there is no ring
inversion. As expected, 1,2-dimethylcyclopropane has two isomers, the cis isomer 45 and the trans
isomer 46.
CH2
CH2
CH2
CH2
CH2
CH2
CH2
42
CH2
CH2 CH2
43
44
Me
Me
Me
Me
45
46
11(13)
CH2
CH2
Ch 4 Cyclohexane
Q4.17
Write down the structure of all isomers of cyclopropane dicarboxylic acid C3H4(COOH)2.
For many years cyclobutane 43 was regarded as a square molecule. It is now known that at
least in the gas and liquid phases, 43 has a folded structure, and consequently a ring inversion 43a
43b, similar to that of cyclohexane, takes place.
43a
43b
Q4.18
Explain the ring inversion of trans- and cis-1,2-dimethylcyclobutane. Predict which direction
the equilibrium will shift for each isomer.
A4.17
The idea is much the same as with the case of dimethylcyclohexane: two methyl groups on the
same carbon atoms or on the different carbon atoms.
H
COOH
COOH
HOOC
COOH
H
HOOC
H COOH
H
cyclopropane 1,1dicarbocylic acid 47
trans -cyclopropane 1,2dicarbocylic acid 48
cis -cyclopropane 1,2dicarbocylic acid 49
A4.18
Me
trans isomer
Me
Me
50:50
Me
50a
50b
Me
Me
cis isomer
largely right
51a
Me
Me
51b
Cyclopentane 44 is also a nonplanar molecule, and like cyclobutane, it has a folded, envelope
type structure 52 or half-chair structure 53. For 52, there is no flip-flop of the triangle part, and
instead, a process in which the carbon at the tip of the triangle alternates one after the other. In the
envelope form, four carbon atoms, and in the half-chair form, three carbon atoms define a plane.
12(13)
Ch 4 Cyclohexane
52
53
13(13)
You might also like
- Conformational Analysis of CyclopentaneDocument11 pagesConformational Analysis of CyclopentaneNimra MalikNo ratings yet
- UntitledDocument46 pagesUntitled양우경No ratings yet
- Cycloalkane Ring Strain and ConformationsDocument18 pagesCycloalkane Ring Strain and ConformationsbgbscgvNo ratings yet
- Org Chem Text - Chapter 1 - Sec1-14 - 1-14Document3 pagesOrg Chem Text - Chapter 1 - Sec1-14 - 1-14TET2005No ratings yet
- Cyclohexane Conformational AnalysisDocument17 pagesCyclohexane Conformational AnalysiscclatrumNo ratings yet
- Module 5: Conformational analysis of alkanes and cyclohexanesDocument8 pagesModule 5: Conformational analysis of alkanes and cyclohexanesARMAN AKRAM BIN OMAR / UPMNo ratings yet
- Cyclic Aliphatic Compounds: NomenclatureDocument19 pagesCyclic Aliphatic Compounds: NomenclatureWinnie SantiagoNo ratings yet
- Ch221 Class 8 Chapter 4: Stereochemistry of Alkanes and Cycloalkanes, ContinuedDocument11 pagesCh221 Class 8 Chapter 4: Stereochemistry of Alkanes and Cycloalkanes, ContinuedSanjay Mani TripathiNo ratings yet
- Angle Strain Torsional Strain Ring StrainDocument6 pagesAngle Strain Torsional Strain Ring StrainchuasioklengNo ratings yet
- What Are Cyclo-Alkanes?Document6 pagesWhat Are Cyclo-Alkanes?gfgfccNo ratings yet
- Conformation & Conformational IsomersDocument4 pagesConformation & Conformational Isomerspulkit asatiNo ratings yet
- Chapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Document8 pagesChapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Amin JamjahNo ratings yet
- Chapter 3Document27 pagesChapter 3Siddarth PalletiNo ratings yet
- Cyclo-Alkanes: Pharmaceutical Organic Chemistry - Ii (Theory)Document6 pagesCyclo-Alkanes: Pharmaceutical Organic Chemistry - Ii (Theory)Tushar GiradkarNo ratings yet
- Cycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheoryDocument5 pagesCycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheorySayed AltamashNo ratings yet
- Visualizing the Conformations of MoleculesDocument6 pagesVisualizing the Conformations of MoleculesLakshmi VR Babu SyamalaNo ratings yet
- Chapter 4 With Video LinksDocument37 pagesChapter 4 With Video LinksDoom RefugeNo ratings yet
- Cycloalkanes are Nonplanar Due to Strain FactorsDocument29 pagesCycloalkanes are Nonplanar Due to Strain FactorsjuanNo ratings yet
- Cyclohexane Conformational AnalysisDocument1 pageCyclohexane Conformational Analysis휘승No ratings yet
- Lesson StructureDocument19 pagesLesson Structurer karthickNo ratings yet
- Conformations and Strains of Alkanes and CycloalkanesDocument92 pagesConformations and Strains of Alkanes and CycloalkanesMutia HadiyantiNo ratings yet
- Cyclo Al KanesDocument3 pagesCyclo Al KanesIbraim ZekirNo ratings yet
- DecalinsDocument25 pagesDecalinstessyNo ratings yet
- Structure of Cyclohexane (C5H12)Document11 pagesStructure of Cyclohexane (C5H12)Mohd Asim UddinNo ratings yet
- Chapter 4 Version 2Document8 pagesChapter 4 Version 2cwodNo ratings yet
- Structural Isomers - How Many Structures from a Simple FormulaDocument19 pagesStructural Isomers - How Many Structures from a Simple FormulaAmalia SillerNo ratings yet
- Long Exam 1Document8 pagesLong Exam 1Allan DNo ratings yet
- Chapter 3: Structure and Stereochemistry of AlkanesDocument42 pagesChapter 3: Structure and Stereochemistry of AlkanesKera Maria AllengerNo ratings yet
- Relative Stabilities of CycloakanesDocument8 pagesRelative Stabilities of CycloakanesKamal KishoreNo ratings yet
- 05 Orbitals Hybrid GeomDocument11 pages05 Orbitals Hybrid GeomKeri Gobin SamarooNo ratings yet
- Tutorial07b OrganicCycloalkanesDocument24 pagesTutorial07b OrganicCycloalkanesHana NisrinaNo ratings yet
- Chair & BoatDocument9 pagesChair & BoatpardeepbthNo ratings yet
- Valence Bonding TheoryDocument35 pagesValence Bonding TheoryHarihar Ghime GhimeNo ratings yet
- Conformational IsomersDocument8 pagesConformational Isomersshirodkar_16593No ratings yet
- PPT-5 Carbon NanostructuresDocument34 pagesPPT-5 Carbon NanostructuresMayank GautamNo ratings yet
- Chapter 4Document33 pagesChapter 4채종희No ratings yet
- SCH 102 Lecture Week 8 2018-9Document27 pagesSCH 102 Lecture Week 8 2018-9Radhika TopwalNo ratings yet
- Coulson and MoffittDocument2 pagesCoulson and MoffittTanya 60No ratings yet
- Organic Chemistry: M. R. Naimi-JamalDocument81 pagesOrganic Chemistry: M. R. Naimi-JamalHamidatun NisaNo ratings yet
- 4 CH241 CycloalkanesDocument73 pages4 CH241 CycloalkanesLuis CastilloNo ratings yet
- Ring formation through C5-OH attackDocument1 pageRing formation through C5-OH attackanonymousNo ratings yet
- 1515564176CHE P1 M17 EtextDocument15 pages1515564176CHE P1 M17 EtextElangovan NatarajanNo ratings yet
- Monocyclic DisubstitutedDocument3 pagesMonocyclic DisubstitutedPs SatchithNo ratings yet
- Conformational Analysis:: Chapter - 6 Stereochemistry NotesDocument12 pagesConformational Analysis:: Chapter - 6 Stereochemistry NotesMohit KambojNo ratings yet
- GeneralChem LS 25 PDFDocument25 pagesGeneralChem LS 25 PDFSunil NahataNo ratings yet
- Conformation AnalysisDocument41 pagesConformation AnalysisJoseNo ratings yet
- Crystal Structure IIDocument9 pagesCrystal Structure IIDeyanire LENo ratings yet
- StudentDocument31 pagesStudentnanaNo ratings yet
- Aromatic Compounds: NamingDocument13 pagesAromatic Compounds: NamingTanzimNo ratings yet
- Chapter 1 SolutionsDocument18 pagesChapter 1 Solutionsrka02No ratings yet
- Cyclo AlkanesDocument38 pagesCyclo Alkanesdinesh111180No ratings yet
- Shape of MoleculesDocument2 pagesShape of MoleculesJAYLEN TRACEYNo ratings yet
- Alkenes Unsaturated Double Bonds Geometrical Isomers Markownikoff RuleDocument2 pagesAlkenes Unsaturated Double Bonds Geometrical Isomers Markownikoff RuledanielmahsaNo ratings yet
- Alkynes and Compounds Containing C C GroupsDocument21 pagesAlkynes and Compounds Containing C C GroupsMani PillaiNo ratings yet
- CH 03Document39 pagesCH 03Anand MurugananthamNo ratings yet
- Combined OrganicDocument82 pagesCombined OrganicSachin KumarNo ratings yet
- T. Yildirim, O. Gulseren and S. Ciraci - Exohydrogenated Single-Wall Carbon NanotubesDocument5 pagesT. Yildirim, O. Gulseren and S. Ciraci - Exohydrogenated Single-Wall Carbon NanotubesKiomaxNo ratings yet
- Understanding Fischer ProjectionsDocument3 pagesUnderstanding Fischer Projectionspfeffer92No ratings yet
- Benzene and Other Aromatic Compounds: AromaticityDocument10 pagesBenzene and Other Aromatic Compounds: AromaticityMabelle DucusinNo ratings yet
- Watch and Clock Escapements A Complete Study in Theory and Practice of the Lever, Cylinder and Chronometer Escapements, Together with a Brief Account of the Origin and Evolution of the Escapement in HorologyFrom EverandWatch and Clock Escapements A Complete Study in Theory and Practice of the Lever, Cylinder and Chronometer Escapements, Together with a Brief Account of the Origin and Evolution of the Escapement in HorologyNo ratings yet
- Double Nourishing Lotion Moisturizes SkinDocument2 pagesDouble Nourishing Lotion Moisturizes SkinAminahNatashaNo ratings yet
- Perfect Comp.Document74 pagesPerfect Comp.AminahNatashaNo ratings yet
- Essay CompetitionDocument3 pagesEssay CompetitionAminahNatashaNo ratings yet
- Diabetis InspidusDocument40 pagesDiabetis InspidusAminahNatasha100% (1)
- Practice Chapter 3 Conformations of Alkanes and CycloalkanesDocument22 pagesPractice Chapter 3 Conformations of Alkanes and CycloalkanesAminahNatashaNo ratings yet
- Cyclohexane Ring StereochemistryDocument3 pagesCyclohexane Ring StereochemistrymadhavdhruvNo ratings yet
- Anatomyandphysiologyofurinarysystem 110509111246 Phpapp02Document38 pagesAnatomyandphysiologyofurinarysystem 110509111246 Phpapp02louradelNo ratings yet
- Peptic Ulcer Disease and H. PyloriDocument8 pagesPeptic Ulcer Disease and H. PyloriAminahNatashaNo ratings yet
- Peptic Ulcer Disease and H. PyloriDocument8 pagesPeptic Ulcer Disease and H. PyloriAminahNatashaNo ratings yet
- Pertemuan 1 - Introduction and FunctionalizationDocument57 pagesPertemuan 1 - Introduction and FunctionalizationGhina IzdiharNo ratings yet
- Alkyl Halides & Aryl Halides-02 - Solved ProblemsDocument13 pagesAlkyl Halides & Aryl Halides-02 - Solved ProblemsRaju SinghNo ratings yet
- Register of Pesticides March 26 2020 Active IngredientDocument26 pagesRegister of Pesticides March 26 2020 Active Ingredient20 Võ Xuân KỳNo ratings yet
- Zeta-Potential Measurements On Micro Bubbles GeneratedDocument9 pagesZeta-Potential Measurements On Micro Bubbles Generatedggg123789No ratings yet
- Exp 2 CO2 Absorption-Effect of Solvent RateDocument6 pagesExp 2 CO2 Absorption-Effect of Solvent RateLil Wayne JrNo ratings yet
- 3 - D Printer Carbon Fiber Reinforced WithDocument17 pages3 - D Printer Carbon Fiber Reinforced With19 CH 056 Vaishali VivekNo ratings yet
- Abiraterone Acetate TabletsDocument3 pagesAbiraterone Acetate TabletsRaquel BcNo ratings yet
- GBSA Oil Seals CatalogDocument4 pagesGBSA Oil Seals CatalogAnonymous ItzBhUGoiNo ratings yet
- Methods Book 2005: Raw SugarDocument3 pagesMethods Book 2005: Raw Sugariwan hermawanNo ratings yet
- 2015 Chandra - Basic Concepts of BiotechnologyDocument518 pages2015 Chandra - Basic Concepts of BiotechnologyPavani ReddyNo ratings yet
- Liquid Soap ProcessDocument3 pagesLiquid Soap ProcessAnnette40% (5)
- Shell Gadus S2 High Speed Coupling Grease Technical Data SheetDocument3 pagesShell Gadus S2 High Speed Coupling Grease Technical Data Sheetjuan felipe diazgranados santosNo ratings yet
- High-N Causes Cast Iron CracksDocument3 pagesHigh-N Causes Cast Iron CracksGh AbadiNo ratings yet
- Chemetall - (Data Sheet) Oakite 33Document3 pagesChemetall - (Data Sheet) Oakite 33Pubcrawl100% (1)
- Surfactants - Fundamental Properties and Applications (1) (Autosaved)Document28 pagesSurfactants - Fundamental Properties and Applications (1) (Autosaved)SHRAWAN KUMAR JAISWALNo ratings yet
- Thermal EOR Methods GuideDocument42 pagesThermal EOR Methods GuidePetroleum PetroleumNo ratings yet
- Iron and Steel Manufacturing ProcessDocument28 pagesIron and Steel Manufacturing ProcessMarnel Roy Mayor78% (32)
- Standardization of Acids and BasesDocument37 pagesStandardization of Acids and BasesNarayanRajNo ratings yet
- Periodic Table Chart A4 WebDocument2 pagesPeriodic Table Chart A4 WebvibinNo ratings yet
- 165 Gmaw Zug Asme (Imam Mustofa 3g) WPQDocument4 pages165 Gmaw Zug Asme (Imam Mustofa 3g) WPQMuhammad Fitransyah Syamsuar PutraNo ratings yet
- Astm G21 13Document5 pagesAstm G21 13AbinashBeheraNo ratings yet
- Chemistry 1 11 Q1 M1Document15 pagesChemistry 1 11 Q1 M1Jericho Avendaño100% (1)
- Lecture No 70, 71 Formulation of Semi Solids and Gels and JelliesDocument34 pagesLecture No 70, 71 Formulation of Semi Solids and Gels and JelliesAdinath ShirsatNo ratings yet
- Science 7 Parallel Test 2021-2022Document6 pagesScience 7 Parallel Test 2021-2022malifi ciadoNo ratings yet
- ICE Working Group Explores 2nd Generation Biofuels and Güssing Demo PlantDocument26 pagesICE Working Group Explores 2nd Generation Biofuels and Güssing Demo PlantTom MOt'sNo ratings yet
- Sliding TB 105mmDocument42 pagesSliding TB 105mmSaud AffanNo ratings yet
- Sample Not Drawn byDocument3 pagesSample Not Drawn bykarthikNo ratings yet
- PyrometallurgyDocument3 pagesPyrometallurgyMojalefa MotloutsiNo ratings yet
- Directory of Petrochemicals (Large and Medium Scale) Units - 2016-17Document59 pagesDirectory of Petrochemicals (Large and Medium Scale) Units - 2016-17AartiNo ratings yet
- Youngs Modulus For Different MaterialsDocument3 pagesYoungs Modulus For Different Materialschaitanya kulkarniNo ratings yet