Professional Documents
Culture Documents
Introduction To Oil and Gas Production PDF
Uploaded by
Sufian R EllabbadOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Introduction To Oil and Gas Production PDF
Uploaded by
Sufian R EllabbadCopyright:
Available Formats
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
TABLE OF CONTENT
Sec.
No.
Page
No.
SUBJECT
INTRODUCTION
DEVELOPMENT OF PETROLEUM
PETROLEUM RESERVOIR
12
WELLHEAD
32
THE WELL
38
WELL COMPLETION
43
WELL TREATMENT ( STIMULATION )
49
WORKOVER
54
WELL TESTING
56
10
FLUID TREATMENT
88
INTRODUCTION TO OIL&GAS PRODUCTION
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
1. INTRODUCTION
1.1 LIBYA CRUDE OIL PRODUCTION HISTORY:
The search for oil in Libya may be considered the shortest and most successful
in oil exploration history. The first concessions were granted in November
1955, first commercial discovery followed in May 1959 and production
commenced in October 1961.
Initial efforts were concentrated in the Fezzan encourage by Algerian successes
in the polignac basin near the Libyan border. Only minor quantities of oil were
found and after the commercial discovery at Dahra in May 1959 followed by
Zelten in June 1959, emphasis quickly changed to the SIRTE basin area.
In 1971 oil was found offshore in the Gulf of Gabes near the Tunisian border
but no commercial fields have been proved at that time.
1.2 SOC FIELD PRODUCTION OPERATING AREAS
1. ZELTEN PRODUCTION AREA:
This consists of the South Field production wells, and Ralah, Waha,
Wadi and Jebel field production. These fields produce crude oil with
associated gas which is piped to the main Zelten GOSPs for separation of the
oil, gas and water.
The water, after separation is dumped to the Zelten lakes. The oil is metered
and pumped to Brega via a 36" transmission line. The separated gas at various
pressure levels is compressed and separated from produced condensate. The
gas is dehydrated by contact with Tri-Ethylene Glycol (TEG) and enters a 36"
pipeline to Brega LNG plant.
Condensate produced in Zelten is metered and joins the gas in the 36" line to
Brega. A 30" line is also used for condensate storage when operating conditions
are abnormal.
Gas and condensate are also received in Zelten from the Waha Company.
The gas is mainly used for plant fuel and gas lift operations. The condensate is
piped to the 36" gas transmission line to Brega.
Zelten areas also include the North Field operations. These consist of Meghil,
Sorra and Lehib fields. Gas Lift and Wireline operations are also conducted in
the Zelten production fields
In the South field many natural flow and gas lifted wells are piped to field
manifolds and then to the GOSP via single flow-lines.
INTRODUCTION TO OIL&GAS PRODUCTION
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
2. JEBEL FIELD
This field lies about 40 Km south of Zelten and consists of crude oil &
associated gas producing wells. 24 wells are produced by Gas Lift and a further
7 wells by natural flow.
The pressure of the reservoir is maintained by injection of source water
which is taken from a nearby water table.
The source water is taken from the water table wells by down-hole pumps.
This water is then piped to three gas turbine driven pumps which then deliver it
to the injection wells at over 3000 PSI. Part of this pump discharge flow is
directed to drive the down-hole water-pump turbines. The produced fluids (gas,
oil & water) are piped to the field manifold and on to Zelten GOSP.
In addition to the above field manifolds, a number of nearby wells are piped
directly from the South field areas to the GOSP along individual flowlines.
Zelten GOSP consists of Five (5) separation plants - GOSP 1, 2, 3, 4 & 5.
(The GOSP receive the 3-phase flow from up to 227 wells). All except GOSP
4 are two-stage separation units. GOSP 4 is a medium pressure single stage
separation plant.
From the 2nd stage and GOSP 4 separators, the liquids are further separated in
two more stages - degassing boots and degassing (surge) tanks. The final stage
- the surge tanks - is a 3-phase separation process. Very low pressure surge gas,
oil and water are separated out. The gas is compressed and piped to the gas
plant. The oil is metered and pumped to Brega via the 36" Brega oil line and
the water is pumped to the Zelten lake.
3. MEGHIL FIELD
Meghil plant lies 9 Km north of Zelten and mainly produces nonassociated gas.
However, due to reservoir depletion, some oil is also now being produced from
two wells in East Meghil. This oil production is directed to Zelten GOSP.
The produced non- associated gas from 3 further wells, is fed into the 36"
Brega pipeline after being dehydrated by Glycol. The gas is also piped to Zelten
for back-up fuel supply and other utilities.
Produced condensate is piped to the 36" Brega line or into the 30" condensate
storage line.
INTRODUCTION TO OIL&GAS PRODUCTION
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
4. SORRA FIELD
Sorra plant lies 25 Km north of Zelten and a single well (PP 6) produces
non-associated gas. This gas is at about 1500 Psi. It is dehydrated by glycol
and mainly piped to Zelten to the gas lift gas distribution system. Some gas is
available to the 36" Brega line as required.
Produced condensate is piped to the 36" Brega line or into the 30" condensate
storage line.
5. LEHIB FIELD
Lehib is a crude oil & associated gas producing field. There are 9 wells
feeding the production to the Lehib plant. The plant consists of 1st & 2nd stage
GOSP and degassing boots and tanks for gas and liquid separation. Boot gas at
8 Psi is compressed in two stages to 45 and 180 Psi and added to the 2nd stage
GOSP gas. The 180 Psi 2nd stage gas is then compressed and added to the 1st
stage H.P. gas at about 425 Psi. The total gas flow is then passed to the
dehydration unit which uses glycol desiccant. Very low pressure gas from the
surge tanks is flared. The dry gas and produced condensate is piped to the 36"
line to Brega and the produced oil to the 36" Brega oil line.
6. HATEIBA
The Hateiba plant receives the flow of Non-associated gas from up to 12
wells.
The plant has six trains of Low Temperature Separation units (LTS).
Separation of water depends on the auto-refrigeration caused by pressure drop
across chokes which produces HYDRATES. The hydrates are liquefied using
hot water circulation which causes the water and condensate to separate. The
water is drained away while the condensate is piped to the 36"gas pipeline to
Brega. The gas is piped to a 30" pipeline to the Petrochemical complex for
ammonia and methanol production with spill-over into the 36" when required.
7. ATTAHADY FIELD
This is the most recently developed field. The field is situated in Sirte basin
about 90 km south of the Brega industrial complex.
At present there are 28 wells, though more may be added in the future.
Attahaddy plant is designed to receive raw gas from up to 45 gas wells.
INTRODUCTION TO OIL&GAS PRODUCTION
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
The plant consists of two identical trains; each is capable of receiving and
processing 175 MMSCF/D of raw gas from the gas wells.
The field produced the following:
270 MMSCF/D of export gas. (Approximately)
30000 barrels per day of condensate. (Approximately)
BASICALLY THE GAS CONDITIONING FACILITY CONSISTS OF THE
FOLLOWING UNITS:
Primary Separation to separate condensate and water from
the gas.
Acid Gas Removal Unit to reduce the CO2 content to less
than 2% by volume.
Gas dehydration.
Metering of both the gas and condensate produced.
Mercury removal unit. (For gas production only)
The gas and condensate production from plant are transported
via two separate trunk lines (30" and 12" respectively) to be
tied in at Km 91.5, each trunk line is tied in to a separate
transmission line to send production to Brega.
Gas analysis shows no presence of H2S in raw gas entering the Attahaddy
gas plant.
8. SAHL
The Sahl plant consists of 12 wells producing non-associated gas. The gas
is piped into the facility and is cooled to condense water and condensate. The
gas is then passed through Amine contactors where H2S is absorbed by MethylDi-ethanol amine (MDEA). The sweet gas is then passed through a glycol
contactor for dehydration and then metered and piped to the 30" gas
transmission line to the Petrochemical complex with a spillover control to the
36" line to the LNG plant. The MDEA and glycol are regenerated by
distillation with heat supplied by a Hot-oil circulation system at 500 0F.
9. ASSAMOUD
Assamoud has 9 wells producing non-associated gas which is dehydrated
by glycol and passed into the 30" Sahl gas line to the Petrochemical pipeline
and/or the LNG plant pipeline. Sahl and Assamoud condensate is piped into the
36" or 30" pipelines at Km 90.
INTRODUCTION TO OIL&GAS PRODUCTION
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
10. RAGUBA
Raguba field is a part of SOC operations, it is located 180KM to the south
of EL - BREGA .
It was established in 1959 and was given code E and concession 20 . Its
producing method is natural flow and gas lift. In 1963 the field starts producing
Oil with associated Gas in the rate of 150000 B/D Oil.
The total wells drilled in Raguba Field Area are 103 wells , these wells
consists of :
1. Natural Flow Wells
2. Gas Lift Wells
3. Gas Wells
The main producing Zones in Raguba Field are:
1. Waha &Gargaf Formation, which is Oil Zone..
2. Mabruk formation, which is Gas Zone.
The oil associated with the gas and water are produced from wells with
different pressure amounts. They are gathered in a manifold at GOSP.
The Gas, Oil and Water are separated in GOSP plant, the Water is drained away
to the lake, and the oil is metered and pumped via 20 inch transfer line into the
36-inch. oil transmission line to Brega , and the gas is compressed and
dehydrated by glycol injection and piped to the 36 inch. Brega gas line .
Gas lift facilities are also used in Raguba field .
INTRODUCTION TO OIL&GAS PRODUCTION
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
2. DEVELOPMENT OF PETROLEUM
2. 1. OCCURRENCE.
There are two generally accepted theories to explain the origin of oil, the
organic and inorganic theories. The inorganic theory holds that hydrogen and
carbon were brought together under great pressure and temperature deep in the
earth to form oil and gas, which then found its way through porous rocks to
collect in natural trap in the underground formation of the earth.
The organic theory, on the other hand presumes that both the hydrogen
and the carbon that make up petroleum came from plants and animals living on
land and in the sea, It is thought that this organic material probably was mostly
former sea and swamp life rather than true land life. Also, it possibly was
mostly the very small, rather than the larger forms of life.
ORGANIC THEORY OF ORIGIN
The organic theory is the explanation most accepted by scientists. The
argument for the organic theory is found in the evidence left in underground
rocks of the earth by ancient seas which through great periods of time have
covered much of the present land area.
Throughout millions of years rivers flowed down to these seas and
carried with them great volumes of mud and sand to be spread out by currents
and tides over the sea bottom near the gradually charging shorelines. Each day
through the sands of new deposits were distributed layer upon layer over the sea
floors.
Under the increasing weight of the accumulating new beds, the ocean
floors slowly sank, so that there was built up the thick series of mud and sand
layers. These sea bottom mud and sands were squeezed by the weight of
thousands of feet of overlying layers of mud and sands and eventually become
what are called sedimentary rooks (the sandstones and shale, and the lime
stones and dolomite).
The large amount of very small plants and animal life, which came into
the sea with the river silts and mud. And possibility much great volume of
similar tiny marine life remains already on the sea bottom is the principal source
material of petroleum according to the organic theory.
INTRODUCTION TO OIL&GAS PRODUCTION
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
These small organisms, dying, dying and settling to the bottom were later
buried by silt, sealed from the air and further protected from ordinary decay by
salty seawater.
As time went on, pressure, temperature, Bacteria and reactions caused
these remains to change into oil and natural gas.
2. 2. GENERAL COMPOSITION:
Petroleum is a mixture of naturally occurring hydrocarbons, which may
exist in the solid, liquid or gaseous states, depending upon the conditions of
pressure, and temperature to which it subjected.
Virtually all petroleum is produced from the earth in either liquid or
gaseous form and commonly, these materials are referred to as either crude oil
or natural gas, depending upon the state of the hydrocarbon mixture.
Crude oil is the material most sought after of these naturally occurring
hydrocarbons, but natural gas is commonly produced along with the crude oil.
Petroleum consists chemically of approximately 11 to 13 wt% hydrogen and 84
to 87 wt% carbon.
Traces of oxygen, sulfur, nitrogen and helium may be found as impurities
in crude petroleum, hydrogen and carbon combined in various molecular sizes
to form a family of hydrocarbons.
Each hydrocarbon is named for the number of carbon and hydrogen
atoms in its molecule. Hydrocarbons are named using conventional chemical
standards, which are based on Latin language. For example the prefix (meth) in
the name methane indicates the molecules contain one carbon atom.
Fig.1 is a simplified portrayal of a molecule of methane. Table 1 shows
the names, compositions and molecular weight of the first 12 hydrocarbon
family compounds arranged in order of increasing molecular weight.
INTRODUCTION TO OIL&GAS PRODUCTION
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Fig.1 A methane molecule is composed of four hydrogen atoms, which
are bonded to one atom.
Table.1 Physical constants of hydrocarbons and other compounds
INTRODUCTION TO OIL&GAS PRODUCTION
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Hydrocarbons take various forms called states, which depend on pressure
and temperature. At the pressure and temperature beneath the earths surface,
the hydrocarbons methane through pentane take the form of gas (the mixture of
them called produced gas), hexane and heavier hydrocarbons are liquid. (Their
mixture is called crude oil). At atmospheric pressure and room temperature,
methane through butane occurs as gas, pentane and heavier hydrocarbons occur
as liquids.
Hydrocarbon deposits often contain acid gases such as carbon dioxide
and hydrogen sulfide. Some liquid petroleum deposits contain sulfur
compounds also.
2. 3. Physical properties:
Hydrocarbons, like all other forms of matter, have physical properties.
Some of the most important physical properties are given in table 1.
Other physical properties commonly considered are color, refractive index,
odor, freezing point & flash point.
The density of a substance is a measurement of the weight of that
substance. The densities given in table 1 are for the liquid state. As expected,
the density increases as the number of carbon and hydrogen atoms in
hydrocarbon molecules increases.
The specific gravity of liquids is defined as the ratio of the density of the
liquid to the density of water, both at specified of pressure and temperature. The
specific gravity of crude oils ranges from about 0.75 to 1.01. In petroleum
industry, a certain scale which is preferred to as the API (American petroleum
institute) scale is used. This scale relates the specific gravity through a modulus
to an expression of density called API gravity. Expressed mathematically
=
or
API =
141.5
131.5 + API
141.5
131.5
Where is the specific gravity and API is the API gravity.
INTRODUCTION TO OIL&GAS PRODUCTION
10
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Since the density of the liquid is a function of temperature and pressure, it
is necessary to designate standard conditions for reporting specific gravity and
API gravity. The petroleum industry has adopted as standards a temperature of
a 60F and atmospheric pressure.
Viscosity is a measurement of the fluidity of liquids. A viscosity
measurement indicates the resistance the liquid offers to change its shape.
Viscosity is measured in centipoises. The viscosity of crude oil ranges from
about 0.3 centipoises for a gas saturated oil at reservoir conditions to about 1000
centipoises for gas-free crude oil at atmospheric pressure and 100 F.
Other physical properties of liquid petroleum are frequently correlated
with API gravity and viscosity. Gas gravity is widely use to characterize natural
gases. Gas gravity is the ratio of the density of a gas at atmospheric pressure and
temperature to the density of air at the same condition of pressure and
temperature. Gas gravities for natural gases range from 0.6 to 1.1, depending on
the relative concentration of the heavier hydrocarbons present in the gas.
Natural gases are also described as dry or wet gases depending on the
amount of condensable hydrocarbons presented in the mixture. Pentane and
heavier components are considered to be condensable hydrocarbons, as at
atmospheric pressure and temperature pure pentane exists as a liquid. The
higher hydrocarbons - methane, ethane, propane and butane exist in the
gaseous state at atmospheric conditions.
INTRODUCTION TO OIL&GAS PRODUCTION
11
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3. PETROLEUM RESERVOIRS
3. 1. TYPES OF RESERVOIR ROCK
Petroleum is almost invariably found in sedimentary rock. As layer upon
layer of sediment and animal / vegetable deposits were buried, they were
compressed by the weight of the layers above. Pressure, heat and other factors
(chemical, bacterial and radioactive) changed the organic material into todays
natural gas and oil.
Sedimentary rocks may occurs as loose mud and sand or be hard and
compacted, depending on how much pressure has been exerted and how old the
layers are.
Two types of sedimentary rock of interest in the exploration and
development of petroleum reservoir are carbonate and clastic rocks. Carbonate
rocks were formed when the high temperature and pressure inside the earth
causing inorganic and some organic material to combine into an inorganic rock.
Limestone and dolomite are carbonate rocks in which petroleum is often
discovered.
Clastic rocks were formed when particles of older rocks (particles such as
sand and clay) were cemented together. The cementing material may be clay or
perhaps organic material that was connected to inorganic material.
Sand particles are comparatively large and irregularly shaped remnants of
older rocks which were worn by wind and wave action. When irregularly
shaped and sized sand particles were cemented together, avoid spaces were
created between them. Rock formed this way is called sandstone.
Clay particles are usually extremely small and more regularly shaped.
When these particles are commented, few void spaces exit. Rock formed by
cemented clay particles is called shale and is often almost impervious.
The principle difference between clastic and carbonate rocks is in the
chemical composition of rocks. Carbonate rocks may be dissolved easily by
most acids, while clastic rocks are inert to the action of acid.
INTRODUCTION TO OIL&GAS PRODUCTION
12
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3. 2. RESERVOIR ROCKS CHARACTERISTICS:
For rock to contain petroleum and later allow petroleum to flow, it must
have certain physical characteristics. The most important characteristics are
porosity and permeability.
Porosity:
If the rock has openings, voids and spaces in which liquids and gases may
be stored, it is said to be porous. For a given volume of rock, the ratio of the
pore space to the total volume of the rock is called porosity. The porosity may
be expressed as a decimal fraction but is most often expressed as a percentage.
For example, if 100 cubic feet of rock contains many tiny pores and
spaces, which together have a volume of 10 cubic feet, the porosity of the rock
is 10%. To visualize the concept, imagine a box full of balls of equal size
stacked on top of each other. The arrangement and size of the spheres affects
the porosity as shown in fig.2.
Actual porosity in a reservoir may range from 3% to 40% depending on the
difference in the sizes of the grains and the way they joint together.
Fig. 2 The arrangement and size of the spheres affects the porosity.
Cubic arrangement can have a maximum porosity of 47.6%.
A rhombohedral arrangement can yield a porosity of 25.9%
INTRODUCTION TO OIL&GAS PRODUCTION
13
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
The pores in most porous rock are very small, often about the diameter of
a pencil lead or smaller. Large pores are called vugs. Remember that petroleum
is not found in large open spaces like tanks, but rather in small pores that
difficult to see with the naked eye.
Even if rock has space for liquid and gas, these fluids could never be
recovered if the individual spaces were not connected. Fig.3 indicates the path
oil and gas might take to reach a well.
Fig.3 The flow of petroleum in a reservoir proceeds from one pore to the
next unit the fluid reaches the wellbore
The connection between pores may be in the form of tiny channels,
cracks in the rock, or other pores. As the size of these connection increases, so
does the ease with which liquid and gas may pass from one pore to another. As
the connection size decreases, it becomes more difficult for liquid and gas to
move from one pore to another.
Permeability:
Is the rock characteristic that describes the ease with which liquid and gas
may move through porous rock. High permeability indicates relative ease in
moving through rock, low permeability indicate the opposite. The porosity of
rock, the pore size of connecting channels between pores are all related to
permeability, which is measured in the unit called the darcy.
However, in many rocks the permeability is so low that it is more
convenient to express it in the millidarcy (onethousandth of one darcy).
INTRODUCTION TO OIL&GAS PRODUCTION
14
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
In most reservoirs, the average permeability is less than one darcy. Liquid
and gas pass through rock volumes as a result of a difference in pressure. Oil
and gas flow from high-pressure areas to low-pressure areas, and permeability is
a way of expressing the resistance (or lack of it) that rock offers to flow. The
material composing porous rock is called the rock matrix, i.e., the inorganic
material of the structure, not the pores or connecting channels.
An interesting characteristic of the rock matrix is its compressibility. The
rock matrix, compressed by millions of tons of rock above, will compress
slightly, perhaps a few inches. Later, as pressure in the reservoir is reduced, the
rock matrix may expand a few thousandths of an inch.
3. 3. RESERVOIR FORMATIONS:
A reservoir is simply a volume of porous rock. Rock that is not porous
cannot store any thing and so cannot be a reservoir. Not all reservoirs contain
petroleum. If the rock formation was not covered by an impervious or
impermeable layer such as shale, petroleum may have never formed or it may
moved to another reservoir over millions of years. Therefore, to contain
petroleum, a reservoir must be bounded on top by a layer of impermeable rock,
which traps the petroleum products.
Petroleum reservoirs are massive rock structures that may be hundreds of
feet thick and cover several square miles. Or, they may be only 10 feet thick and
cover only a few thousand square feet. Depending on porosity and reservoir
size, the amount of petroleum contained may be several thousand to several
million cubic feet.
Before the reservoir can form, four qualifications must be met:
1. A source bed must exist. This is the original layer that contained
the potentially petroleum producing organisms and was submitted
to the proper temperature and pressure.
2. The petroleum from the source rock must accumulate in a reservoir
rock the rock filled with holes and pores so the oil and gas can be
collected (porosity).
3. The reservoir rocks pores must be interconnected so the oil or gas
can move within the rock (permeability).
INTRODUCTION TO OIL&GAS PRODUCTION
15
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
4. Some kind of closure or entrapment must exist that will prevent
further upward movement of the fluids and will allow them to
collect in one area.
If any one of these four points or characteristics is not present in an
underground formation, a petroleum reservoir will not exist.
3. 4. RESERVOIR CONTENTS:
The principle reservoir fluids are oil, water and gas. Oil and water are
liquids, natural gas is a gas, but all three are fluids because they flow. Most
reservoirs that contain liquids also contain gases. Conversely, some reservoirs
that contain gases also contain some liquids.
3.4.1 WATER:
Almost invariably, reservoirs that contain petroleum also contain some
connate water. Connate water may well have been the water originally
associated with the organic material or it may have moved into the reservoir
from some other location.
This water is dispersed throughout the reservoir, although it tends to
accumulate more near the bottom of the reservoir because it is heavier than
hydrocarbons. This separation is never complete, though, some water always
remains with the oil and gas.
A reservoir or part of reservoir that contains only water is called an
aquifer. The water that occurs at the bottom of the reservoir is called bottom
water. The water that collects at the perimeter of the reservoir is called edge
water. The bottom water and the edge water are important factors in getting the
oil out of the ground and up the well.
The water associated with petroleum reservoirs almost always contains
some salt. Thus, petroleum is usually associated with salty or brackish water.
3.4.2 OIL:
Oil is lighter than water, so it tends to accumulate above the water layer.
When oil and water move through a pore, a thin coat of either oil or water will
be left in the pore. In many cases the melting liquid, or wetting phase, as it is
called, is left in the pore permanently. If the wetting phase is oil, the oil is left
permanently in the reservoir, that is, this significant volume is permanently left
behind after a petroleum deposit has been depleted.
INTRODUCTION TO OIL&GAS PRODUCTION
16
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
If the wetting phase is water as shown in fig.4 no real lose is suffered by
leaving the water behind. However, because the wetting liquid occupies
volume, the effective porosity of the reservoir is reduced.
Fig.4 Wetting water clings to the rocks, making them water wet
3.4.3. GAS:
Natural gas is always associated with oil in a reservoir. Given proper
conditions of pressure and temperature, the substance will stay in solution
(dissolved) in the oil. When the temperature and pressure are lowered, the gas
comes out of solution. Free gas (gas not in solution) tends to accumulate near
the top of the reservoir.
3. 5. FLUID MIGRATION IN RESERVOIR:
Oil and gas are usually not found where they were formed. Source rocks,
in which the original tissue from the living organisms was trapped, are finegrained and relatively impervious. They rarely hold oil and gas in a thing but
small quantities. Instead, the oil and gas move from the source rock upward
toward the surface.
Some escapes through faults to surface. Large quantities of the oil and
gas never reach the surface, however. They migrate upward until they reach an
impermeable barrier or cap rock and accumulate in place to form a reservoir.
This barrier and the resulting reservoir are called trap. It is possible gas and oil
may migrate from a reservoir that had no trapping structure into a reservoir that
did have such a structure. Thus, petroleum may move from its original reservoir
into a completely different reservoir. Another possibility is that free gas may
migrate out of a reservoir but the oil and solution gas remain. In such cases, it
may appear that two petroleum formations developed.
INTRODUCTION TO OIL&GAS PRODUCTION
17
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
The upward movement of oil and gas is also accompanied by a separation
of the oil, gas and water. The oil and gas rise as they displace the seawater that
originally filled the pore spaces of the sedimentary rock. As they reach
impenetrable barrier, the materials separate, gas is found in the highest part, the
oil, and salt water at the bottom.
3. 6. TYPES OF RESERVOIRS:
There are many different shapes sizes and types of geologic structures or
traps that provide reservoirs in which petroleum is found. Perhaps the simplest
means of classifying reservoirs is to group them according to the conditions
causing their occurrence, as in the six divisions following:
3.6.1 DOMES AND ANTICLINES:
Reservoirs formed by folding of the rock layers or strata usually have the
shape of structural domes or anticlines as shown in fig.5.
Fig.5 Oil, gas and water tend to separate into three layers
These traps were filled by upward migration or movement of oil or gas
(or both) through the porous strata or beds to the location of the trap. Here
further movement was arrested by a combination of the form of the structure
and the seal or cap rock provided by the formation covering the structure. It is
common to find traps, which apparently are big enough to hold larger quantities
of oil or gas that have accumulated, and which remain partially filled with salt
water underneath the oil or gas.
INTRODUCTION TO OIL&GAS PRODUCTION
18
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3.6.2. FAULT TRAPS:
Reservoir formed by breaking or shearing and offsetting of strata (called
faulting) are illustrated in fig.6.
Fig.6 A fault is a crack in the earth along which layers move
The escape of oil from such trap is prevented by non-porous rocks that
have moved into a position opposite the porous petroleum-bearing formation.
The oil is confined in traps of this type because of the tilt of the rock layers and
faulting.
3.6,3. UNCONFORMITIES:
The type of reservoir formed as a result of an unconformity is shown in fig.7.
Fig.7 Oil is trapped under an unconformity in this illustration.
Here the upward movement of oil has been halted by the impermeable
cap rock laid down across the cut-off (possibly by water or wind erosion)
surfaces of the lower beds.
INTRODUCTION TO OIL&GAS PRODUCTION
19
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3.6.4. DOME AND PLUG TRAPS:
Accumulation of oil are found in porous formations on or surrounding
great plugs or masses of salt or serpentine rock that have pierced, deformed, or
lifted the overlying rock layers. Some typical accumulations of this type are
shown in fig.8, illustrating a non-porous salt mass that has formed dome-shaped
traps in overlying and surrounding porous rocks.
Fig.8 Salt domes often deform overlying rocks to form traps like the one shown
here
Fig.9 illustrates a porous serpentine plug that has formed a reservoir within
itself by intruding into non-porous surrounding formations.
Fig.9 Serpentine plugs sometimes form reservoirs similar to that shown here.
INTRODUCTION TO OIL&GAS PRODUCTION
20
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3.6.5. LENSES TYPE TRAPS:
Another type of reservoir is one that is sealed in its upper regions by
abrupt changes in the amount of connected pore space within a formation. This
may be caused in the case of sandstones by irregular depositing of sand and
shale at the time the formation was laid down.
In these cases, oil is confined within porous parts of the rock by the nonporous parts of the rock surrounding it. A sound reservoir of this type is shown
in fig.10. A limestone reservoir of this type is shown in fig.11.
Fig.10 Bodies of sand in a non-porous formation often form traps like this one
Fig.11 Limestone formations often have areas of high porosity that form traps
like this one.
INTRODUCTION TO OIL&GAS PRODUCTION
21
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3. 7. RESERVOIR DRIVE MECHANISMS:
Liquid and gas are moved through reservoir as a result of pressure
difference in the reservoir. They move from an area of high pressure to an area
of low pressure. When a well is drilled into a reservoir, the wellbore becomes an
area of low pressure. If there is a path for flow liquid and gas move to the
wellbore see fig.12.
Fig.12 A wellbore, which penetrates a reservoir, is an area of the low pressure
towards which fluids will move
Pressure differences cause one fluid to move and push another fluid in
front of it. The action of one fluid pushing another is called a drive mechanism.
Through the producing life times of some reservoirs, more than one drive
mechanism is responsible for the movement of fluids.
3.7.1. SOLUTION GAS DRIVE:
If the formation contains oil and gas, the gas dissolved in the oil forms
bubbles. As the pressure in the reservoir is reduced, the gas emerges and
expands, driving the oil through the reservoir towards the wells and assisting in
lifting it to the surface.
This oil production process is illustrated in fig.13 and is generally
considered the least effect type, yielding maximum recoveries indicated
between 15 to 20 percent of the oil originally contained in the reservoir.
INTRODUCTION TO OIL&GAS PRODUCTION
22
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Fig.13 Solution-gas drive reservoir
As liquid is removed from a reservoir, reservoir pressure declines and the
gas-saturated oil expands. As reservoir pressure decreases, some solution gas
breaks from the oil to become free gas. Because of the permeability involved, it
takes a long time for this gas to bubble to the top of the reservoir to become a
gas cap.
A feature of solution gas expansion drive is the rate at which gas flows
from the well. The gas production rate will often decline during the producing
life of a reservoir. The primary reason for this effect is that as the pressure
drops, more and more gas becomes free gas that is trapped and remains in the
reservoir.
INTRODUCTION TO OIL&GAS PRODUCTION
23
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3.7.2. GAS CAP DRIVE:
In many cases, there is more gas in a reservoir than the oil can retain in solution.
This extra gas, since it is lighter than oil, rises to the top of the reservoir and
forms a cap (Fig.14).
Fig.14 Gas-cap drive reservoir
In gas-cap drive reservoir, the well is drilled into the oil zone. As the oil
begin to flow into the wellbore, the pressure decreases and the expanding gas
pushes down on the oil, forcing the oil up the wellbore as the gas attempts to
make its way up the well also.
The gas-cap drive production process is substantially more effective than
solution gas drive alone, yielding indicated oil recoveries range from 25 to 50
percent.
A characteristic of the gas cap expansion drive mechanism is that as
liquids are removed from a reservoir, the rate at which gas moves to the well
stays at a constant rate because nothing prevents the flow of this gas. At some
point, some of the free gas from the gas cap may begin to enter the well.
INTRODUCTION TO OIL&GAS PRODUCTION
24
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3.7.3. WATER DRIVE:
In the previously mentioned mechanisms, water, like oil, was pushed to a
well if a flow path existed. This condition exists only in a closed reservoir
where there is no path for water to leave. When the water is a part of an aquifer,
it is possible for the water to be pushing other fluids. This situation is called a
water drive, as illustrated in fig.15.
Fig.15 Water-drive reservoir
INTRODUCTION TO OIL&GAS PRODUCTION
25
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
There are two ways water drive can be the primary drive mechanism in a
reservoir.
a. Water is only slightly compressible. Even so, if the volume of an
aquifer is hundreds or thousands of times the size of a petroleum
reservoir, the water causes a significant pressure in the petroleum
reservoir. When large aquifers lie under petroleum reservoir, the
compressed water in the aquifer will exert pressure against the
fluids in the reservoir and causes the fluid to move to the wellbore.
This type of water drive characterized by very low gas production
rates. However, the reservoir pressure dose decline. Recovery from
this type of drive mechanism is low, about 10-15%.
b. Another water drive mechanism occurs when an aquifer receives
its driving energy from an external source. This source may be
another undiscovered petroleum reservoir, a reservoir containing a
gas on an outcrop of the aquifer formation.
Fig.16 shows a discovered petroleum reservoir driven by an aquifer connected
to another reservoir.
Fig.16 Gas cap expansion in a secondary reservoir can push oil by first exerting
pressure on an aquifer
Gas expansion in the secondary reservoir pushed the water through the
aquifer. In turn, the water pushed the fluids in the petroleum reservoir upward
and into a wellbore as shown in fig.17.
INTRODUCTION TO OIL&GAS PRODUCTION
26
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Fig.17 As the gas cap in a secondary reservoir expands, water is pushed
out of the secondary reservoir, but water is pushed up into the
petroleum reservoir.
Fig.18 shows a petroleum reservoir driven by an aquifer connected to an
outcrop. The aquifer is filled with water to the surface of the earth. The weight
of the water in the aquifer above the reservoir exerts pressure on the petroleum
reservoir.
Fig.18 The action of gravity on water in an outcropping formation can exert
pressure on a petroleum reservoir and form a bottom water drive
In active water drive mechanism water moves upward into a petroleum
reservoir as oil and gas are removed. Since the amount of material in the
reservoir remains constant, there is little or no change in reservoir pressure
during the productive life of the reservoir.
This lack of pressure change is characteristic of a water drive mechanism.
The rate at which gas flows to the well is also constant during the life of the
reservoir. Since there is little change in pressure, the ratio of solution or free gas
to oil does not change.
INTRODUCTION TO OIL&GAS PRODUCTION
27
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Another characteristic of a water drive mechanism is related to the rate at
which water flows into a well. Late in the life of water drive mechanism as the
levels of water in the reservoir rise more and more water begins to enter the
wellbore. Recovery with a water drive mechanism is about 25-30%.
3.7.4. COMBINATION DRIVE MECHANISMS:
Rarely is a petroleum reservoir discovered which has only one drive
mechanism acting on it. One mechanism is usually the predominant energy
source, and the other mechanism is the secondary energy sources. Also as the
productive life of a reservoir continues, another drive mechanism may develop
or become predominant.
For example, some reservoirs may be driven by a gas cap and an aquifer.
Another example of drive mechanism combination occurs when a reservoir has
both a gas cap and significant volume of solution gas. In the early stages of
reservoir life the expansion of the gas cap furnishes most of the drive energy
because the pressure has not yet changed enough for there to be a sufficient
change in solution gas volume.
However, as the life of the reservoir continues, solution gas expansion
becomes important in supplying motivating energy.
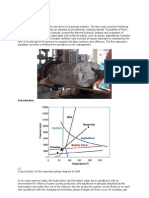
3. 8. RESERVOIR DEPLETION:
It was said that a reservoir was depleted when no more oil and gas could
be recovered. This means that the reservoir energy has been used to remove as
much oil and gas from a reservoir as possible.
3.8.1. PRIMARY RESERVOIR DEPLETION:
For many years the reservoir was operated until naturally occurring
reservoir conditions would no longer support the removal of oil and gas. This
method of producing a reservoir is referred to as primary recovery.
For the average reservoir, wells are seldom operated long enough to
completely deplete the reservoir during primary recovery before supplemental
techniques are implemented. However by monitoring production and recovery
data, it is possible to predict recovery as if primary recovery operation is
15-20%.
INTRODUCTION TO OIL&GAS PRODUCTION
28
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3.8.2. SECONDARY RECOVERY TECHNIQUES:
As the technology of petroleum production improved, it was found that
there were several methods; of increasing recovery with man-made drive
mechanisms. These methods were grouped under classification of secondary
recovery techniques.
The first secondary recovery technique is the water injection. The water
produced with oil is often pumped back into the reservoir. Since no commercial
use is available for the water. Thus disposal of water become the first secondary
recovery technique in which energy was returned to the reservoir by pumping
water into the reservoir.
Injected water pushes oil, gas and connate water horizontally across the
reservoir. Fig.19 shows a water injection operation, or a secondary water flood
operation, as it is commonly called. As water is injected, the water bank
expands and pushes the oil bank, containing oil, gas and connate water, ahead to
offsetting production wells.
Fig.19 As water is injected into the water bank, the water bank is expands
outward and pushes petroleum ahead towards offsetting production wells.
By carefully selecting the wells used for injection of the wells (injection
rate and pressure), secondary water flood operations can be efficient recovery
techniques. The second secondary recovery technique is the gas injection. Gas
can be compressed and injected into the top of a reservoir to form or supplement
a gas cap.
INTRODUCTION TO OIL&GAS PRODUCTION
29
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Fig.20 shows a reservoir being produced by gas injection. Gas is injected
into the gas cap expansion.
Fig.20 Gas may be injected into a gas cap to supplement the reservoir energy
If there is no gas cap in the reservoir, this technique is used to create a
secondary gas cap. As water or gas injection proceeds during a secondary
recovery operation, the gas and water saturations and the relative permeability
change. Thus, some oil and gas is left behind as the gas cap expands or as a
flood front passes. Oil and gas are still in the reservoir when so much gas or
water is being produced that it is no longer feasible to continue secondary
injection.
Recovery under secondary recovery operation is improved over that
during primary recovery operations. Whereas the ultimate recovery rate
during primary recovery operation was 15-20%. The recovery rate during
secondary recovery operations increases to 30-50%.
3.8.3. ENHANCED RECOVERY TECHNIQUES:
In the last few years other methods of production operation have been
found that even further increase recovery. These newer techniques are called
enhanced recovery techniques. They are sometimes implemented after
secondary recovery techniques have been used and, in some newly drilled wells,
even before primary recovery operation have been carried to depletion.
From research and testing of enhanced recovery techniques it has been
found that it is possible to control the relative permeability and mobility of
fluids in a reservoir.
INTRODUCTION TO OIL&GAS PRODUCTION
30
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
In such techniques, it is possible to avoid bypassing oil and gas during
a recovery operation.
The first method of enhanced recovery: is to inject into the reservoir
a mixture of petroleum gases consisting primarily of methane, ethane and
propane. This gas is injected alternately with water under carefully controlled
pressure conditions to form a fluid that mixes easily with both water and oil.
The second method of enhanced recovery: is to inject a mixture of
carbon dioxide and some times hydrogen sulfide alternately with water. Again,
a fluid that mixes both oil and water is formed.
The third method enhanced recovery: is to mix special chemicals,
called polymers, with water. These polymers cause the same mixing actions as
the other injection techniques.
Enhanced recovery techniques depend on forming flood fronts much like
those formed during secondary water flood operations. As the flood fronts pass
a part of the reservoir, only a fraction of the oil and gas is left behind.
RESEARCH AND TESTING INDICATES THAT A RECOVERY
RATE OF MORE THAN 60% CAN BE EXPECTED FROM ENHANCED
RECOVERY TECHNIQUES.
INTRODUCTION TO OIL&GAS PRODUCTION
31
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
4.WELLHEAD
WELLHEAD: Is the equipment used to maintain surface control of the well.
It is attached to the top of the tubular material used in oil wells to support the
strings of pipe, and to provide seals between the strings of pipe, and to control
the production of wells.
The kind of wellhead configuration to be used is determined by well conditions.
The high pressure wellhead is required where formations are extremely high.
Where production and pressures are very low, the simple wellhead may be used.
The wellhead is formed of combinations of parts called :
1.
2.
3.
4.
5.
Casing Head
Tubing Head
Christmas Tree
Stuffing Box
Pressure Gauges
4.1.WELLHEAD VALVES:
1. Surface casing valve: Located in the lower most casing head, usually 2``
size, used for checking if there is a flow of fluid or pressure between the
surface casing and oil string.
2. Casing valves: 2`` or 4`` size, located on the casing head, used to control
the flow of oil from oil string. These valves should never be loosened or
removed.
3. Master valve: 3`` or 6`` size, located on the top of the tubing head. Used
to control the flow of oil from the tubing and control the well. This valve
should never be used to close the well except in case of emergency.
4. Tubing valves: 2`` size, located on the wings of the wellhead, used to
control the flow of oil from the tubing as the master valve. These valves
can be changed under supervision without the well being killed.
5. Crown valve: 2`` size located on the top of the wellhead and some times
called swabbing valve.
It is secondary master valve used to swab through . It actually does not
control any flow except when running wire line tools, swabbing or
acidizing operations.
This valve can be changed under supervision without killing the well.
6. Choke valve: Is a device attached down stream from the tubing or casing
valves to restrict, control, or regulate the flow of oil. It ma be:
a. A positive choke
b. Adjustable choke
INTRODUCTION TO OIL&GAS PRODUCTION
32
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
4.2.SAFETY PRECAUTIONS:
1.
2.
3.
4.
5.
6.
7.
8.
No smoking within 300 ft from the wellhead.
Spark resistant tools must be used.
Cars and Trucks must be kept at a safe distance.
Be aware of danger of working with high pressure.
Never put a pipe wrench on a Cameron valve.
Do not drop tools on pressure gauges and valves.
The valves should be opened slowly to avoid a pressure surge.
When opening and closing valves, the hand wheel is turned until
movement is stopped, and then backed off one half rotation. The valve
well not seal until this is done.
INTRODUCTION TO OIL&GAS PRODUCTION
33
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
34
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
35
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
A High- Pressure Double -Wing Christmas Tree
Flow from the well can go through flow valves on either
INTRODUCTION TO OIL&GAS PRODUCTION
36
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
37
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
5.THE WELL
THE WELL: Is a means of recovering a natural resource, Crude oil and natural
gas reservoir.
Crude oil and natural gas reservoir are found in the formation below the surface
of the earth and the well is drilled to these formations. Pipe is then run into the
hole to allow fluid to flow to the surface.
THE MAIN COMPONENTS OF THE WELL ARE:5.1. CASING:
A drilled hole must be stabilized to prevent fresh water sand
contamination, lost circulation, hole sloughing or charging shallow sands with
abnormal pressure.
To do this, successively smaller diameter casing strings are set in the well
starting with:
1.
2.
3.
4.
Conductor pipe
Surface pipe
Intermediate string
The production or oil string.
The depth that each string is set is determined by the particular conditions
at the well site.
For example, surface casing can be set at depths from 200 ft to 5000 ft and
an oil string can be set from depths of 2500 ft to 25000 ft or more.
A sketch of a well is shown as in fig. bellow.
Each time a casing string is set and brought to the surface, a Blow Out
Preventer
( BOP ) of appropriate size and pressure rating is flanged onto the casing by a
casing head to control pressure in the drilling well.
Casing must be designed to meet the physical conditions imposed on the pipe.
A well with 10000 PSI surface pressure requires much heavier casing
than a well with 20000 PSI surface pressure.
By the same reasoning, the collapse resistance of the casing must be much
higher for string that is to be set at 20000 ft than string to be set at 2000ft.
INTRODUCTION TO OIL&GAS PRODUCTION
38
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
5.2. TUBING:
Because the casing and liner must remain in the well for along time and
their repair or replacement would be costly, another string of pipe is placed in
the well through which the oil is usually produced. This string called Tubing.
The tubing is suspended from the wellhead ( surface ) and usually reaches
to within a few feet of the bottom of the well.
Tubing is also used as the flow string because casing is usually too large to
permit the well to flow efficiently, or, in some cases , to maintain continuous
flow.
Tubing packers are some times used in the tubing string to seal off the
space between the tubing and the oil string of casing.
This is done particularly in wells where there are high reservoir pressure.
By sealing off this space, the casing is not exposed to high pressure, and the
chances of casing failure are reduced.
Tubing anchors and packers also support part of the weight of the tubing
in the casing and prevent the tubing string from moving up and down.
Tubing size diameter range from 1 to 4 inches.
5.3. SAFETY VALVES:
When a well is first put on production, it usually flows because of
pressure in the reservoir. Often wells are located where an accident could cause
danger either to the environment, people or facilities.
To provide needed protection there are several types of safety valves used to
shut in the well in case of an accident or equipment failure.
One type of safety valves is a subsurface safety valve, which is set in the
tubing string and will be close off when a predetermined rate of flow is
exceeded.
An alternate type is the surface controlled subsurface safety valve, that is
actuated by an external hydraulic system.
Another commonly used type of safety valve is installed on the wellhead.
This valve is known as a fail safe device, that is ,the valve is held open
against a spring by an external means, usually gas pressure.
Loss of pressure will cause the valve to close. This could be caused by a
broken flow line, fire, failure of a production vessel to operate properly or a
remote manual bleed off of the pressure.
INTRODUCTION TO OIL&GAS PRODUCTION
39
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
40
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
41
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
42
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
6. WELL COMPELTION
6. 1. INTRODUCTION:
The primary objective of the completion process is to develop a well,
which will yield the highest possible productivity in years come. The main
factors, which influence decisions during the design of the completion program
include:
1.
2.
3.
4.
5.
6.
7.
8.
9.
Investment required.
Desirable producing rate.
Reserves in various zones.
Reservoir drive mechanism.
Stimulation needs.
Sand control requirements.
Workover aspects.
Artificial lift consideration.
Possibility of future additional recovery projects.
6. 2. TYPES OF COMPLETIONS:
6.2.1. BOTTOM HOLE COMPLETION TECHNIQUES.
The three basic bottom hole completion techniques are:
1. open hole completion techniques
2. liner completion techniques
3. perforated casing completion techniques
The three completion types are presented in figs.21, 22, 23.
INTRODUCTION TO OIL&GAS PRODUCTION
43
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Fig.21 Open hole completions
Fig.22 Liner completions
INTRODUCTION TO OIL&GAS PRODUCTION
44
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Fig.23 Perforated completions
6.2.2. NUMBER OF ZONES TO BE COMPLETED PER WELL
According to the number of zone completed per well, completion type
can also be divided into single and multi-zone completion. Single-zone wells
are certainly the simplest, and the initial wells in a field will normally be of this
type until basic reservoir and geological information is obtained.
However, in multi-reservoir and in massive horizons containing extensive
barriers such as shale. It may be that separate completions will be required for
efficient operations and adequate depletion of productive materials.
INTRODUCTION TO OIL&GAS PRODUCTION
45
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
A. SINGLE COMPLETIONS:
Where only one productive interval exists or where several zones can be
coming into one wellbore, a single type completion will apply. In this type of
completion (fig.24) a tubing string is normally installed inside the casing for
production purposes.
Fig.24 Single completion installations
Even in high productivity wells where production is directly up the
casing, tubing will be installed to permit circulation of kill fluids, for maximum
production, the tubing is run open ended and the well flowed through both
tubing and annulus.
INTRODUCTION TO OIL&GAS PRODUCTION
46
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
B. MULTIPLE COMPLETIONS:
Multiple completions are a means of segregating Production from several
zones in the wellbore. In fields where zone separation is desirable or necessary,
the use of multiple completions reduces the number of wells required, and result
in lowest investment per completion. Some of the reasons why zone segregation
may be required or perforated are as follow.
i. Higher producing rate and faster payout. This is the most common
reason for multiple completions.
ii. Separating different types of reservoirs.
iii. Proper reservoir control.
Disadvantage:
i. More costly than single completion.
ii. Increase the risk of equipment failure and fishing jobs.
INTRODUCTION TO OIL&GAS PRODUCTION
47
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
48
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
7. STIMULATION TECHNIQUES
7. 1. PURPOSE OF STIMULATION:
1. To improve porosity and permeability of reservoir.
2. To remove formation damage resulted from drilling and cementing
operation.
3. To clean up wellbore itself by removing residue of mud or cement
or residues from perforating operations.
4. To enhance fluid flow rate.
5. To remove scale from pores and channels of formations resulted
from chemical reaction of two incompatible liquids.
7. 2. STIMULATION METHODS:
There are in general three stimulation methods (Acidizing, Hydraulic fracturing
and Explosive fracturing).
7.2.1. ACIDIZING:
The purpose of any stimulation technique is to increase the porosity and/or
permeability of the reservoir. Acidizing increases these parameters by reacting
with and removing some of the rock material itself.
1. Hydrochloric acid (HCl):
Used in field is normally 15% by weight HCl. This acid will dissolve
limestone, dolomite and other carbonates. When HCl comes with contact
calcium carbonate, the following products resulted:
Calcium chloride (very soluble salts).
Carbon dioxide (very soluble gas).
2. Hydrofluoric acid ( HF ):
Used in oil gas or service wells is normally 3% HF acid plus 12% HCl. It
is employed exclusively in sandstone matrix acidizing to dissolve formation
clays.
INTRODUCTION TO OIL&GAS PRODUCTION
49
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3. Acetic acid ( CH3COOH):
Acetic acid is a weekly-ionized slow reacting organic acid. It is
frequently used as a perforating fluid in limestone because it is relatively easy to
inhibit against corrosion.
4. Formic acid ( HCOOH):
Formic acid is a weekly ionized, slow reacting organic acid. However
formic acid is more difficult to inhibit against corrosion.
5. Sulfuric acid ( H2SO4):
Sulfuric acid is a granular powdered material, reacts about as fast as HCl.
The primary advantage of salfonic acid is that it can be handed to the location as
a dry powder and then mixed with water. This acid is not recommended for
temperature above 180F.
Acid additives:
Acidizing can cause a number of well problems. Acids may
i. Release fines.
ii. Great precipitants.
iii. Form emulsions.
iv. Corrode steel.
ADDITIVES ARE AVAILABLE TO CORRECT THESE AND A
NUMBER OF OTHER PROBLEMS.
INTRODUCTION TO OIL&GAS PRODUCTION
50
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
51
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
7.2.2. HYDRAULIC FRACTURING:
OBJECTIVE:
The objective of hydraulic fracturing for well stimulation is to increase
well productivity by creating a highly conductive path (compared to reservoir
permeability) some distance away from the wellbore into the formation. Usually
the conductivity is maintained by propping with sand to hold the fracture faces
apart.
Acid fracturing involves most of the same considerations as hydraulic
fracturing except that conductivity is generated by removing portions of the
fracture face with acid, leaving etched channels after the fracture closes.
FRACTURE INDICATION:
A hydraulic fracture treatment is accomplished by pumping a suitable
fluid into the formation at a rate faster than the fluid can leak off into the rock.
Fluid pressure (or stress) is built up sufficient to overcome the earth
compressive stress holding the rock material together. The rock then parts or
fractures along a plane perpendicular to the minimum compressive stress in the
formation matrix.
INTRODUCTION TO OIL&GAS PRODUCTION
52
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
53
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
8. WORKOVER
WORKOVER:
Is remedial operation performed on the reservoir and is carried out where
more extensive repairs are required, or the producing formation requires
treatment to improve production from the formation.
The work-over operations are carried out by using a work over rig. And
using a specific fluid which are circulated through the well bore during the
operations for the following purposes:1. To provide hydrostatic pressure necessary to control the formation
pressure.
2. To remove the drill cutting ( or solids ) from the well bore.
3. To cool the drill bit.
The work over operations include:1.
2.
3.
4.
5.
6.
7.
8.
Sand removal from the well bore.
Liner removal for repairing.
Casing repairs.
Drilling deeper.
Cementing operations.
Side tracking.
Separation of water zone.
Change completion type.
In Raguba field most of the work-over operations are carried to separate the
water zone and to perforate the upper zones, and also to repair tubing and
cementing.
INTRODUCTION TO OIL&GAS PRODUCTION
54
FIELD TRAINING/RAGUBA 2010
INDUSTRIAL TRAINING
FIG. 25
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
55
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
9. WELL TESTING
Generally some kind of test must be made to determine the performance
of an oil or gas well. The procedure followed is called testing.
There are many types of well test methods, and each is conducted to obtain
certain information about the well.
Some types of test are made often, and some may be only on rare occasions.
PREPARATION FOR WELL TESTING:
Preparing oil and gas wells for testing involves stabilizing the production rate
and pressure.
The surface indication of the wells stabilization are:
1. Constant Wellhead Flowing Pressure ( WHFP ).
2. Constant Gas production rate.
3. Constant fluid production rate.
The reason for stabilizing flow rate is to insure that the data obtained are
representative of actual performance, i.e. a retest under the same conditions will
yield the same results.
9.1. OIL WELL TESTING:
TYPES OF WELL TEST :
There are several tests that are performed to determine the best way to
produce the well.
Some of these tests are routine or normal production test, potential test, Gas-Oil
Ratio ( GOR ) test, and Bottom Hole Pressure (BHP), Bottom Hole
Temperature ( BHT), Liquid level determination.
It is very important that any test be done accurately, so that the data
obtained will reflect the ability of the well to produce and presents the true case
history of it.
Some of these tests are required by the petroleum engineering, which
assigns a producing allowable, and in some instances, they run for field
information.
INTRODUCTION TO OIL&GAS PRODUCTION
56
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
57
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
SOME OF THE MOST FREQUENTLY MADE WELL TESTS ARE AS
FOLLOWS:1. DRILL STEM TEST ( DST ) :
When the well is being drilled, a DST is often run in the formation of
unknown performance to determine if the formation contain oil, water or gas.
This procedure requires special tools and packers to be run in the drill pipe to
isolate the interval to be tested.
The test results show the following:
a)
b)
c)
d)
Bottom Hole Shut in Pressure ( BHSIP )
Bottom Hole Flowing Pressure ( BHFP )
Bottom Hole Temperature ( BHT )
Type of fluid the well might be capable of producing.
The results of DST on the well will often determine if the casing string should
be run to complete the well as producer, or if the well is a dry hole.
2. ROUTINE OR NORMAL TEST :
This test is the most frequently performed, periodically conducted at some
specified interval, usually monthly.
This test required the measurement of oil, water ,and gas rate that a well will
produce in 24 hour period under certain fixed conditions.
This test is made on each newly completed well and at other times as might
be requested by the petroleum engineering group and operators to keep accurate
records of production from the well. Also the results used to analyze the well
problems and predicting future of the well performance.
These tests can be taken with various items of equipment, ranging from
simple tank measuring equipment and Gas meter, to a completely automated
test facility ( test separator, control and readout panel, etc. )
INTRODUCTION TO OIL&GAS PRODUCTION
58
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3. POTENTIAL TEST :
The potential test is a measurement of the amount of oil and gas a well
produce during a specified period of time ( such as 24 hour ) under certain fixed
conditions.
The information obtained from these tests is used in assigning a producing
allowable which must be followed by the operator of the well.
The oil is measured by test tank, or by meters , and the gas is measured at the
same time with equipment such as an orifice meter.
A potential test normally is required on a newly completed well and often
during its production life.
The same equipment as in routine test can be used .
4. GAS-OIL RATIO TEST ( GOR ) :
Gas-Oil Ratio test is required at periodic interval for all wells. The test is made
to determine the volume of gas produced per barrel of oil so as to ascertain
whether or not a well, in making its allowable, is producing Gas in excess of the
permissible limit, and when the GOR exceeds the permissible limit.
Total amount of Gas in SCF/ D
GOR = -------------------------------------------- = SCF/B
Total amount of Oil in B/D
SCF
= Standard Cubic Feet.
B
D
= Barrel of Oil produced.
= Day.
An example would be : An Oil well produced 350 BBLS. of Oil and 140000
SCF of Gas during the 24 hour test period.
What is the Gas-Oil Ratio?
140,000 SCF
GOR = ---------------------- = 400 SCF/BBLS.
350 BBLS
GOR behavior of wells is used in evaluating well and reservoir performance.
INTRODUCTION TO OIL&GAS PRODUCTION
59
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
The GOR sometime used as a basis for recombining Oil and Gas samples
obtained at surface for establishing the reservoir fluid composition. The
properties of the reservoir fluid can then be determined.
5. SPECIAL TESTS :
There are some other types of tests, which may be performed on an Oil well to
determine or measure Pressure and Temperature in the well, such as surface
( WHT), ( WHCIP), (WHFP),and Bottom-Hole Pressure and Temperature
( BHCIP ), ( BHFP), ( BHT ) , pressure Build-up, Drawdown, PDS.
Also Fluid Level Test is normally performed on wells which will not flow
naturally, and must be made to flow by artificial lift.
PROBLEMS IN OIL WELL TESTING:
1. Emulsions: If the fluid produced from a well consists of both oil and
water, there is a possibility that they well not separate. The resulting fluid
is commonly called an emulsion. If the volume of this emulsion is
measured as oil, the test will be in error by the amount of water in the
emulsion, so that the oil measured during the test should be tested for its
water content
2. Liquid meter accuracy: Liquid meter should be checked periodically for
accuracy. This is usually accomplished by discharging fluid from the
meter into a test tank. If the meter is found in error, it should be repaired
before it put back in service, or correction factor used in the volume
calculation.
REPORTING OF WELL TEST DATA:
Much of well tests usefulness will be lost unless all necessary data are reported.
This principle way of reporting test data is through of the use of a form or
record sheet, such reports may vary, depending upon the purpose of the tests
data required . It is the responsibility of well tester to report data accurately and
completely. ( see the report form attached ).
INTRODUCTION TO OIL&GAS PRODUCTION
60
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
INDUSTRIAL TRAINING
61
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
9.2. GAS - WELL TESTING :
Gas Wells are Wells which produce dry Gas, or Gas condensate, and
a small amount of Water.
To test a Gas Well, it is necessary to separate the Gas, Condensate and Water
and then measure the amount of each that is produced during the time interval.
The primary measurement being the Gas volume.
Gas production from a Well is usually expressed in terms of thousands of
standard cubic feet per day ( MSCF/ D ), or millions of standard cubic feet per
day
( MMSCF / D ).
SCF is the volume of Gas at standard atmospheric pressure ( 14.7 PSIA ) and
Temperature ( 520 0 R ).
Condensate or Water production is usually expressed as stock tank barrels
per million standard cubic feet of Gas ( BCPMM ).
Most Gas Wells produce less than 100 stock barrels of condensate per MMSCF
of Gas.
PREPARATION FOR TEST:
For determining the stabilized shut-in Wellhead Pressure ( WHSIP), and
prior to any special testing of the well, the well should be flowed at a rate
sufficient to clean the bore of liquids and other materials and then should be
shut-in.
The clean up of a well is very important. Some wells, usually low volume
producing gas wells, take several days of flowing to clean up. A Shut-in
Bottom-Hole Pressure ( BHSIP) can be calculated from the wellhead pressure if
the well does not have an accumulation of liquid. If a bottom hole pressure gage
is used , it may not be necessary to thoroughly clean the well bore of liquids
before making the pressure measurement. Well that stabilized slowly should be
shut-in 48 to 72 hours, if possible. pressure at the wellhead should be measured
by using a dead weight gage.
Most gas wells may be considered stabilized when the rate of pressure
build-up per day does not exceed 1% of the shut-in pressure.
INTRODUCTION TO OIL&GAS PRODUCTION
62
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
TYPES OF GAS WELL TESTS :
1. ROUTINE GAS WELL TEST :
Periodic measurement of Gas, Condensate, and Water production may be
considered a routine test.
For many wells, gas production is continuously metered for the individual well.
This means that these wells are being continuously tested because their gas
production rates and cumulative production of the gas and liquids are
continuously measured.
It is also necessary to measure the Wellhead Flowing Pressure ( WHFP )
and Wellhead shut-In Pressure ( WHSIP ), because the ability of the well to
produce depends upon the available pressure draw-down ( difference between
the WHSIP and WHFP ).
2. OTHER TYPES OF TESTS:
There are some other tests, which may be performed on a Gas-Well to
determine different data on a particular Gas well .
These tests assist the engineers in determining such things as Well-bore
damage, permeability of the reservoir, Shut-In pressure, and flowing pressure.
Because well testing facilities are similar to the fields separation
facilities and this will learn you and show separators work, how they are built,
and adjust them to keep your separators in good operating conditions.
PROBLEMS IN GAS WELL TESTING:
There are several problems that are unique in testing gas wells:1. Liquid Loading: This problem usually occurs when testing low
producing gas wells with high liquid-gas ratios. Indications of liquid
loading are wide variations of surface pressure.
2. Hydrate Formation: This problem usually occurs in a high pressure gas
well, if adequate production equipment is available, this problem can be
eliminated by maintaining the wellstream temperature above the hydrate
formation temperature.
3. Wet gas streams: Sometime it is necessary to meter a gas stream at the
wellhead. Such streams often will deposit liquid in the flowline
downstream of the point where the orifice meter was installed.
INTRODUCTION TO OIL&GAS PRODUCTION
63
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
4. Sour Gas ( H2S ): Extreme caution should be exercised in handling gases
and fluids containing Hydrogen Sulfide ( H2S). This hazardous substance
is highly toxic, and under certain concentration can cause illness and
death. Special precaution should be taken when testing wells where
Hydrogen Sulfide is present.
REPORTING DATA:
a) Fluid Measurements:
1.
2.
3.
4.
Gas production rate for all stream, in MSCF/D or MMSCF/D.
Condensate or oil rate , in stock tank barrels per day STB/D.
Water rate, B/D.
Condensate Gravity, API ( @ 600F).
b) Pressure Measurement, in PSIG:
1.
2.
3.
4.
5.
Flowing Wellhead Pressure ( WHFP).
Shut-in Wellhead Pressure ( WHSIP).
Flowing Bottom-Hole Pressure (BHFP).
Shut-in Bottom-Hole Pressure ( BHSIP).
Atmospheric Pressure.
c) Temperature Measurements, in 0F:
1.
2.
3.
4.
Bottom-Hole Temperature ( BHT).
Wellhead Temperature ( WHT).
Stock-tank liquid Temperature.
Atmospheric Temperature.
d) Choke size, Inches.
e) Separation conditions, PSIG and 0F:
1. Number of separators.
2. Separator Pressure and Temperature.
3. Stabilizer Pressure and inlet and outlet Temperature.
f) Time Data- Minutes, Hours, Days:
1. Duration of flowing .
2. Length of time on test choke size before test period.
3. Shut-in time for pressure measurement.
INTRODUCTION TO OIL&GAS PRODUCTION
64
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
9.2. SEPARATORS
A separator is a vessel in which a mixture of non-miscible fluids are
separated from each other. For example, the 3-phase (gas, oil and water) fluids
produced from the wells.
In our process operations, we use horizontal separators in the GOSP to
separate the well fluids arriving from the outlying producing fields. Other
horizontal and vertical separators are used as Knock-Out drums, Gas scrubbers,
Flash tanks etc..
Generally, a vessel of this type is used to separate two or three fluid
phases.
A vessel used to separate gas and liquid is called a 'Two-Phase' separator' while
a 'Three Phase' vessel' will separate Gas, Oil and Water.
The number of phases represents the number of fluid streams leaving a
separator. A GOSP TEST separator is an example of the measurement of the
gas and liquids produced. It is used to check the production rate of gas, oil and
water from a single well.
GOSP (GAS - OIL SEPARATION PLANT)
These consist of a number of horizontal vessels containing baffles which
perform the separation process. In most cases the separation takes place in two
stages - High pressure (1st stage) and Low pressure (2nd stage). As the threephase flow enters and flows through the separators, the gas is released and
leaves the top of the vessels while the oil and water are piped from the bottom
under level control.
In the Raguba GOSP, 1st Stage separators are normally operated as High
Pressure (HP) systems at about 250 psi. However, operational requirements (lower pressure wells etc), need some 1st stage separators to be operated at a
lower pressure (about 75 to 85psi).
The oil and water from the 1st stage separation, enters the 2nd stage
where further separation takes place. This is the case for all GOSP separators
except C which is a single stage separation process used for the low pressure
and wet crude.
INTRODUCTION TO OIL&GAS PRODUCTION
65
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
SEPARATION PRINCIPLES:
Separators consist of the following main parts: 1. A primary separation section to remove the bulk of the liquid from the gas.
2. Sufficient liquid capacity to handle surges of liquid from the line.
3. Sufficient length or height to allow the small droplets to settle out by
gravity (to prevent undue entrainment).
4. A means of reducing turbulence in the main body of the separator so
that proper settling may take place.
5. A mist extractor to capture entrained droplets or those too small to settle by
gravity.
Where a vessel is simply separating total liquid from gas, it is called a ' TwoPhase Separator ' When the process requires the separation of two liquids and a
gas, the separator is called a ' Three-Phase Separator '. ' Two or Three Phase
separation ', refers to the number of streams leaving the vessel and not the inlet
fluid stream.
The main principles used to achieve physical separation of gas and liquids are: GRAVITY SETTLING and COALESCING
Any separator may employ one or more of these principles, but the fluid phases
must be ' Immiscible ' (cannot mix), and have ' Different Densities ' for
separation to occur.
GRAVITY SETTLING:
During the separation process, the gas is moving in an upward direction into
the vapor section of the separator and the liquid particles are tending to fall to
the vessel bottom under the influence of gravity. Gas will separate more quickly
from a liquid when it is flowing HORIZONTALLY.
In a ' VERTICAL ' separator, the gas is moving vertically upwards and the
liquid droplets, due to gravity, are falling vertically downwards. The contraflow of the two fluids therefore interferes with the flow paths and separation is
slower.
Generally, because of the above factors, the vapor section of a Horizontal
separator will be of a smaller volume than that of a Vertical vessel.
INTRODUCTION TO OIL&GAS PRODUCTION
66
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
COALESCING
Very small droplets such as fog or mist cannot be separated practically
by gravity.
These droplets can be coalesced to form larger droplets that will settle by
gravity. Coalescing devices in separators force gas to follow a ' twisting' path.
The momentum of the droplets causes them to collide with other droplets
or with the coalescing device, forming larger drops. These can then separate
out of the gas phase due to the influence of gravity.
Wire mesh screens, Vane elements, and filter cartridges are typical examples of
coalescing devices.
Separation vessels usually contain FOUR MAJOR SECTIONS, plus the
necessary pressure and liquid level controls :
1. Primary Separation Section :
For removing the bulk of the liquid from the inlet stream. For example,
free liquids, slugs and large droplets. This is usually accomplished by a change
in the direction of fluid flow, either by baffles or deflection plates near the inlet
nozzle or by using a tangential inlet nozzle as in ' Tangential Feed ' or ' Cyclone
' separators which operate by a centrifugal force being set up in the vessel.
2. Secondary Separation Section :
For removing the maximum amount small liquid droplets without an
elaborate design. The major separation principle in this section is by gravity
settling of the liquid droplets from the vapor stream.
3. Mist Extraction Section :
For removing maximum amount of tiny liquid droplets remaining in the
vapor stream. The mist extractor may be of the impingement type; (mesh pads)
and/or may use the centrifugal force principle; (the vane type).
INTRODUCTION TO OIL&GAS PRODUCTION
67
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
4. Liquid Accumulation Section :
For the receiving and disposing of the liquid collected. Sufficient volume
and proper level control equipment should be provided to handle surges that
may occur in normal operations.
The length of a horizontal separator has a greater effect on capacity than
the height of a vertical type. In the horizontal vessel the path of any droplet
ideally has a trajectory similar to that of a shell from a gun. Therefore, the
length required depends on : 1. Droplet size.
4. Vessel diameter
2. Gas velocity.
3. Droplet density.
5. Degree of turbulence
FACTORS AFFECTING SEPARATION
The following table shows some of the factors that affect separation :-
SEPARATION FACTOR
EFFECT OF THE FACTOR
1. Difference in fluid densities
The greater the difference in densities,
the easier the separation.
2. Residence time
The longer the fluids are in the
separator, the better the separation.
3. Coalescing element surface area
The greater the area of the coalescing
element, the better the separation.
INTRODUCTION TO OIL&GAS PRODUCTION
68
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
TYPES OF SEPARATORS
Separators can be designed to handle fluids according to the fluid
composition, and they come in different sizes and shapes as separation
requirement as follow:
1. Horizontal separators.
a) Horizontal Single - Tube separators
a.1) Two - phase separators. a.2) Three - phase separators.
b) Horizontal Two -Tube separators.
2. Vertical separators.
a) Two - phase separators. b) Three - phase separators.
3. Spherical separator.
4. Filter separations.
5. Scrubber or knockout drums.
6. Coalescer ( liquid - liquid ) separator.
7. Slug catcher.
8. Flash tank.
9. Metering separators.
1. HORIZONTAL SEPARATORS
This type of separators has a much greater gas- liquid interface area,
consisting of a large, long baffled gas separation section, that permits much
higher gas velocities.
METHOD OF OPERATION
In operation, gas flows horizontally and at the same time, falls towards
the liquid surface. The gas flows in the baffle surface and forms a liquid film
that is drained away to the liquid section of the separator. The baffle need only
be longer than the distance of liquid trajectory travel at the design gas velocity.
When well fluids strike the angle baffle, forward motion is stopped temporarily,
heavy liquids fall immediately to the bottom of the separator. Gases and oil
spray continue through the de-foaming element, or the primary mist extractor.
In the mist extractor, baffles change the direction of flow, gas will flow
easily around the baffles. But, oil drops tend to coalesce on the baffles, and flow
down the baffles to the liquid section. Gas continues through the secondary mist
extractor, where most of the remainder of liquids is removed from the gas. Oil
and water leave the separator through the liquid outlet.
SEE FIGURES ( 1 ), ( 2 ) , ( 3 ) and ( 4 ).
INTRODUCTION TO OIL&GAS PRODUCTION
69
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Figure: 1
INTRODUCTION TO OIL&GAS PRODUCTION
70
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Figure: 2
INTRODUCTION TO OIL&GAS PRODUCTION
71
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Figure: 3
INTRODUCTION TO OIL&GAS PRODUCTION
72
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Figure: 4
INTRODUCTION TO OIL&GAS PRODUCTION
73
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
2. VERTICAL SEPARATORS
This type of separators is often used on low to intermediate gas - oil ratio well
streams, and where relatively large slugs of liquid are expected.
METHOD OF SEPARATION
The stream of gas and liquid flow through and impinges against several layers
of vanes. Because it has greater {inertia}, the liquid has greater resistance to
change in direction of flow.
Gas assumes the new direction of flow more readily than the liquid. Gas flows
through the space between the vanes, while the liquid coalesce on the vane
surfaces. Coalescing package mist extractor remove liquid mist from gas
streams.
SEE FIGURES ( 5 ) ( 6 ) ( 7 ) and ( 8 ).
INTRODUCTION TO OIL&GAS PRODUCTION
74
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
ZELTEN TRAINING
Figure: 5 - Vertical Separator with Spiral Inlet
INTRODUCTION TO OIL&GAS PRODUCTION
75
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
ZELTEN TRAINING
Figure: 6 - Vertical Separator
INTRODUCTION TO OIL&GAS PRODUCTION
76
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
ZELTEN TRAINING
Figure: 7 - Vertical Separator
INTRODUCTION TO OIL&GAS PRODUCTION
77
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
ZELTEN TRAINING
Figure: 8 - Simple Three Phase vertical Separation
INTRODUCTION TO OIL&GAS PRODUCTION
78
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3. SPHERICAL SEPARATORS
These separators are occasionally used for high pressure service, where compact
size is desired and liquid volumes are small.
METHOD OF OPERATION
Fluid flowing through the inlet, is deflected into two streams, breaking up the
stream allow gas to rise out of the liquid. The separator is divided into two
sections by a plate or floor. Liquid flow to the lower section through drains in
the floor. The liquid spreads into a thin layer across the plate or floor.
Because the liquid is thinly spread, it is easier for any remaining gases to escape
the liquid, and pass through the mist extractor, and out of the separator, through
the gas outlet. Liquid level in the separator is regulated by the float connected to
the dump valve.
SEE FIGURE ( 9 ) ( 10 ).
INTRODUCTION TO OIL&GAS PRODUCTION
79
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
ZELTEN TRAINING
Figure: 9 - Spherical Separator
INTRODUCTION TO OIL&GAS PRODUCTION
80
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
ZELTEN TRAINING
Figure: 10 - Spherical Separator
INTRODUCTION TO OIL&GAS PRODUCTION
81
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
4. SCRUBBER OR KNOCK OUT DRUMS
A vessel designed to handle streams with high gas- to-liquid ratios. The liquid is
generally entrained as mist in the gas or is free, flowing along the pipe wall.
The vessels are used to separate a two or three phase inlet fluid into liquids and
gas.
The vessel inlet flow generally hits an inlet deflector plate to begin the
separation process.
Between the inlet and the gas outlet, some form of demisting element may be
installed which can be a wire mesh screen or pad or an angled vane type.
The demister construction presents a large surface area to the liquid mist
entrained in the gas which causes small droplets of liquid to coalesce into larger
drops, which fall to the vessel bottom by gravity. The gas outlet nozzle exits the
gas from the vessel above the demister screen.
SEE FIGURE ( 11 ).
INTRODUCTION TO OIL&GAS PRODUCTION
82
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Figure: 11 - Knock-out Drum
INTRODUCTION TO OIL&GAS PRODUCTION
83
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
5. FILTER SEPARATORS
A filter separator usually has two compartments. The first compartment
contains filter-coalescing elements. As the gas flows through the elements, the
liquid particles coalesce into larger droplets and when the droplets reach
sufficient size, the gas flow causes them to flow out of the filter elements into
the center core.
The particles are then carried into the second compartment of the vessel
( containing a vane type of knitted wire mesh mist extractor ) where the larger
droplets are removed.
A lower barrel or boot may be used for surge or storage of the removed liquid.
SEE FIGURE ( 12 ).
INTRODUCTION TO OIL&GAS PRODUCTION
84
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Figure: 12 - Dry Gas Filter Separator
INTRODUCTION TO OIL&GAS PRODUCTION
85
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
6. COALESCER ( LIQUID LIQUID ) SEPARATOR
Avery small droplets such as fog or mist cannot be separated practically by
gravity. These droplets can be coalesced to form larger droplets that will settle
by gravity.
Coalescing devices in separators force gas to follow a tortuous path. The
momentum of the droplets causes them to collide with other droplets or the
coalescing device, forming larger droplets. These larger droplets can then settle
out of the gas phase by gravity.
Wire mesh screens, vane elements, and filter cartridges are typical examples of
coalescing devices.
SEE FIGURE ( 13 ).
INTRODUCTION TO OIL&GAS PRODUCTION
86
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Figure: 13 - Coalescer Separator
INTRODUCTION TO OIL&GAS PRODUCTION
87
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
10. FLUID TREATMENT
Well fluids are often a complex mixture of liquid hydrocarbons, gas, and
some impurities. It is necessary to remove the gas and some impurities from the
liquid hydrocarbon before they are stored, transported, and sold.
Liquid hydrocarbons and objectionable impurities must be removed from
natural gas before the gas goes to a sales line.
Impurities that might be found in some gases are hydrogen sulphide, carbon
dioxide, free water, water vapour, mercaptans, nitrogen, helium and solids.
All of the impurities cause various types of operating problems.
Water vapour in gas at high pressure can cause serious operating problems.
These problems can best be illustrated by the following typical example :
Gas is separated at 1000 psig and 90 0F, at this pressure and temperature,
gas at water saturation contains 46 pounds of water vapour per million cubic
feet of gas.
If the gas is cooled to 70 0F, it will contain only 25 pounds of water vapour per
million cubic feet of gas.
This means 21 pounds of water vapour have condensed to free water. This free
water has a tendency to cause corrosion and reduce flow rates in the gas
transmission line. Free water also promotes the formation of gas hydrates,
which are ice-like crystalline compounds formed in natural gas in the presence
of free water under conditions of high pressure and temperature.
These hydrates form at various temperature, often well above the freezing
temperature of water and these hydrates causes a total or partial blockage in the
gas gathering or distribution line or pipeline.
INTRODUCTION TO OIL&GAS PRODUCTION
88
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
10.1. OIL TREATING :
When crude oil produced, various amounts of gas, water and other
impurities are mixed with the oil. Some of this mixture comes as free oil, some
as free water, and some as homogeneous mixture known as an emulsion.
The gas, water and other impurities ( known as basic sediment and water ) must
be removed before selling the oil. This separation process is called oil treating.
To select a treating system, a number of factors should be considered to
determine the most desirable method of treating the crude oil to pipeline
requirement.
SOME OF THESE FACTORS ARE:1.
2.
3.
4.
5.
6.
7.
Tightness of emulsion.
Specific gravity of the oil, gas and produced water.
Corrosiveness of the oil, gas and produced water.
Scaling tendencies of the produced water.
Quantity of fluid to be treated and percent of water in the fluid.
Desirable operating pressure for the equipment.
Paraffin forming tendencies of the crude oil.
Oil field emulsions are usually of the water-in-oil type, however, a few of the
emulsions are oil-in-water type and are called reverse emulsions.
Emulsions are complex and each should be considered individually.
In order to break a crude oil emulsion and obtain clean oil, it is necessary to
displace the demulsifies and its film. This brings about the coalescence of
droplets of water and furnishes a means and time period of undisturbed setting
of the coalesced water drops.
10.2 GAS TREATING:
Natural gas produced from a gas wells or separated from oil, contains
substantial amount of water vapour or other impurities such as hydrogen
sulphide, sulphur compounds and carbon dioxide. These gases or water vapour
are treated prior to sale or entry to process plant to prevent corrosion and
erosion of equipment and pipeline, also to prevent a water hydrates which cause
a total or partial blockage of the pipeline.
A gas which contains a high concentration of sulphur compounds is called Sour
gas or acid gas.
To remove these impurities from natural gas, processes of absorption and
adsorption were used.
INTRODUCTION TO OIL&GAS PRODUCTION
89
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
10.2.1 GAS DEHYDRATION
1. GENERAL DESCRIPTION:
Most produced natural gas contains water. Some of this water is FREE
WATER, liquid phase, and may be removed by passing the gas through a
separator or scrubber. After scrubbing the gas will still contain WATER
VAPOUR. This is the water that we are concerned with in this section.
The term DEHYDRATION means to remove water from a substance or the
drying of the substance.
The process of water vapour removal from the natural gas stream is carried out
by a process of ABSORPTION, using a liquid desiccant, or ADSORPTION,
using a solid desiccant.
In Brega, in a section called the VAPOUR DRYING SECTION, the water
vapour is removed from the natural gas by adsorption using a desiccant called
MOLECULAR SIEVE.
In the field locations, we use absorption processes, with a liquid desiccant called
GLYCOL (generally TRI-ETHYLENE GLYCOL). The process is carried
out in towers called ABSORBERS or CONTACTORS.
1.A. GENERAL DEHYDRATION PROCESS:
The wet gas enters the absorber tower bottom and flows upwards through
contacting devices, usually bubble caps. The glycol, called LEAN GLYCOL,
enters the tower top and flows down across the bubble cap trays, which gives
intimate contact between the gas and glycol.
Dry gas leaves the top of the tower and goes for further processing, while the
wet glycol, now called RICH GLYCOL, leaves the tower bottom and passes to
the glycol REGENERATOR or RECONCENTRATOR where by
DISTILLATION, the water is vaporized out and passed to the atmosphere as
steam. The rich glycol is then re-circulated around the dehydration unit.
The presence of water vapour in natural gas can lead to many problems. The
dehydration process of natural gas is therefore carried out for the following
reasons:
INTRODUCTION TO OIL&GAS PRODUCTION
90
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Water vapour reduces the ability of gas to flow in the flow lines
and process systems.
Water vapour causes corrosion in lines and equipment.
At low temperature, water vapour forms hydrates, complicated
molecules of hydrocarbon liquid and water, causing blockage of
lines and equipment.
Water vapour must therefore be removed from the gas stream. Natural gas may
contain from 10 to 300 pounds of water vapour per million cubic feet of natural
gas produced, 10 to 3000lb. Water/mmcf, depending on the temperature and
pressure of the natural gas. The warmer the gas, the more water vapour it will
contain.
Tri-Ethylene Glycol (TEG) dehydration systems are the most common means
used for the process. When the lean TEG is brought into contact with the wet
natural gas, it absorbs the water vapour from the gas stream.
1.B. DESCRIPTION OF THE PROCESS AND EQUIPMENT:
Referring to Figure 1, the wet natural gas enters the bottom of the glycol
contactor tower and rises through the column where it is brought into contact
with the lean glycol flowing downwards across the bubble cap trays.
In the contactor, the up-flowing gas gives up water vapour to the glycol flowing
down from the top tray. At the tower top the dry gas passes through mist
extractor elements and then passes from the contactor to other processes.
The mist extractor coalesces fine particles of liquid into large droplets, which
fall back into the glycol passing down the tower. In this way, glycol carry over
with the gas stream is minimized.
The absorption of water vapour during the process gradually dilutes, weakens,
the glycol. The rich, dilute, solution collects in the bottom of the glycol
contactor tower from where it is discharged to the glycol regeneration unit, by
way of a level control system.
INTRODUCTION TO OIL&GAS PRODUCTION
91
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
At the regeneration unit, the wet glycol flows first to the glycol flash tank,
where a pressure drop takes place causing dissolved gases to leave the glycol as
it passes into the tank.
Flash tank pressure is controlled by a PCV in the gas line. The releases gas may
be piped to flare or fuel system or may be passed into the still column.
The rich glycol leaves the bottom of the flash tank under level control and
passes through a REFLUX COIL paced in the top of the still column.
After the reflux coil, the glycol is filtered and then passed through a double pipe
heat exchanger to be heated by the regenerated lean glycol leaving the unit
reboiler. This in turn cols the lean glycol. The rich glycol now enters the still
column at the top tray, below the reflux coil, and flows down across the bubble
cap trays
As the glycol flows down the tower, the absorbed water is stripped out by hot
rising vapours from the reboiler.
The glycol, as it collects in the still column bottom section, is now partially
regenerated and is referred to as SEMI-LEAN glycol. It is then passed into the
reboiler for final water removal.
Generally, the reboiler is of the fire tube type and contains a weir, which
ensures that the fire tube is completely immersed in glycol.
In systems that have separate reboiler and still column, the weir also maintains
the level in the still column bottom.
In the reboiler, the glycol flows over the weir and enters a stripping section
containing Raschig rings or other contacting devices. Extra stripping action may
be provided by injection of dry stripping gas into the reboiler stripping section.
On leaving the reboiler, the lean glycol passes through the glycol/glycol
exchanger into the glycol accumulator, or storage drum, from where the
circulation pumps take suction and discharge the glycol back to the contactor
via the glycol cooler, to complete the circuit.
In some small field units, the still column may be a packed type and is usually
an integral part of the reboiler, see Figure 2.
INTRODUCTION TO OIL&GAS PRODUCTION
92
FIELD TRAINING/RAGUBA 2010
INDUSTRIAL TRAINING
Figure 1. Complete glycol unit, Absorption and regeneration
TRAINING & DEVELOPMENT
INTRODUCTION TO OIL&GAS PRODUCTION
93
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Figure 2. Skid mounted field glycol regeneration unit
INTRODUCTION TO OIL&GAS PRODUCTION
94
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
The following figures represent the glycol dehydration systems in Raguba and
Sahil Gas Plants. (Glycol injection & Absorption Tower System)
INTRODUCTION TO OIL&GAS PRODUCTION
95
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Sahil Plant - Simplified Flow sheet
INTRODUCTION TO OIL&GAS PRODUCTION
96
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
2. MAIN OPERATING VARIABLES AND LIMITS
In order to understand the operating mechanism of the glycol dehydration
process, it is necessary to consider and understand the effect of the following
four major variables:
1.
2.
3.
4.
TEMPERATURE.
PRESSURE.
GLYCOL FLOW RATE.
GLYCOL CONCENTRATION.
1. TEMPERATURE:
Temperature of the incoming wet natural gas to the contactor is very important,
and is the key factor affecting the potential use of the glycol dehydrator.
This can be seen clearly by looking at the following points:
The higher the gas temperature, the more water it will contain in vapour
form.
If the temperature of the wet natural gas is around 140oF or above, the
natural gas does not want to give up the water vapour to the glycol. On
the other hand, if the natural gas temperature is 40oF or below, the glycol
becomes viscous and does not want to pick up the water vapour.
Therefore, dehydration will take place at temperatures between 50 to
130oF. The best results are obtained between 80 and 110oF.
The temperature of the lean glycol entering the top tray of the contactor
should be 10 to 15oF above the temperature of the gas to be treated. If the
glycol temperature is too much higher than the gas temperature, the
glycol will tend to foam and be carried out of the contactor tower with the
gas.
Conversely, if the glycol temperature is much lower than the gas temperature,
liquid hydrocarbons, condensate, will tend to form and fall to the bottom of the
contactor tower, causing problems in the glycol regeneration system.
INTRODUCTION TO OIL&GAS PRODUCTION
97
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
2. PRESSURE:
At constant temperature, the lower the pressure, the higher the water content of
the inlet gas. Other than affecting the water content of the inlet gas stream,
pressure has very little effect on the mechanics of glycol dehydration.
3. GLYCOL CIRCULATION FLOW RATE:
Determining the proper glycol circulation rate is not an easy task due to several
limitations and considerations involved. There are many factors that must be
considered, but, for simplicity, over a normal pressure range up to 1200 psi,
about 3 to 5 gallons of glycol must be circulated for every pound of water
removed at a 55oF dew point depression.
This quantity of glycol is based on equilibrium conditions, plant design, glycol
concentration and other factors.
4. GLYCOL CONCENTRATION:
Since the main objective of natural gas dehydration is maximum dew point
depression, relatively high glycol concentrations must be used. The usual
practice is to introduce, at the top of the glycol contacting tower, a solution of
regenerated glycol with a concentration ranging from 97 to 99%, and to remove
the solution from the base of the contactor tower at a glycol concentration of 80
to 90%.
In general, high glycol concentrations will give a larger dew point depression,
the larger the better, if the glycol circulation flow-rate is proportional to the
water content of the feed gas.
INTRODUCTION TO OIL&GAS PRODUCTION
98
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
3. GLYCOL REGENERATION PROCESS AND EQUIPMENT
The glycol regeneration process is very important to maintain the correct
concentration of the lean glycol. Refer to figure 1 and 2 for the equipment used
in the glycol regeneration process.
The glycol reboiler is the main piece of equipment that plays this role in the
regeneration process. The reboiler supplies heat to separate the glycol and water
by simple distillation. The Zelten reboiler is a fire tube reboiler.
The system consists of a U shaped, combustion chamber with one gas burner,
set into the shell of the reboiler and includes an outlet stack for the waste
combustion gases.
The shell contains a weir that maintains the level of glycol above the fire-tube in
order to prevent overheating of the tube and subsequent damage and/or glycol
decomposition by excess heat.
See figure 3.
INTRODUCTION TO OIL&GAS PRODUCTION
99
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
Figure 3. The glycol reboiler
INTRODUCTION TO OIL&GAS PRODUCTION
100
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
The temperature of the reboiler should be in the range of 375 to 390F.
This temperature will usually give good distillation of the rich glycol and
evaporate all water out of it.
The glycol should never be heated above 400F as it begins to decompose
above that temperature.
When making adjustment to reboiler temperature, never increase the
temperature setting by more than five degrees at a time.
Too great an increase will cause the control system to open the fuel gas valve
too wide, giving a large burner flame which in turn will cause flame
impingement on the inside of the fire-tube.
This will lead to Hot-spots and cause damage to the fire-tube and breakdown
of the glycol into corrosive organic acids.
If coke, salts or tar deposits form on the fire tube, the heat transfer into the
glycol is reduced, the control system will increase the fuel to maintain the
glycol temperature and tube failure can result. Localized overheating, especially
where salt deposits accumulate, will decompose the glycol.
Salt deposits can be detected by shutting off the burner on the glycol reboiler
system at night and looking down the firebox. A bright red glow will be visible
at the hot spots on the fire tube walls where salt deposits have collected. An
analysis of the glycol will determine the degree of this contamination.
It is highly recommended that, during a plant start-up, make sure the reboiler is
up to the desired operating temperature before flowing gas through the
contactor.
Some fires have been caused by leaks in the gas lines near the firebox. The best
precaution is to have valves and regulators in the gas line at a suitable distance
from the firebox. Another very effective measure is the addition of a flame
arrestor around the firebox. If the flame arrestor is properly designed, even
severe gas leaks in the immediate vicinity of the firebox will not ignite.
INTRODUCTION TO OIL&GAS PRODUCTION
101
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
4. OPERATING PROBLEMS AND GLYCOL CARE:
Most operating and technical problems usually occur when the circulating
glycol solution gets dirty. In order have a long, trouble free life with the glycol
system, it is necessary and very important to recognize these problems and
know how to prevent them.
SOME OF THE MAJOR PROBLEMS ARE:
1.
2.
3.
4.
5.
6.
7.
8.
GLYCOL LOSS
FOAMING
THERMAL DECOMPOSITION OF THE GLYCOL
DEW POINT CONTROL
GLYCOL pH CONTROL
SALT CONTAMINATION
GLYCOL OXIDATION
SLUDGE FORMATION
1. GLYCOL LOSS
The physical loss of glycol is probably the most important operating problem in
the dehydration system. Most dehydration units are designed for a loss of less
than 0.10 gallons of glycol per million cubic feet of natural gas. However, if the
system is not operated properly, the loss might be much higher than this.
The glycol contactor and the glycol regenerator are the most common places in
a dehydration system where about 90% of the glycol loss occurs. High gas
velocity through the glycol contactor will cause a carryover of glycol into the
pipeline, and a poor mist eliminator in the top of the glycol contactor will pass
some glycol even at normal gas velocities.
The glycol losses occurring in the glycol regenerator are usually caused by
excessive reboiler temperature, which causes vaporization or thermal
decomposition of glycol. Also, excessive top temperature in the still column
allows vaporized glycol to escape from the still column to atmosphere with the
water vapour.
INTRODUCTION TO OIL&GAS PRODUCTION
102
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
2. FOAMING
Foaming of glycol is another problem frequently encountered. It can increase
glycol loss and reduce the plant capacity. Entrained glycol will carry over from
the contactor with the sales gas. Also, foaming can cause poor contact between
the gas and the glycol solution; therefore, the drying efficiency is decreased.
The best cure for glycol foaming is the proper care of glycol solution. The most
important measures in the programme are effective gas cleaning ahead of the
glycol system and good filtration of the glycol solution.
De-foaming agents, such as Mono-ethanolamine (MEA), are widely used to
control the problem. However, it is very important to point out that the use of
these does not solve the basic problem, and it is only a temporary measure until
the cause of the foaming can be determined and eliminated.
SOME FACTORS THAT CAUSE FOAMING ARE:
Low glycol solution concentration to the contactor.
High differential temperature between wet gas inlet and lean glycol
inlet to contactor.
High glycol pH, Note: basic glycol solution pH > 9 tends to foam
and emulsify.
Hydrocarbon liquids, condensate.
Finely divided suspended solids.
Salt contamination.
Field corrosion inhibitors.
3. THERMAL DECOMPOSITION OF GLYCOL
It has been established that the glycol reboiler temperature is limited by the
TEG decomposition temperature, and glycol vaporization losses. Laboratory
data indicates that TEG is thermally stable up to about 400 oF. Excessive heat as
a result of one or more of the following conditions will decompose the TEG and
form corrosive compounds:
A high reboiler temperature above the glycol decomposition level.
Localized overheating caused by deposits of salt or tarry
compounds on the reboiler fire tube or by flame impingement on
the fire tube.
INTRODUCTION TO OIL&GAS PRODUCTION
103
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
4. DEW POINT CONTROL
Dew Point is the temperature at which water vapour first starts to condense to
liquid. In industry, the dew point is used to indicate the water vapour content in
a gas stream. For the dew point to have a meaning as a descriptive term, the
pressure at which it is measured must be stated.
When dew point depression of the treated gas is too low, there can be several
causes such as; low glycol circulation rate, low glycol concentration, foaming,
blocked or dirty contacting devices in the tower, high gas velocity in the
contactor, etc.
As a conclusion, the dew point depression indicated the extent to which the
moisture content of the gas is lowered. For example, a 50 o dew point depression
below a saturation temperature of 80oF at 600 psia, would indicate that the
natural gas, after dehydration, would have to be cooled to 30oF before any
condensation would occur.
The greater the dew point depression, the more water vapour removed.
5. GLYCOL pH CONTROL
The pH of a glycol solution is a measure of its acidity or alkalinity, and is
measured on a scale of 0 14. A pH of less than 7 is an acid solution, 7 is
neutral and greater than 7 is alkaline.
The corrosion rate of equipment increases rapidly with decrease in the glycol
pH. The formation of organic acids, resulting from the oxidation of glycol,
thermal decomposition products or acid gases picked up from the gas stream,
are the most troublesome corrosive compounds.
Therefore, the pH of the glycol should be checked periodically and kept on the
alkaline side by neutralizing the acidic compounds with borax, Ethanolamines
or other suitable alkaline chemicals to maintain the pH at 7.5 to 8.0. A glycol
solution that is too alkaline, i.e. a pH greater than 9.0, tends to foam and
emulsify.
INTRODUCTION TO OIL&GAS PRODUCTION
104
FIELD TRAINING/RAGUBA 2010
TRAINING & DEVELOPMENT
INDUSTRIAL TRAINING
10.2.2 GAS SWEETENING :
Many natural gases are produced from wells containing hydrogen
sulphide, sulphur compounds, and carbon dioxide. These gases are treated prior
to sale or entry to process plant. A gas which has concentration of sulphur
compounds called Sour gas ( also called Acid gas ).
The gas produced from sahl field is Sour because it contains a high
concentration of hydrogen sulphide (H2S) and some carbon dioxide (CO2 ).
The H2S and CO2 are known as acid gases, and are corrosive, if water or oxygen
are present.
The hydrogen sulphide must be removed from the natural gas before it can be
used, due to the fact that it is highly corrosive and deadly toxic.
To remove sulphur compounds from the produced gases in sahl field,
a chemical solution called Methyl Di-Ethanol Amine ( MDEA ) is used.
There are a number of absorption gas treating processes, but the most
common is the amine process in which acid gases react chemically. The rich
amine solution is heated under low pressure to regenerate the liquid by driving
off the acid gases.
The sour natural gas stream from the gas scrubber enters the bottom of
the amine absorber tower . The lean Amine solution enters at the top tray of the
absorber which contains 20 trays.
As the gas flow upward through the absorber tower, it contacts the down
flowing lean amine solution. The H2S is absorbed from the gas by Amine
solution flowing across the trays and downward through the tower.
The Amine solution becomes more saturated ( richer ) with H2S as it
passes across each tray and to bottom of the absorber tower from where it is
piped to the amine regeneration section for reused again. While the sweet gas
passes from the top via a mist extractor element and is piped to the dehydration
section of the plant.
( see the simplified plant diagram @ page 95 )
INTRODUCTION TO OIL&GAS PRODUCTION
105
FIELD TRAINING/RAGUBA 2010
You might also like
- Pet Econs Calculations - LatestDocument167 pagesPet Econs Calculations - Lateststanlnleybuduka100% (1)
- Enhanced Gas RecoveryDocument4 pagesEnhanced Gas RecoveryAlaa Nagie100% (1)
- Petroleum Refining and PetrochemicalsDocument1 pagePetroleum Refining and PetrochemicalsadityamduttaNo ratings yet
- PWC Oil and Gas Review 2015Document60 pagesPWC Oil and Gas Review 2015Amos DesmondNo ratings yet
- Production OperationDocument28 pagesProduction OperationAbuzeid100% (1)
- Crude Oil Properties and Condensate Properties and CorrelatiDocument20 pagesCrude Oil Properties and Condensate Properties and CorrelatiPablo Mendoza100% (1)
- 3 ArtificialliftsystemsDocument29 pages3 Artificialliftsystemsstephaniebritosuare2No ratings yet
- JPTDocument144 pagesJPTbilalNo ratings yet
- SubPUMP 2011Document2 pagesSubPUMP 2011Bealca AdiestamientoNo ratings yet
- Sesi 2. Introduction Oil & Gas AccountingDocument27 pagesSesi 2. Introduction Oil & Gas AccountingAnkga SuhendaNo ratings yet
- Petroleum Project Economics 05Document16 pagesPetroleum Project Economics 05ediwskiNo ratings yet
- Project Report RajkishanDocument58 pagesProject Report RajkishanRajkishan GuptaNo ratings yet
- Final CO2 Capture and Storage (CCS)Document34 pagesFinal CO2 Capture and Storage (CCS)Kaushal PatelNo ratings yet
- Petroleum ProductionDocument36 pagesPetroleum Productionadeeyo100% (1)
- How Rich Is RichDocument8 pagesHow Rich Is RichJulio C MarchenaNo ratings yet
- 0 - Special Topics in Oil and Gas ProductionDocument10 pages0 - Special Topics in Oil and Gas ProductionAliNo ratings yet
- 2008 IPTC Conference Preview FINALDocument72 pages2008 IPTC Conference Preview FINALHing Chai ShingNo ratings yet
- Shale Gas 2016 - FinalDocument33 pagesShale Gas 2016 - FinalNandani SudamaNo ratings yet
- Shell CaseDocument12 pagesShell CasewchaitanNo ratings yet
- Crude Oil PropertiesDocument8 pagesCrude Oil Propertiesjoiya1001No ratings yet
- Well Test AnalysisDocument6 pagesWell Test AnalysisnrnakNo ratings yet
- Gas Transportation Storage I 2018Document52 pagesGas Transportation Storage I 2018Johny ImitazNo ratings yet
- Shale ReviewDocument17 pagesShale ReviewAnonymous QZd4nBoTNo ratings yet
- Artificial Lift Methods Comparison Table PDFDocument11 pagesArtificial Lift Methods Comparison Table PDFBerty SimanjuntakNo ratings yet
- Economic Viability of UGS in NigeriaDocument87 pagesEconomic Viability of UGS in NigeriaNwachukwu Caleb Kelechi100% (3)
- PetroSkills - Surface Production OperationsDocument2 pagesPetroSkills - Surface Production OperationsMausam GauravNo ratings yet
- Tullow Oil 2007 Interim Results Presentation (Final)Document61 pagesTullow Oil 2007 Interim Results Presentation (Final)Ankur SharmaNo ratings yet
- Gas Treating and ProcessingDocument3 pagesGas Treating and ProcessingRoger AP100% (1)
- Oil Sands Millennium ProjectDocument9 pagesOil Sands Millennium ProjectMIRCEA1305No ratings yet
- Petroleum and Its ProductsDocument42 pagesPetroleum and Its ProductsmahmoudmakladNo ratings yet
- Enhanced Oil Recovery - DocxsDocument4 pagesEnhanced Oil Recovery - Docxsbu7amudNo ratings yet
- OGJ Worldwide Refining Survey 2010Document67 pagesOGJ Worldwide Refining Survey 2010Zahra GhNo ratings yet
- Peak OilDocument55 pagesPeak OilMahant MotheyNo ratings yet
- Acquiring Upstream Assets Via Joint Ventures - Akin Gump Straus Hauer & Feld LLPDocument39 pagesAcquiring Upstream Assets Via Joint Ventures - Akin Gump Straus Hauer & Feld LLPshortmycdsNo ratings yet
- CO2 FloodingDocument15 pagesCO2 FloodingUzumaki28No ratings yet
- CHAPTER 1: What Is Oil & Gas Exploration?: 1. Upstream A. Exploration I. Geophysical SurveysDocument43 pagesCHAPTER 1: What Is Oil & Gas Exploration?: 1. Upstream A. Exploration I. Geophysical SurveysMuhammad Ridhwan100% (2)
- Petroleum QuizDocument62 pagesPetroleum Quizchoksi himanshu100% (1)
- Week 3 Tar SandDocument31 pagesWeek 3 Tar SandAzkaaNo ratings yet
- Gas LiftBestPracticesDocument80 pagesGas LiftBestPracticesRaed fouad100% (1)
- Underground Gas Storage: ISO 9001: 2008 Certified CompanyDocument6 pagesUnderground Gas Storage: ISO 9001: 2008 Certified CompanycuervohijoguachoNo ratings yet
- Iraq ESP1Document6 pagesIraq ESP1juliocanel2009No ratings yet
- KBC White Paper - Maximising Margin in A Competitive EnvironmentDocument4 pagesKBC White Paper - Maximising Margin in A Competitive EnvironmentAndrew J RobertsNo ratings yet
- The Essential of Gas Lift Technologies - Part One 00Document126 pagesThe Essential of Gas Lift Technologies - Part One 00Giuseppe MoriccaNo ratings yet
- Real Ccs Audit Report TemplateDocument32 pagesReal Ccs Audit Report TemplateLALUKISNo ratings yet
- Drive Mechanism PDFDocument28 pagesDrive Mechanism PDFvinthegreat84No ratings yet
- Flow AssuranceDocument7 pagesFlow AssuranceMubarik AliNo ratings yet
- Reservoir Fluid and Its PropertiesDocument24 pagesReservoir Fluid and Its PropertiesMod Hah GhasdcNo ratings yet
- 6 Petroleum and Natural Gas Life Cycle AnalysisDocument8 pages6 Petroleum and Natural Gas Life Cycle AnalysisDimitris HatzidimosNo ratings yet
- Flowing WellsDocument18 pagesFlowing WellsJorge RidezNo ratings yet
- Chap2 - Fundamentals and Principles of Natural Gas ProcessingDocument22 pagesChap2 - Fundamentals and Principles of Natural Gas Processingghgh140No ratings yet
- Training in Production and Facilities Operation Final Rev - PPT - 5405839 - 01Document117 pagesTraining in Production and Facilities Operation Final Rev - PPT - 5405839 - 01Gabriela PaulinaNo ratings yet
- Fundamental of Exploration and ProductionDocument34 pagesFundamental of Exploration and ProductionVelya Galyani Pasila Galla100% (1)
- Well Production ProblemsDocument22 pagesWell Production ProblemsMohammed Khaled Al-ThobhaniNo ratings yet
- An Introduction To EORDocument4 pagesAn Introduction To EORMnesNo ratings yet
- Final Examination Introduction To Oil and Gas Sustainable DevelopmentDocument22 pagesFinal Examination Introduction To Oil and Gas Sustainable DevelopmentAhmad AdninNo ratings yet
- Mdea - Fact SheetDocument4 pagesMdea - Fact SheetdanjakobNo ratings yet
- Petroleum SystemDocument19 pagesPetroleum SystemBright MoonNo ratings yet
- Artificial LiftDocument11 pagesArtificial LiftsiriuslotNo ratings yet
- Artificial Lift Design of Mishrif Formation in Nasiriyah Oil FieldDocument21 pagesArtificial Lift Design of Mishrif Formation in Nasiriyah Oil FieldSufian R EllabbadNo ratings yet
- Safety & Environmental ControlDocument120 pagesSafety & Environmental ControlSufian R Ellabbad100% (1)
- Producing Oil From Dead Oil Wells Using Injected LPGDocument5 pagesProducing Oil From Dead Oil Wells Using Injected LPGSufian R EllabbadNo ratings yet
- Using The Artificial Gas Lift To Increase The Productivity of Noor OilDocument6 pagesUsing The Artificial Gas Lift To Increase The Productivity of Noor OilSufian R EllabbadNo ratings yet
- Review of Artificial Lift PDFDocument9 pagesReview of Artificial Lift PDFSufian R EllabbadNo ratings yet
- Evaluation Continous Gas LiftDocument10 pagesEvaluation Continous Gas LiftRima Apriani JamilahNo ratings yet
- Safety & Environmental ControlDocument120 pagesSafety & Environmental ControlSufian R Ellabbad100% (1)
- Access BasicsaccessDocument11 pagesAccess Basicsaccesskamal_potter1707No ratings yet
- Well Logging - Chapter OneDocument31 pagesWell Logging - Chapter OneSufian R EllabbadNo ratings yet
- ChemistryDocument117 pagesChemistrySufian R EllabbadNo ratings yet
- Examinations of The Performance of A Gas Lift For Oil Well ProductionDocument7 pagesExaminations of The Performance of A Gas Lift For Oil Well ProductionOmid ShahbaziNo ratings yet
- Coiled TubingDocument32 pagesCoiled TubingGeetha Bakki100% (2)
- Well Test SetupDocument54 pagesWell Test SetupADNANNo ratings yet
- Production OptimizationDocument36 pagesProduction OptimizationLawrence MbahNo ratings yet
- Optimization of Gas-Injected Oil WellsDocument11 pagesOptimization of Gas-Injected Oil WellsSufian R EllabbadNo ratings yet
- Well StringDocument2 pagesWell StringSufian R EllabbadNo ratings yet
- C164 Francesco IFACWCDocument6 pagesC164 Francesco IFACWCSufian R EllabbadNo ratings yet
- 1 IntroductionToArtificialLiftMethodsDocument29 pages1 IntroductionToArtificialLiftMethodsMahesh MahajanNo ratings yet
- Presentation BP Casing PressDocument25 pagesPresentation BP Casing PressSufian R EllabbadNo ratings yet
- API RP 19G13 - Section 5.6 - Very Deep Gas-Lift - Version 2011-04-03Document51 pagesAPI RP 19G13 - Section 5.6 - Very Deep Gas-Lift - Version 2011-04-03Sufian R EllabbadNo ratings yet
- Gasliftequipments PDFDocument51 pagesGasliftequipments PDFSufian R EllabbadNo ratings yet
- Om Series Kickover PDFDocument2 pagesOm Series Kickover PDFSufian R EllabbadNo ratings yet
- Basic Hydraulic Fracturing: James A. CraigDocument51 pagesBasic Hydraulic Fracturing: James A. CraigSufian R EllabbadNo ratings yet
- 2013 Gas-Lift Form - Abstracts and NotesDocument23 pages2013 Gas-Lift Form - Abstracts and NotesSufian R EllabbadNo ratings yet
- Why You Should Consider Diagnostic Surface Management For OilDocument2 pagesWhy You Should Consider Diagnostic Surface Management For OilSufian R EllabbadNo ratings yet
- How A Well FlowsDocument34 pagesHow A Well FlowsfddddddNo ratings yet
- Esp Skimetic For Waha OrderDocument1 pageEsp Skimetic For Waha OrderSufian R EllabbadNo ratings yet
- The Effects On Production RatesDocument1 pageThe Effects On Production RatesSufian R EllabbadNo ratings yet
- Gas Lift QuizDocument7 pagesGas Lift QuizTripoli TR TRNo ratings yet
- Nodal Analysis Indentifies ESP Wellhead Choke ProblemsDocument14 pagesNodal Analysis Indentifies ESP Wellhead Choke ProblemsSufian R EllabbadNo ratings yet
- PDFDocument253 pagesPDFWilmar ValenzuelaNo ratings yet
- Indo-US Relations in 21st CenturyDocument10 pagesIndo-US Relations in 21st CenturyDr. Afroz Alam100% (1)
- Annibal Bonavides PetrobrasDocument18 pagesAnnibal Bonavides PetrobraswilsongringoNo ratings yet
- ACDC Home Improvement ApplicationDocument16 pagesACDC Home Improvement ApplicationNativeVillageofEyakNo ratings yet
- Chemical Hazards in RefineryDocument11 pagesChemical Hazards in RefineryRuqiyya Israfilova100% (1)
- Oil & Gas - Introduction To Upstream - 09112011Document14 pagesOil & Gas - Introduction To Upstream - 09112011Efemena DorohNo ratings yet
- Ikoku Ch1 Eng 01 52 EstimReservDocument37 pagesIkoku Ch1 Eng 01 52 EstimReservVictor Gomez100% (1)
- Health Care, Oil and Gas SectorDocument15 pagesHealth Care, Oil and Gas SectorDhruvil GorsawalaNo ratings yet
- Environmental Key Performance IndicatorsDocument68 pagesEnvironmental Key Performance Indicatorssb3stripes100% (6)
- Romanian Association of Drilling Contractors ACFRDocument83 pagesRomanian Association of Drilling Contractors ACFRFuBasho33% (3)
- Basic Principles of Wind Energy ConversionDocument10 pagesBasic Principles of Wind Energy ConversionTEAM BLACK50% (2)
- Objective: Aramco. Shell Brunei, Phe Onwj, Santos, Emp, Medco Energy Natuna, Medco Tomori, Ophir EnergyDocument2 pagesObjective: Aramco. Shell Brunei, Phe Onwj, Santos, Emp, Medco Energy Natuna, Medco Tomori, Ophir EnergyPurnomoEbeNo ratings yet
- 4915 PDFDocument79 pages4915 PDFyyamidNo ratings yet
- BAUER Compressors For Industry enDocument40 pagesBAUER Compressors For Industry enhao100% (1)
- Sample Grant Proposal Wind Energy Low IncomeDocument20 pagesSample Grant Proposal Wind Energy Low Incomeprodigl100% (1)
- JPT 2018-03 PDFDocument100 pagesJPT 2018-03 PDFJohnNo ratings yet
- Land Transportation HistoryDocument62 pagesLand Transportation HistoryDominicSavioNo ratings yet
- Gas ConversionDocument3 pagesGas ConversionKarim MuhammedNo ratings yet
- 2007 Nfpa 88a PDFDocument13 pages2007 Nfpa 88a PDFN V Sumanth VallabhaneniNo ratings yet
- Mark Diesen Dorf Renewable EnergyDocument13 pagesMark Diesen Dorf Renewable EnergymaronnamNo ratings yet
- Oilfield Technology April 2013Document118 pagesOilfield Technology April 2013asadashfaqlodhiNo ratings yet
- Acronyms DefinitionsDocument27 pagesAcronyms DefinitionsjohnNo ratings yet
- Deppl: A Consortium Partner Of: Engineering Research & Development Consultant's GroupDocument27 pagesDeppl: A Consortium Partner Of: Engineering Research & Development Consultant's GroupMehdi Hajd KacemNo ratings yet
- Overview LNG Central Java V.1.2.REV1Document10 pagesOverview LNG Central Java V.1.2.REV1sigit l.prabowoNo ratings yet
- Chem Unit 1 PDFDocument32 pagesChem Unit 1 PDFomjee7408No ratings yet
- Vaghode Pratik MarotiDocument26 pagesVaghode Pratik Marotisaish kailas chaudhariNo ratings yet
- Root Cause Analysis (RCA) of Fractured ASTM A53 Carbon Steel Pipe at Oil & Gas CompanyDocument8 pagesRoot Cause Analysis (RCA) of Fractured ASTM A53 Carbon Steel Pipe at Oil & Gas CompanynaderbahramiNo ratings yet
- Etd Tamu 2004A PETE Seo 1Document132 pagesEtd Tamu 2004A PETE Seo 1Philip Jezreel SugaboNo ratings yet
- GTG 1733PW LM6000 220MwBarge 50HzDocument11 pagesGTG 1733PW LM6000 220MwBarge 50Hzwidyo saptoto100% (1)
- Comparative Study of Performance of LPG Fuelled Si Engine at Different Compression Ratio and Ignition TimingDocument7 pagesComparative Study of Performance of LPG Fuelled Si Engine at Different Compression Ratio and Ignition TimingIAEME PublicationNo ratings yet
- Internal Combustion: How Corporations and Governments Addicted the World to Oil and Subverted the AlternativesFrom EverandInternal Combustion: How Corporations and Governments Addicted the World to Oil and Subverted the AlternativesRating: 4 out of 5 stars4/5 (2)
- Advanced Production Decline Analysis and ApplicationFrom EverandAdvanced Production Decline Analysis and ApplicationRating: 3.5 out of 5 stars3.5/5 (4)
- Well Control for Completions and InterventionsFrom EverandWell Control for Completions and InterventionsRating: 4 out of 5 stars4/5 (10)
- Well Integrity for Workovers and RecompletionsFrom EverandWell Integrity for Workovers and RecompletionsRating: 5 out of 5 stars5/5 (3)
- Pocket Guide to Flanges, Fittings, and Piping DataFrom EverandPocket Guide to Flanges, Fittings, and Piping DataRating: 3.5 out of 5 stars3.5/5 (22)
- Asphaltene Deposition Control by Chemical Inhibitors: Theoretical and Practical ProspectsFrom EverandAsphaltene Deposition Control by Chemical Inhibitors: Theoretical and Practical ProspectsNo ratings yet
- Case Studies of Material Corrosion Prevention for Oil and Gas ValvesFrom EverandCase Studies of Material Corrosion Prevention for Oil and Gas ValvesNo ratings yet
- Casing and Liners for Drilling and Completion: Design and ApplicationFrom EverandCasing and Liners for Drilling and Completion: Design and ApplicationRating: 5 out of 5 stars5/5 (3)
- An Operations Guide to Safety and Environmental Management Systems (SEMS): Making Sense of BSEE SEMS RegulationsFrom EverandAn Operations Guide to Safety and Environmental Management Systems (SEMS): Making Sense of BSEE SEMS RegulationsNo ratings yet
- Machine Learning Guide for Oil and Gas Using Python: A Step-by-Step Breakdown with Data, Algorithms, Codes, and ApplicationsFrom EverandMachine Learning Guide for Oil and Gas Using Python: A Step-by-Step Breakdown with Data, Algorithms, Codes, and ApplicationsRating: 4 out of 5 stars4/5 (4)
- Advanced Biomass Gasification: New Concepts for Efficiency Increase and Product FlexibilityFrom EverandAdvanced Biomass Gasification: New Concepts for Efficiency Increase and Product FlexibilityRating: 3 out of 5 stars3/5 (2)
- The Petroleum Engineering Handbook: Sustainable Operations: Sustainable OperationsFrom EverandThe Petroleum Engineering Handbook: Sustainable Operations: Sustainable OperationsRating: 3.5 out of 5 stars3.5/5 (5)
- Well Testing Project Management: Onshore and Offshore OperationsFrom EverandWell Testing Project Management: Onshore and Offshore OperationsNo ratings yet
- Oil: An Overview of the Petroleum IndustryFrom EverandOil: An Overview of the Petroleum IndustryRating: 4.5 out of 5 stars4.5/5 (3)
- Oil and Gas Artificial Fluid Lifting TechniquesFrom EverandOil and Gas Artificial Fluid Lifting TechniquesRating: 5 out of 5 stars5/5 (1)