Professional Documents
Culture Documents
Ribeiro Et Al 2003
Uploaded by
baglamaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ribeiro Et Al 2003
Uploaded by
baglamaCopyright:
Available Formats
Acta Oecologica 24 (2003) S117S123
www.elsevier.com/locate/actoec
Taxonomic survey of the microphytobenthic
communities of two Tagus estuary mudflats
Loureno Ribeiro a,*, Vanda Brotas a, Grard Mascarell b, Alain Cout b
a
Faculdade de Cincias, Instituto de Oceanografia, Universidade de Lisboa, Campo Grande, 1749-017 Lisbon, Portugal
b
Laboratoire de Cryptogamie, Musum National dHistoire Naturelle, 12, rue Buffon, 75005 Paris, France
Abstract
A taxonomic survey of the microphytobenthic communities was conducted in two intertidal mudflats of the Tagus estuary. These mudflats
are located in different littoral zones of the estuary and had distinct sediment characteristics and salinity range. Sampling was performed
monthly during low tide over a 14-month-period. Sediment cores were collected and brought to the laboratory, where the microalgae were
harvested using the lens-tissue technique under light stimulation. Samples were examined using both light and scanning electron microscopy.
The epipelic pennate diatoms dominated the microphytobenthic assemblages on both mudflats but cyanobacteria and euglenophytes were also
found. Of the 52 taxa identified, 47 were pennate diatoms, five of which seem to be previously unreported for Portugal. The species more
frequently found in the samples were Gyrosigma fasciola, Cylindrotheca cf. signata, Cylindrotheca closterium, Staurophora amphioxys and
Navicula gregaria.
2003 ditions scientifiques et mdicales Elsevier SAS. All rights reserved.
Keywords: Microphytobenthos; Species identification; Tagus estuary
1. Introduction
Microphytobenthic communities are the main primary
producers in intertidal flats of many estuaries. In the case of
the Tagus estuary, they have an estimated primary production
of 11 000 tonnes carbon per year (Brotas and Catarino,
1995), which is about two-fold of the annual primary production of the phytoplankton indicated by (Cabeadas et al.,
2000). The extensive biofilms that develop on the surface of
intertidal sediments are also an active element of the biogeochemistry cycles of sediment-air and sediment-water column interfaces (Cabrita and Brotas, 2000; Dong et al., 2000).
While the taxonomical composition of the phytoplankton
communities of the Tagus estuary has been assessed several
times in the last 30 years ((Moita and Vilarinho, 1998), and
references herein), a thorough taxonomic characterisation of
the benthic microalgae of the Tagus and other Portuguese
estuaries is yet to be achieved. Nevertheless, the overall
structure of the microphytobenthic communities found in the
Tagus intertidal flats and salt marshes, as well as their seasonal and spatial variation, was already established (Brotas
and Plante-Cuny, 1998; Brotas et al., 1995). The former
* Corresponding author.
E-mail address: lourosr@fc.ul.pt (L. Ribeiro).
2003 ditions scientifiques et mdicales Elsevier SAS. All rights reserved.
DOI: 1 0 . 1 0 1 6 / S 1 1 4 6 - 6 0 9 X ( 0 3 ) 0 0 0 1 2 - 2
work, where these communities were studied using HPLC
pigment analysis, concluded that diatoms were dominant but
euglenoids and cyanobacteria were also frequently present.
Brotas and Catarino (Brotas and Catarino, 1995) refer a set of
the 10 most common epipelic species found in the intertidal
mudflats of the Tagus estuary.
The benthic estuarine microalgae have always posed
problems to algal taxonomists (Riznyk, 1973). Sampling, as
well as the separation of the microphytobenthos from the
sediment, and cleansing procedures are hampered by several
methodological difficulties. An additional obstacle comes
from the fact that the taxonomical literature regarding this
brackish water group is still quite scarce and scattered, when
compared to the fresh water and planktonic studies.
The objective of this work was to contribute to the knowledge on the taxonomical composition of the microphytobenthic assemblages of the Tagus intertidal estuarine areas,
giving special attention to the motile epipelic fraction.
2. Materials and methods
The Tagus estuary (3844'N, 0908'W) is the largest estuary in Western Europe, covering an area of approximately
320 km2. The estuary has a vast inner bay with extensive
intertidal areas. These areas are essentially constituted of
S118
L. Ribeiro et al. / Acta Oecologica 24 (2003) S117S123
Fig. 1. Map of the Tagus estuary showing the sampled stations (Pancas and
Rosrio). Hatched areas, saltmarsh. Thin line, lower limit of intertidal zone.
mudflats, which have an estimated total area of 100 km2
(Brotas and Catarino, 1995). The Tagus estuary is mesotidal
with a mean tidal rage of 2.4 m, varying from about 1 m at
neap tides to about 4 m at spring tides.
Sampling was carried out at two stations, Pancas (P) and
Rosrio (R), situated on two mudflats in the southeastern
margin of the estuary (Fig. 1). Pancas is located in the inner
estuary on the upper eullitoral zone, while Rosrio is located
in the outer estuary on the lower eullitoral zone. Various
physicochemical parameters were analysed during sampling
and were previously published (Cabrita and Brotas, 2000).
Pancas had an annual salinity range of 3-33 and had
muddy sediment (97.5% of silt and clay, in average), whereas
Rosrios salinity range was 16-37 and its sediment, although muddy, was much more sandier (59.9% of silt and
clay, in average) and showed a considerably variation during
the sampling period.
Both stations were sampled monthly between March 1997
and April 1998. A second site was sampled at each station,
every 3 months, to account for local variability. A total of 34
samples were collected during the sampling period.
The method chosen for harvesting the microphytobenthic
community was the lens-tissue technique (Eaton and Moss,
1966). This selective method collects the motile epipelic
fraction of the microphytobenthos, which is known to migrate vertically in response to light stimulus and tidal
rhythms. Sediment samples were taken using cylindrical
Plexiglas corers (13 cm long 8 cm i.d.) during low spring
tides and brought to the laboratory for further processing.
The sediment corers were then placed in a box filled with
estuarine water and several 2 cm2 pieces of lens-tissue were
laid on the sediments surface and left undisturbed for 24 h.
During the exposure period, the corers were artificially illuminated for 2 h, after which two lens-tissue pieces were
picked and placed in a 10 ml beaker containing a mixture of
filtered estuarine water and glutaraldehyde (final concentration 2.5% glutaraldehyde).
For each sample several slides were screened under a M20
WILD optic microscope, equipped with a Camera Lucida.
Drawings of every taxon encountered were made for subsequent identification. The slides were made directly from the
samples, without any kind of cleansing treatment, in order to
preserve other algal groups beside diatoms. Specific samples
that covered the majority of the taxa observed during the light
microscopy sessions were selected for electron microscopy.
Aliquots of these samples were placed in a transparent inkwell, treated with hydrogen peroxide 33% and rinsed with
bi-distilled water. The liquid containing the cleaned frustules
was then siphoned by a 10 ml syringe and filtered using a
Millipore Membrane Filter with an average pore diameter
of 1.2 m. The filters were dehydrated in pure ethanol and
critical point dried. Each dried filter was placed on an aluminium pin stub and gold-palladium coated. An alternative
method was also used and consisted in isolating the cleaned
frustules with a capillary pipette, under a compound dissecting microscope, and mounting them directly on the surface of
the pin stub. The diatom micrographs (ILFORD FP4 LM 125
ASA/120 film) were taken on a JEOL 840 Scanning Electron
Microscope using an acceleration voltage of 8 kV.
The taxa were identified following the descriptions by
Bourrely (Bourrely, 1981), Cox (Cox, 1996), Germain (Germain, 1980), Krammer and Lange-Bertalot (Krammer and
Lange-Bertalot, 1986; Krammer and Lange-Bertalot, 1988),
Patrick and Reimer (Patrick and Reimer, 1966; Patrick and
Reimer, 1975), Peragallo and Peragallo (Peragallo, 1897),
Ricard (Ricard, 1987), Round et al. (Round et al., 1990) and
Witkowski et al. (Witkowski et al., 2000).
3. Results
A total of 48 diatom taxa were identified to the genus or
species level (Table 1). With the exception of Cyclotella
meneghiniana, they were all pennate monoraphid or biraphid
diatoms and belonged to 24 different genera. Regarding other
algal groups, two unidentified species of Euglena, as well as
one Oscillatoria sp., were found in both stations. A species of
the cyanobacteria Merismopedia occurred only in Rosrio.
Out of 43 taxa found in Pancas, 29 occurred in Rosrio
also, which had a slightly lower number of species (38).
Although the majority of the taxa encountered were common
to both stations, Pancas had 14 specific taxa and Rosrio
nine.
The additional sampling made every 3 months in Pancas
and Rosrio did not show any apparent difference in terms of
taxa composition within each station. Although these
samples were collected 400 and 250 m apart from the sites
sampled monthly, in Pancas and Rosrio, respectively, only
L. Ribeiro et al. / Acta Oecologica 24 (2003) S117S123
S119
Table 1
List of the taxa found on two mudflats of the Tagus estuary. Pancas (P) and
Rosrio (R)
Table 2
Taxa found in more than 2/3 (66%) of the samples of each station and
considering both stations together
Taxa
Bacillariophyta
Achnanthes brevipes Agardh
Amphora coffeaeformis (Agardh) Ktzing
Amphora spp.
Bacillaria paxillifer (O.F. Mller) Hendey
Berkeleya sp.
Caloneis westii W. Smith
Cocconeis sp.
Cosmioneis pusilla (W. Smith) Mann and Stickle
Cyclotella meneghiniana Ktzing
Cylindrotheca closterium Reinmann and Lewin
C. cf. signata Reinmann and Lewin
Cymatopleura solea (Brbisson) W. Smith
Diploneis didyma (Ehrenberg) Cleve
Entomoneis alata (Ehrenberg) Ehrenberg
E. paludosa (W. Smith) Reimer
Epithemia sp.
Frustulia interposita (Lewis) De Toni
Gyrosigma cf. acuminatum (Ktzing) Rabenhorst var. gallica
Grunow
G. (Ktzing) Cleve
G. balticum (Ehrenberg) Rabenhorst
G. distortum (W. Smith) Griffith and Henfrey
G. fasciola (Ehrenberg) Cleve
G. limosum Sterrenburg and Underwood
Gyrosigma sp.
Navicula cryptocephala Ktzing
N. gregaria Donkin
N. phyllepta Ktzing
N. cf. digito-radiata (Gregory) Ralfs
Navicula spp.
Nitzschia acicularis (Ktzing) W. Smith
N. alexandrina (Cholnoky) Lange-Bertalot and Simonsen
N. navicularis (Brbisson) Grunow
N. reversa W. Smith
N. sigma (Ktzing) W. Smith
N. tubicula Grunow
Nitzschia sp.
Petrodyction gemma (Ehrenberg) D.G. Mann
Plagiotropis sp. 1
Plagiotropis sp. 2
Pleurosigma angulatum (Quekett) W. Smith
P. angulatum (Quekett) W. Smith var. aestuarii (Brbisson)
Staurophora amphioxys (Gregory) D.G. Mann
Surirella brebissonii Krammer and Lange-Bertalot
Surirella cf. brightwellii W. Smith
Tryblionella apiculata Gregory
T. gracilis W. Smith
T. hungarica (Grunow) D.G. Mann
Vanheurckia lewisiana (Greville) Brbisson
Pancas
T. gracilis
C. cf. signata
G. limosum
N. gregaria
S. amphioxys
G. attenuatum
G. fasciola
N. sigma
G. distortum
N. naviculares
G. cf. acuminatum
S. cf. brightwellii
Cyanophyta
Oscillatoria spp.
Merismopedia sp.
Euglenophyta
Euglena sp. 1
Euglena sp. 2
Station
P
P
R
R
R
R
R
P
P
P
P
P
P
P
P
P
P
R
R
R
R
R
R
R
R
R
R
P
R
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
R
R
R
R
R
R
R
R
R
R
R
R
R
R
R
R
R
R
R
R
P
P
R
R
Rosrio
C. closterium
G. fasciola
C. cf. signata
S. amphioxys
N. gregaria
D. dydima
G. limosum
S. cf. brightwellii
Plagiotropis sp2
Both stations
C. cf. signata
G. fasciola
S. amphioxys
N. gregaria
C. closterium
G. limosum
S. cf. brightwellii
D. dydima
N. sigma
two to three taxa were not common to both sets of samples
from the same station. For this reason it was decided to
consider the samples of each station as a whole in order to
attain a maximal number of taxa per station.
The taxa most frequently found in the studied samples are
shown in Table 2, where a set of 15 different species is
presented. This table displays encountered species from decreasing order of occurrence (ratio of number of samples
where species occurred to total number of samples observed), from 96 to 66%.
A close observation of Tables 1 and 2 indicates that several taxa were frequently found in both stations, e.g. Cylindrotheca signata, Gyrosigma fasciola (Fig. 2), Gyrosigma
limosum (Fig. 4), Navicula gregaria (Fig. 9) and Staurophora amphioxys (Fig. 8). Other taxa, Cylindrotheca closterium (Fig. 10), Diploneis dydima and Plagiotropis sp. 2,
were more frequently found in Rosrio. Some of the most
frequently found species in Pancas occurred exclusively in
this station, namely Tryblionella gracilis (Fig. 15), Gyrosigma attenuatum (Fig. 6), Gyrosigma distortum (Fig. 3)
and Nitzschia navicularis.
Many species listed in Table 1 have never been reported
for the Tagus estuary, moreover, five species may be considered as new records for Portugal: Frustulia interposita
(Fig. 7), Nitzschia alexandrina (Fig. 12), Nitzschia tubicula
(Fig. 13), S. amphioxys, Vanheurckia lewisiana. To our
knowledge, both F. interposita and V. lewisiana are also first
reports for Europe.
Being a qualitative study, it is difficult to infer the possible
seasonal fluctuations in the assemblages without quantitative
data (cell counts). The optic microscopy observations suggested the existence of an increase in cell number during
spring-summer, followed by a decrease in the autumn-winter
period. However, in terms of species occurrences, most of the
reported taxa did not show any kind of marked seasonality.
All taxa listed in Table 2 were present throughout the year,
for they were reported for at least nine of the 14 months
surveyed. The majority of the other taxa reported, such as
Gyrosigma cf. acuminatum (Fig. 5) Nitzschia sigma
(Fig. 11), Tryblionella apiculata (Fig. 15), Entomoneis palu-
S120
L. Ribeiro et al. / Acta Oecologica 24 (2003) S117S123
Figs. 29. Diatom SEM micrograph. Fig. 2. G. fasciola, Fig. 3. G. distortum, Fig. 4. G. limosum, Fig. 5. Gyrosigmasp. [cf. acuminatum var. gallica],
Fig. 6. G. attenuatum, Fig. 7. Frustulia interposita, Fig. 8. Staurophora amphioxis, Fig. 9. Navicula gregaria.
dosa (Fig. 16) and Surirella cf. brightwellii (Fig. 17), although not as frequent, did not show any apparent preference
for a given season during the sampling period.
4. Discussion
The microphytobenthic taxa found on both mudflats were
essentially epipelic pennate diatoms, as previously reported
(Brotas and Catarino, 1995; Brotas and Plante-Cuny, 1998)
for several similar sites in the Tagus estuary. The taxonomic
composition of the microphytobenthic assemblages of the
two studied intertidal mudflats confirms the cosmopolitan
nature of the majority of the estuarine microalgae, in particular epibenthic diatoms. Most of the identified diatoms in this
study are also listed in the revision papers of Round (Round,
1971), McIntire and Moore (McIntire and Moore, 1977) and
L. Ribeiro et al. / Acta Oecologica 24 (2003) S117S123
S121
Figs. 1017. Diatom SEM micrograph. Fig. 10. Cylindrotheca closterium, Fig. 11. Nitzschia sigma, Fig. 12. N. Alexandrina, Fig. 13. N. tubicula,
Fig. 14. Tryblionella apiculata, Fig. 15. T. gracilis, Fig. 16. Entomoneis paludosa, Fig. 17. Surirella sp. [cf. brightwellii].
Admiraal (Admiraal, 1984) for world-wide benthic estuarine
and littoral diatoms. Other taxa have also been reported in
several floristic and ecological studies from French (Riaux
and Germain, 1980; Rinc and Robert, 1983), Italian (Tolomio et al., 1999), British (Round, 1960; Zong and Horton,
1998) and North American (Amspoker and McIntire, 1978;
Riznyk, 1973) estuarine tidal flats and salt marshes.
Although motile epipelic species often dominate muddy
estuarine tidal flats (Admiraal, 1984) they are not exclusive
of these substrata. In fact, several diatoms reported in this
S122
L. Ribeiro et al. / Acta Oecologica 24 (2003) S117S123
work, such as C. meneghiniana, C. closterium or Entomoneis
alata, have been reported in publications concerning the
Tagus estuary phytoplankton (Sousa e Silva et al., 1969).
Admiraal (Admiraal, 1984) defined as plankton-benthic diatoms the species that are frequently found in estuarine plankton as well as on the sediment. This author considered them
an essential feature of estuaries and coastal ecosystems,
allowing the intermingling of benthic and pelagic food
chains.
Although Pancas and Rosrio had a similar number of
species and shared more than 2/3 of the taxa, the differences
in the taxonomic structure of the assemblages of the two
stations should not be discarded. As much as 33% of the taxa
reported in Pancas and 24% in Rosrio were exclusive to
each station. In Pancas, some of the most frequently found
species (e.g. T. gracilis, G. attenuatum) only appeared in this
station. Pancas and Rosrios stations were clearly distinct in
a number of environmental factors: Pancas had lower salinity, lower immersion periods, finer grain size and lower
nutrient content in sediment interstitial water (Cabrita and
Brotas, 2000). These results support the idea that there is a
general overlapping pattern in the species distribution along
the estuary and that some species may have a specific set of
environmental conditions, as has been suggested by several
authors (e.g. (Moore and McIntire, 1977; Underwood et al.,
1998)).
The seasonal succession of the microphytobenthic diatom
assemblages has been documented in several reports, usually
from Northern Europe (Round, 1960; Underwood, 1994).
However, there is no systematic account on the seasonal
occurrence of individual species (Admiraal, 1984). The continuous occurrence of the most frequent taxa during this
study, as well as the apparent lack of periodicity of the other
reported species in both mudflats may point otherwise. One
striking example is C. closterium that occurred abundantly in
every month surveyed, during our study, and has been often
referred as a summer species in British estuaries (Hopkins,
1964; Underwood, 1994). One possible explanation to this
fact is that the studies that reported a seasonal effect in the
overall structure of the diatom communities were made at
higher latitudes, where the annual variation of light and
temperature may have a more decisive effect on the seasonal
succession of benthic diatoms than under a mild climate,
such as in Tagus estuarys.
Studies of the microphytobenthic taxonomy in the Tagus
estuary are fundamental for the understanding the overall
ecology of these communities. Consistent quantitative and
qualitative are still needed to disclose the seasonal and spatial
changes of the epipelic assemblages, as well as their distribution along salinity or nutrient gradients in the Tagus and
other Portuguese estuaries.
Acknowledgements
The authors gratefully acknowledge Professor Yves Rinc
(Universit de Nantes) for the confirmation of the taxonomi-
cal identifications and Michle Dumont (MNHN, Paris) for
technical support, Bruno Jesus for the digitalisation of the
micrographs and Paulo Cartaxana for the text revision. This
work was supported by the Europe Union ELOISE program
under the framework of NICE project, Contract MAS3CT96-0048 and partly by Project Sat-Tagis no.
PDCTM/MAR/15256/99.
References
Admiraal, W., 1984. The ecology of estuarine sediment-inhabiting diatoms.
Progr. Phycol. Res. In: Round, F.E., Chapman, D.J. (Eds.), Biopress,
Bristol, 3, pp. 269332.
Amspoker, M.C., McIntire, C., 1978. Distribution of intertidal diatoms
associated with sediments in Yaquina estuary, Oregon. J. Phycol. 14,
387395.
Bourrely, P., 1981. Les Algues deau douce. Les Algues jaunes et brunnes,
vol. 2. Editions Boube, Paris, pp. 438.
Brotas, V., Catarino, F., 1995. Microphytobenthos primary production of
Tagus estuary intertidal flats (Portugal). Neth. J. Aquat. Ecol 29, 33339.
Brotas, V., Plante-Cuny, M.R., 1998. Spatial and temporal patterns of microphytobenthic taxa of estuarine tidal flats in the Tagus Estuary (Portugal)
using pigment analysis by HPLC. Mar. Ecol. Prog. Ser 171, 4357.
Brotas, V., Cabrita, T., Portugal, A., Serdio, J., Catarino, F., 1995. Spatiotemporal distribution of microphytobenthic biomass in tidal flats of the
Tagus estuary (Portugal). Hydrobiologia 300/301, 93104.
Cabeadas, G., Brogueira, M.J., Cabeadas, L., 2000. Southern Portugal: the
Tagus and Sado estuaries. In: Sheppard, C. (Ed.), Seas at The
Millennium-An Environmental Evaluation. Regional Chapters: Europe,
The Americas and West Africa. Pergamon, Oxford, pp. 151165.
Cabrita, M.T., Brotas, V., 2000. Seasonal variation in denitrification and
dissolved nitrogen fluxes in intertidal sediments of the Tagus estuary,
Portugal. Mar. Ecol. Prog. Ser. 202, 5165.
Cox, E.J., 1996. Identification of Freshwater Diatoms from Live Material.
Chapman and Hall, London, pp. 158.
Dong, L.F., Thornton, D.C.O., Nedwell, D.B., Underwood, G.J.C., 2000.
Denitrification in sediments of the River Colne estuary, England. Mar.
Ecol. Prog. Ser. 203, 109122.
Eaton, J.W., Moss, B., 1966. The estimation of number and pigment content
in epipelic algal populations. Limnol. Oceanogr 11, 584596.
Germain, H., 1980. Flore des Diatomes. Editions Boube, Paris, pp. 444.
Hopkins, J.T., 1964. A study of the diatoms of the Ouse estuary, SussexII.
The ecology of the mudflat diatom flora. J. Mar. Biol. Assoc. UK 44,
333384.
Krammer, K., Lange-Bertalot, H., 1986. Bacillariophyceae. In: Ettl, H.,
Gerloff, J., Heynig, H., Mollenhauer, D. (Eds.), Naviculaceae, Swasserflora von Mitteleuropa, 1. Gustav Fisher Verlag, Stuttgart, pp. 876.
Krammer, K., Lange-Bertalot, H., 1988. Bacillariophyceae. In: Ettl, H.,
Gerloff, J., Heynig, H., Mollenhauer, D. (Eds.), Bacillariaceae, Epithemiaceae, Surirellaceae, Swasserflora von Mitteleuropa, 2. Gustav
Fisher Verlag, Stuttgart, pp. 878.
McIntire, C.D., Moore, W.W., 1977. Marine littoral diatoms: ecological
considerations. In: Dietrich, W. (Ed.), The Biology of Diatoms, Botanical Monograph 13. Blackwell Scientific Publications, London,
pp. 333365.
Moita, M.T., Vilarinho, M.G., 1998. Checklist of phytoplankton species off
Portugal: 70 years (1929-1998) of studies. Portugaliae Acta Biol., Sr. B,
Sist. 18, 550.
Moore, W.W., McIntire, C.D., 1977. Spatial and seasonal distribution of
littoral diatoms in Yaquina estuary, Oregon, USA. Bot. Mar. 20, 99109.
Patrick, R., Reimer, C.W., 1966. The Diatoms of the United States. Acad.
Nat. Sci. Phil. vol. 1, 688 (Monograph 13).
L. Ribeiro et al. / Acta Oecologica 24 (2003) S117S123
Patrick, R., Reimer, C.W., 1975. The Diatoms of the United States. Acad.
Nat. Sci. Phil. vol. 2, 213 (Monograph 13).
Peragallo, H., Peragallo, M., 18971908. Diatomes marines de France et
des districts maritimes voisins. Micrographie-Editeur, Grez-sur-Loing,
pp. 491.
Riaux, C., Germain, H., 1980. Peuplement de diatomes pipliques dune
slikke de Bretagne Nord, Importance relative du genre Cocconeis Ehr.
Cryptogamie Algol 1, 265279.
Ricard, M., 1987. Atlas du Phytoplancton marin. Diatomophyces, 2.
CNRS, Paris, pp. 297.
Rinc, Y., Robert, J.M., 1983. volution des peuplements de diatomes
planctoniques et benthiques dun marais salant lors des variations printanires de salinit. Cryptogamie Algol. 4, 7387.
Riznyk, R.Z., 1973. Interstitial diatoms from two tidal flats in Yaquina
estuary, Oregon, USA. Bot. Mar. 16, 113138.
Round, F.E., 1960. The diatom flora of a salt marsh on the River Dee. New
Phytol 59, 332348.
Round, F.E., 1971. Benthic marine diatoms. Oceanogr. Mar. Biol. Ann. Rev
9, 83139.
S123
Round, F.E., Crawford, R.M., Mann, D.G., 1990. The Diatoms: Biology and
Morphology of the Genera. Cambridge University Press, Cambridge,
pp. 747.
Sousa e Silva, E., Assis, M.E., Sampayo, M.A., 1969. Primary productivity
in the Tagus and Sado estuaries from May 1967 to May 1968. Notas e
Estudos Inst. Biol. Marit. 37, 131.
Tolomio, C., Moro, I., Mocschin, E., Valandro, A., 1999. Rsultats prliminaires sur les diatomes benthiques de substrats meubles deans Lagune
de Venise, Italie (mars 1994-janvier 1995). Diatom Res. 14, 367397.
Underwood, G.J.C., 1994. Seasonal and spatial variation in epipelic diatom
assemblages in the Severn Estuary. Diatom Res 9, 451472.
Underwood, G.J.C., Phillips, J., Saunders, K., 1998. Distribution of estuarine benthic diatom species along salinity and nutrient gradients. Eur. J.
Phycol 33, 173183.
Witkowski, A., Lange-Bertalot, H., Metzeltin, D., 2000. Diatom flora of
marine coasts I. In: Lange-Bertalot, H. (Ed.), Iconographia Diatomologica, 7. Ganter Verlag, Ruggell, pp. 925.
Zong, Y., Horton, B., 1998. Diatom zones across intertidal flats and coastal
saltmarshes in Britain. Diatom Res 13, 375394.
You might also like
- HeatTransferLaboratoryExperiments PDFDocument31 pagesHeatTransferLaboratoryExperiments PDFsarmedNo ratings yet
- Periphytic Diatom Community in A Mediterranean Salt Wedge Estuary: The Ebro Estuary (NE Iberian Peninsula)Document16 pagesPeriphytic Diatom Community in A Mediterranean Salt Wedge Estuary: The Ebro Estuary (NE Iberian Peninsula)Asil KurundarathNo ratings yet
- Iden 8Document6 pagesIden 8Qori Diyah FatmalaNo ratings yet
- Comparison of Calculation Procedures of Primary Productivity by Aquatic Macrophytes in A Shallow Tropical Coastal LagoonDocument11 pagesComparison of Calculation Procedures of Primary Productivity by Aquatic Macrophytes in A Shallow Tropical Coastal LagoonDian OktaviyaniNo ratings yet
- 23 - 2015 - Nature Comm, FitoDocument8 pages23 - 2015 - Nature Comm, FitoPedro L. Recuenco AndrésNo ratings yet
- Sampaio Et Al 2007Document9 pagesSampaio Et Al 2007Bruna FacundesNo ratings yet
- 2003 Elias Et Al IheringiaDocument10 pages2003 Elias Et Al IheringiapablofedericciNo ratings yet
- Grupos Funcionales Del Planton Frente Al Puerto Pesquero de AnconcitoDocument17 pagesGrupos Funcionales Del Planton Frente Al Puerto Pesquero de AnconcitoCear Andrade RuízNo ratings yet
- Distribution of Dinocysts in The Surface Sediments of Sidi Moussa Lagoon: (Atlantic Coast-Morocco)Document6 pagesDistribution of Dinocysts in The Surface Sediments of Sidi Moussa Lagoon: (Atlantic Coast-Morocco)Abdelouahed BenMhamedNo ratings yet
- Karaouzas 2006Document14 pagesKaraouzas 2006Roxana VoicuNo ratings yet
- Distribution and Ecology of Non Marine oDocument15 pagesDistribution and Ecology of Non Marine oJoseph HOTEKPONo ratings yet
- Perforatum GeorgianumDocument50 pagesPerforatum Georgianumcryopodo01No ratings yet
- Linares Et Al 2013Document6 pagesLinares Et Al 2013Marden LinaresNo ratings yet
- Marine FreeDocument7 pagesMarine Freenatu06lumarNo ratings yet
- Ayadi Et Al 2004-OkDocument12 pagesAyadi Et Al 2004-OkrinifiahNo ratings yet
- 0034 7744 RBT 64 01 00203Document10 pages0034 7744 RBT 64 01 00203Milagros Valles FasabiNo ratings yet
- 6697-Texto Del Artículo-19048-2-10-20180919Document16 pages6697-Texto Del Artículo-19048-2-10-20180919Fernando LópezNo ratings yet
- Specific Phytoplankton Signatures and Their Relationship To Hydrographic Co:nditions in The Coastal Northwestern Mediterranean SeaDocument12 pagesSpecific Phytoplankton Signatures and Their Relationship To Hydrographic Co:nditions in The Coastal Northwestern Mediterranean SeaEduardo PalaciosNo ratings yet
- 03 Barreiro Biomasa FitoplancticaDocument9 pages03 Barreiro Biomasa FitoplancticaJosé Manuel Martínez MontesNo ratings yet
- Marine Pollution Bulletin: Rachid Dris, Johnny Gasperi, Mohamed Saad, Cécile Mirande, Bruno TassinDocument4 pagesMarine Pollution Bulletin: Rachid Dris, Johnny Gasperi, Mohamed Saad, Cécile Mirande, Bruno TassinA SierraNo ratings yet
- Pen Rchive Oulouse Rchive Uverte : O A T A O OataoDocument8 pagesPen Rchive Oulouse Rchive Uverte : O A T A O OataoIqioo RedefiniNo ratings yet
- FRRFDocument7 pagesFRRFRamdanyNo ratings yet
- CH 06 PDFDocument23 pagesCH 06 PDFca_rl_4No ratings yet
- Mikropal Prep TechniqueDocument32 pagesMikropal Prep TechniqueMichelle CalistaNo ratings yet
- The Phytoplankton Distribution in Kingston HarbourDocument17 pagesThe Phytoplankton Distribution in Kingston Harbourangelica rodriguezNo ratings yet
- Im1104 0267Document8 pagesIm1104 0267Crista María CanoNo ratings yet
- El Hassi and Muftah. 2024Document24 pagesEl Hassi and Muftah. 2024محمد الحاسيNo ratings yet
- Ja11060 PDFDocument4 pagesJa11060 PDFMahadi HasanNo ratings yet
- Structure and Diversity of Zooplankton Community in Taabo Reservoir (Cote Divoire)Document17 pagesStructure and Diversity of Zooplankton Community in Taabo Reservoir (Cote Divoire)IJAR JOURNALNo ratings yet
- LSM3254 Practical 2-3 FW Stream CommunitiesDocument13 pagesLSM3254 Practical 2-3 FW Stream CommunitiesAbraham KangNo ratings yet
- Plant Communities StructureDocument17 pagesPlant Communities Structureusthb29No ratings yet
- Critique Tulungatung (Yeo)Document37 pagesCritique Tulungatung (Yeo)YEO, REGGIE ALBERT A.No ratings yet
- Jiao 2015Document10 pagesJiao 2015Yayan MardiansyahNo ratings yet
- D 1007032023Document4 pagesD 1007032023IOSRjournalNo ratings yet
- Field Trip ReportDocument28 pagesField Trip ReportTootsie100% (1)
- Depth Distribution of Photosynthetic Pigments and Diatoms in The Sediments of A Microtidal FjordDocument14 pagesDepth Distribution of Photosynthetic Pigments and Diatoms in The Sediments of A Microtidal Fjordjamesth07No ratings yet
- Ecology and Distribution of Aulacoseira Species Bacillariophyta in Tropical Reservoirs From BrazilDocument18 pagesEcology and Distribution of Aulacoseira Species Bacillariophyta in Tropical Reservoirs From BrazilPaola JiradoNo ratings yet
- Lectura Lab6 Salaramirez2008diatom ResearchDocument12 pagesLectura Lab6 Salaramirez2008diatom ResearchFelipe SuarezNo ratings yet
- Effects of Reclamation Projects On Marine Ecological Environment in Tianjin Harbor Industrial ZoneDocument8 pagesEffects of Reclamation Projects On Marine Ecological Environment in Tianjin Harbor Industrial ZoneAnonymous 0o8MyJndNo ratings yet
- 10018-Article Text-56413-1-10-20211221Document9 pages10018-Article Text-56413-1-10-20211221philosophy-thoughtNo ratings yet
- Bouvy 2000 Occurrence of Cylindrospermopsis (Cyanobacteria) in 39 Brazilian Tropical Reservoirs DDocument15 pagesBouvy 2000 Occurrence of Cylindrospermopsis (Cyanobacteria) in 39 Brazilian Tropical Reservoirs DSal TavaresNo ratings yet
- Crabs Engineering Effects On Soil Organic Matter and Nutrients Flow in Subtropical Mangroves ForestDocument7 pagesCrabs Engineering Effects On Soil Organic Matter and Nutrients Flow in Subtropical Mangroves ForestfaithnicNo ratings yet
- 3IJEEFUSAPR20193Document10 pages3IJEEFUSAPR20193TJPRC PublicationsNo ratings yet
- Identification of The Self-Purification Stretches of The Pinios River, Central GreeceDocument14 pagesIdentification of The Self-Purification Stretches of The Pinios River, Central GreeceSergei OstroumovNo ratings yet
- Benthic Habitat Quality Assessment of An Oxygen StressedDocument16 pagesBenthic Habitat Quality Assessment of An Oxygen Stressedscrane@No ratings yet
- Composition and Differential Distribution of Zooplankton in Arcachon BayDocument18 pagesComposition and Differential Distribution of Zooplankton in Arcachon BayBárbara BernardesNo ratings yet
- Gutiérrez Et Al 2011 Marine BiologyDocument15 pagesGutiérrez Et Al 2011 Marine BiologyPato CANo ratings yet
- Diel Variation in The Vertical Distribution of Fish and Plankton in Lake Kinneret: A 24-h Study of Ecological OverlapDocument10 pagesDiel Variation in The Vertical Distribution of Fish and Plankton in Lake Kinneret: A 24-h Study of Ecological OverlapHuynh AnhNo ratings yet
- Cyanobacteria From Ashallow Reservoirin CtedIvoireDocument15 pagesCyanobacteria From Ashallow Reservoirin CtedIvoireAnderson GuerreroNo ratings yet
- tmpBAA5 TMPDocument10 pagestmpBAA5 TMPFrontiersNo ratings yet
- Macroinvertebrate Assemblages As Biological Indicators of WaterDocument13 pagesMacroinvertebrate Assemblages As Biological Indicators of WaterheunjuNo ratings yet
- Arqueas y Comunidades Oxidadoras Anaerobias de Metano en El Golfo de California Vigneron Et Al 2013Document14 pagesArqueas y Comunidades Oxidadoras Anaerobias de Metano en El Golfo de California Vigneron Et Al 2013valenciabastoNo ratings yet
- (PDF) Current State of Zooplankton Diversity in The Pelagic Zone of Lake Tanganyika Offshore of Bujumbura CityDocument7 pages(PDF) Current State of Zooplankton Diversity in The Pelagic Zone of Lake Tanganyika Offshore of Bujumbura CityDr Ir Lambert NiyoyitungiyeNo ratings yet
- Determination of PCB Congener-Specific First Order Absorption/Desorption Rate Constants Using Larvae (Insecta: Diptera: Chironomidae)Document7 pagesDetermination of PCB Congener-Specific First Order Absorption/Desorption Rate Constants Using Larvae (Insecta: Diptera: Chironomidae)Ufuoma AsagbaNo ratings yet
- Microorganisms 08 00632 v2Document15 pagesMicroorganisms 08 00632 v2Alejandro Morales GomezNo ratings yet
- Identification and Estimation of Microcystins in Freshwater MusselsDocument5 pagesIdentification and Estimation of Microcystins in Freshwater Musselsdanial tarikNo ratings yet
- 10.1007@978 981 32 9771 56 PDFDocument19 pages10.1007@978 981 32 9771 56 PDFKanuri VardhanNo ratings yet
- Cotano 2008Document15 pagesCotano 2008Fadhli LatuconsinaNo ratings yet
- Small Dams Also Change The Benthic Macroinvertebrates Community in Rocky RiversDocument9 pagesSmall Dams Also Change The Benthic Macroinvertebrates Community in Rocky RiversAnderson VascoNo ratings yet
- 2 1 6 072016 enDocument29 pages2 1 6 072016 enbaglamaNo ratings yet
- 2009 Korelusova Et Al GeminocystisDocument10 pages2009 Korelusova Et Al GeminocystisbaglamaNo ratings yet
- Guidance On The Vigilance System For CE-marked Medical DevicesDocument4 pagesGuidance On The Vigilance System For CE-marked Medical DevicesbaglamaNo ratings yet
- Botanical Society of America, Inc. American Journal of BotanyDocument6 pagesBotanical Society of America, Inc. American Journal of BotanybaglamaNo ratings yet
- Ribeiro Et Al 2003Document7 pagesRibeiro Et Al 2003baglamaNo ratings yet
- Art:10 1007/BF00361072Document10 pagesArt:10 1007/BF00361072baglamaNo ratings yet
- 00 B 7 D 52 Aec 04 F 0953 A 000000Document16 pages00 B 7 D 52 Aec 04 F 0953 A 000000baglamaNo ratings yet
- 1 PBDocument12 pages1 PBbaglamaNo ratings yet
- Studies in The GramineaeDocument6 pagesStudies in The GramineaebaglamaNo ratings yet
- SMS Phragmites Control Background InformationDocument15 pagesSMS Phragmites Control Background InformationbaglamaNo ratings yet
- v10119 009 0021 3Document10 pagesv10119 009 0021 3baglamaNo ratings yet
- Perez-Garcia Et Al. 11 Bird Conserv IntDocument7 pagesPerez-Garcia Et Al. 11 Bird Conserv IntbaglamaNo ratings yet
- 27 Saumasree Ligule - FinalDocument6 pages27 Saumasree Ligule - FinalbaglamaNo ratings yet
- Nea3508 ChernobylDocument157 pagesNea3508 ChernobylbaglamaNo ratings yet
- Int Manual EU28Document146 pagesInt Manual EU28baglamaNo ratings yet
- Helictotrichon DevesaeDocument7 pagesHelictotrichon DevesaebaglamaNo ratings yet
- Deq Ogl Guide Phragmites 204659 7Document8 pagesDeq Ogl Guide Phragmites 204659 7baglamaNo ratings yet
- The Lasting Effects of Tank Maneuvers On DesertDocument26 pagesThe Lasting Effects of Tank Maneuvers On DesertRobert GatesNo ratings yet
- Anbf33 101Document0 pagesAnbf33 101baglamaNo ratings yet
- Ecological Society of AmericaDocument14 pagesEcological Society of AmericabaglamaNo ratings yet
- Gis PGR ConservationDocument18 pagesGis PGR ConservationbaglamaNo ratings yet
- 2007 47 EC GuidanceDocument6 pages2007 47 EC GuidancebaglamaNo ratings yet
- Phytoremediation of Gypsum DepositeDocument9 pagesPhytoremediation of Gypsum DepositebaglamaNo ratings yet
- On Evolutionary Pathways of Avena SpeciesDocument10 pagesOn Evolutionary Pathways of Avena SpeciesbaglamaNo ratings yet
- w33 1EuroMed 1 Anthemis OretanaDocument7 pagesw33 1EuroMed 1 Anthemis OretanabaglamaNo ratings yet
- Bebber Al 2010 Pnas HerbariaDocument10 pagesBebber Al 2010 Pnas HerbariabaglamaNo ratings yet
- Isoenzyme Variation Patterns and Species Concept (2007) - SH Zarre Et Al.Document7 pagesIsoenzyme Variation Patterns and Species Concept (2007) - SH Zarre Et Al.baglamaNo ratings yet
- Cornicabra TG AG-FoodChem 2004Document8 pagesCornicabra TG AG-FoodChem 2004baglamaNo ratings yet
- Nea3508 ChernobylDocument157 pagesNea3508 ChernobylbaglamaNo ratings yet
- Bme-A Previous Year Questions: Credits Change Accha Hai TeamDocument6 pagesBme-A Previous Year Questions: Credits Change Accha Hai TeamYash RaoNo ratings yet
- Poly Plug 1Document5 pagesPoly Plug 1Aquiles CarreraNo ratings yet
- 175 023400Document2 pages175 023400Abu Anas M.SalaheldinNo ratings yet
- Adatlap PDFDocument12 pagesAdatlap PDFAnuradha SivakumarNo ratings yet
- User's Manual: ULTRA CFR Nd:YAG Laser SystemDocument55 pagesUser's Manual: ULTRA CFR Nd:YAG Laser SystemLeonardo PantojaNo ratings yet
- Manufacturing Processes: by Premchand Kumar Deoghar (Jharkhand)Document49 pagesManufacturing Processes: by Premchand Kumar Deoghar (Jharkhand)PremKumarNo ratings yet
- Effects of Nonuniform Tire Contact Stresses On Pavement ResponseDocument7 pagesEffects of Nonuniform Tire Contact Stresses On Pavement ResponseBarnali DebnathNo ratings yet
- Steel Compression Member-2Document45 pagesSteel Compression Member-2Kristine RomeroNo ratings yet
- Dynamic Balancing Machines, Soft-Bearing vs. Hard-BearingDocument2 pagesDynamic Balancing Machines, Soft-Bearing vs. Hard-BearingS Kumar Pa VeluNo ratings yet
- SBC Report CGD MedchalDocument17 pagesSBC Report CGD MedchalPortland GeoNo ratings yet
- CASSETTE Inverter YKKJE12 55BZTVMCMORXDocument6 pagesCASSETTE Inverter YKKJE12 55BZTVMCMORXJuanNo ratings yet
- Catalog Subzero Insulated Sandwich PanelDocument8 pagesCatalog Subzero Insulated Sandwich PanelTommy Dwi Hartanto100% (1)
- Fcu TechnicalDocument22 pagesFcu Technicaltran duyNo ratings yet
- Distillation Process ProjectDocument71 pagesDistillation Process Projectsunlias100% (2)
- Subject Name: Physics Subject Code: 214: Question Id: 1Document31 pagesSubject Name: Physics Subject Code: 214: Question Id: 1AmanNo ratings yet
- Fiitjee Solution - Answer Keys - Nsep - 2022-23Document17 pagesFiitjee Solution - Answer Keys - Nsep - 2022-23Royal BedukoNo ratings yet
- BET Surface Are MeasurementsDocument16 pagesBET Surface Are MeasurementsKwan ZhangNo ratings yet
- 02 Thermodynamic AirDocument24 pages02 Thermodynamic AirzhangyuluNo ratings yet
- Prestressed Concrete-1 - 2017-2018Document58 pagesPrestressed Concrete-1 - 2017-2018ريام الموسويNo ratings yet
- The Value of Surface Tension of A Liquid at Critical Temperature IsDocument10 pagesThe Value of Surface Tension of A Liquid at Critical Temperature Issagarchidre114No ratings yet
- University BADJI MOKHTAR ANNABADocument6 pagesUniversity BADJI MOKHTAR ANNABAmeghlaouirami5No ratings yet
- Essential in WeldingDocument3 pagesEssential in WeldingBelle SantosNo ratings yet
- Non - Conventional Energy Sources: 2 TopicDocument25 pagesNon - Conventional Energy Sources: 2 Topicshubham sharmaNo ratings yet
- Lab Grade: PH ProbeDocument13 pagesLab Grade: PH ProbeManuel AdrianNo ratings yet
- c5 Pre Frac Injection Tests PDFDocument76 pagesc5 Pre Frac Injection Tests PDFJames WallaceNo ratings yet
- Isolated Footing DesignDocument7 pagesIsolated Footing DesignRamadanNo ratings yet
- Livros de Mecanica Do ContinuoDocument3 pagesLivros de Mecanica Do ContinuomdfdfdNo ratings yet
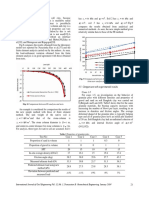
- Kpa C Kpa C Kpa C: Fig. 9 Comparison Between Bearing Capacity Values DeterminedDocument5 pagesKpa C Kpa C Kpa C: Fig. 9 Comparison Between Bearing Capacity Values DeterminedfacedoneNo ratings yet
- JNTU ANATHAPUR B.TECH Mechanical Engineering R09 SyllabusDocument147 pagesJNTU ANATHAPUR B.TECH Mechanical Engineering R09 Syllabuspavankumar72No ratings yet