Professional Documents
Culture Documents
Afhs1204 0576
Uploaded by
karenafiafiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Afhs1204 0576
Uploaded by
karenafiafiCopyright:
Available Formats
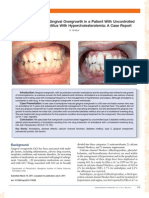
Amlodipine-induced gingival hyperplasia in chronic renal failure: a
case report
*Aldemir NM1, Begenik H2, Emre H2, Erdur FM2, Soyoral Y2
1. Department of Internal Medicine, Faculty of Medicine, Yuzuncu Yil University, Van, Turkey
2. Department of Nephrology, Faculty of Medicine, Yuzuncu Yil University, Van, Turkey
Abstract
Amlodipine is a dihydropyridine calcium channel blocker that is used in the management of both hypertension and angina.
Amlodipine induced side effects are headache, dizziness, edema, flushing, palpitations, and rarely gingival hyperplasia. The
exact reason of amlodipine-induced gingival hyperplasia is not known. We presented a case with chronic renal failure (CRF)
that developed gingival hyperplasia due to amlodipine use, which improved after ceasing the drug.
Keywords: Amlodipine, gingival hyperplasia, chronic renal failure
African Health Sciences 2012; (4): 576 - 578 http://dx.doi.org/10.4314/ahs.v12i4.30
Introduction
Amlodipine is a long-acting calcium channel blocker
belonging to dihydropyridine group, which is used
for the treatment of hypertension and angina.
Pharmacokinetic profile characteristic of this group,
which would also have an increased oral
bioavailability and extended clearance time. A single
intravenous dose of 10 mg resulted in an absolute
bioavailability of 64% and a calculated elimination
half-life of 34 hours1. Side effects of amlodipine
usage involve headache, dizziness, edema, flushing,
palpitation and rarely gingival hyperplasia 2. We
presented here a case with stage-3 chronic renal failure
(CRF), which developed gingival hyperplasia due to
amlodipine use.
Case report
A 39-year-old male patient, who had been under
follow up because of the diagnosis of stage-3
chronic renal failure and hypertension for one year,
appealed to the dentist with the complaint of gingival
hyperplasia. The patient who was planned surgery
was referred to the nephrology policlinic to be given
preoperative recommendations for renal failure. It
was revealed that the patient admitted to our
policlinic had used amlodipine 10 mg 1x1 and
carvedilol 6,25 mg 2x1 for approximately 1 year
and that the patients complaint of gingival
hyperplasia had begun 2 months after the use of
these drugs and had gradually worsened. Since the
gingival hyperplasia was assumed to be due to
amlodipine, the medications were ceased and the
patient was prescribed angiotensin receptor blockers.
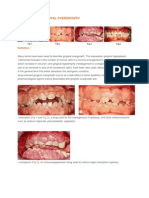
During follow up, a remarkable improvement of
the patients gingival hyperplasia was observed. The
patients figures of during the use of amlodipine
and 3 months after discontinuation have been
showed in figures 1 and 2.
Figure 1: Amlodipine induced gingival
hyperplasia
*Corresponding author:
Mehmet Naci Aldemir
Sanlurfa Education and Research Hospital,
Internal Medicine Department
Sanlurfa, Turkey
Tel: +90 414 318 60 00
E- mail: aldemirmn@gmail.com
576
African Health Sciences Vol 12 Issue 4 December 2012
Figure 2: Regression of gingival hyperplasia
during 3 months after discontinuation of
amlodipine.
Discussion
The drugs causing gingival hyperplasia can be
discussed as three major groups: Anticonvulsants
(phenytoin), immunosuppressive agents
(cyclosporine A) and calcium channel blockers 3.
Among calcium channel blockers, the ones causing
gingival hyperplasia in order of frequency are
nifedipine (6.3%)4, verapamil (4.1%)5 and amlodipine
with lower rates (1.3% to 3.3%)3,4,6.
Amlodipine is a long-acting calcium channel
blocker agent from dihydropyridine group, which
is used for the treatment of hypertension and angina.
The side effects developed due to the use of
amlodipine are headache, dizziness, edema, flushing,
palpitation, and rarely gingival hyperplasia2.
Amlodipine-associated gingival hyperplasia
was first reported in 19932. The majority of the cases
in the literature were published in the journals of
dentistry. Gingival hyperplasia arises approximately
2 to 3 months after the use of amlodipine7. In our
patient, it began to occur after 2 months, the patient
continued to take the drug since the patient did not
know that it might be related to the drug and the
patient appealed to the dentist at first.
In the literature, there are three prevalence studies
along with case reports regarding amlodipineinduced gingival hyperplasia. Gingival hyperplasia due
to the use of amlodipine was found in 5 (3.3%) of
150 patients in the study performed by Jorgensen et
al. in 19973, in 3 (1.7%) of 181 patients in the study
conducted by Ellis et al. in 19994, and in 4 (1.3%) of
301 patients in the study performed by Ono et al. in
20106. To our knowledge, our reported case is the
African Health Sciences Vol 12 Special Issue 4 December 2012
first case with CRF in the literature to develop
hyperplasia due to amlodipine.
Although the exact reason of drug-induced
gingival hyperplasia is not known, some well-known
risk factors are gingival inflammation resulted from
poor oral hygiene, presence of dental plaques, the
dose and duration of the drug used8,9. The underlying
mechanism remains to be fully understood.
However, two main inflammatory and noninflammatory pathways have already been suggested.
The proposed non-inflammatory mechanisms
include defective collagenase activity due to
decreased uptake of folic acid, blockage of
aldosterone synthesis in adrenal cortex and
consequent feedback increase in ACTH level, and
upregulation of keratinocyte growth factor (KGF).
Alternatively, inflammation may develop as a result
of direct toxic effects of concentrated drug in
crevicular gingival fluid (CGF) and/or bacterial
plaques. This inflammation could lead to the
upregulation of several cytokine factors such as TGF110. Consequent gingival hyperplasia causes not only
esthetic problems but also difficulty speaking and
mastication, impairs oral hygiene and leads to
malnutrition8,9. Our patient did not have the habit
of regular tooth brushing (only once a week) and
gingival hyperplasia evolved resulted in nutritional
and speaking problems. Another factor having an
impact on the development of gingival hyperplasia
is gender. It occurs three times more often in men
than in women11. Our patient was a man with chronic
renal failure. It is not known whether chronic renal
failure is associated with adverse effects of
amlodipine.
The primary treatment of drug-induced
gingival hyperplasia involves ceasing the drug,
prescribing another drug from a different group
instead, and a good oral care. In case of establishing
these things, gingival hyperplasia often improves.
Usually, surgical approach is not needed. In the
situations requiring surgery, gingivectomy or
periodontal flap is performed12. Gingival hyperplasia
normally begins at the interdental papillae and is
frequently found in the anterior segment of the labial
surfaces13. A gingival specimen was obtained for
histological examination, of which the findings were
the epithelium with irregular elongation and fusion
of rete ridges and bundles of collagen fibers in the
subepithelial connective tissue6. However, a biopsy
has not been made may be deficiency of for our
case report.
577
Our patient appealed to his dentist and was
referred to our policlinic for preoperative evaluation
because of coexisting CRF. His medication was
ceased since the development of gingival hyperplasia
was assumed to be associated with amlodipine and
it regressed within 3 months without surgical
intervention. Therefore, it is of importance that the
dentists should consider the drugs in the etiology of
these disorders because most of these patients appeal
to the dentists.
Conclusion
When prescribing amlodipine, it should be considered
that amlodipine potentially causes gingival
hyperplasia. The patients should be recommended
pay attention to oral hygiene and appeal to a healthcare
center in case gingival hyperplasia develops.
References
1. Abernethy DR. The pharmacokinetic profile of
amlodipine. Am Heart J. 1989 Nov; 118(5 Pt 2):
1100-1103.
2. Ellis JS, Seymour RA, Thomason JM, Monkman
SC, Idle JR: Gingival sequestration of amlodipine
induced gingival overgrowth. Lancet 1993; 341:
1102-1103.
3. Jorgensen MG: Prevalence of amlodipine related
gingival hyperplasia. J Periodontol 1997; 68: 678
4. Ellis JS, Seymour RA, Steele JG, Robertson P,
Butler TJ, Thomason JM: Prevalence of gingival
over-growth induced by calcium channel
blockers: a community-based study. J Periodontol
1997; 0: 63-67.
5. Miller CS, Damm DD. Incidence of verapamilinduced gingival hyperplasia in a dental
578
6.
7.
8.
9.
10.
11.
12.
13.
population. J Periodontol. 1992 May; 63(5): 453456.
Ono M, Tanaka S, Takeuchi R, Matsumoto H,
Okada H, Yamamoto H, Makiyama Y,
Hirayama T, Sakamaki T, Fujii A, Akimoto Y:
Prevelance of amlodipine-induced gingival
overgrowth. Int J Oral- Med Sci 2010; 9(2): 96100.
Meraw SJ, Sheridan PJ: Medically induced
gingival hyperplasia. Mayo Clin Proc 1998; 73:
1196-1199
Matharu MS, Van Vilet JA, Ferrari MD, Goadsby
PJ. Verapamil induced gingival enlargement in
cluster headache. J Neural Neurosurg Psychiatry
2005; 76: 124127.
Gregoriou A, Schneider P: Phenobarbitalinduced gingival overgrowth? Report of two
cases and complications in management. ASDC
J Dent Child 1996; 63: 408-413.
Lafzi A, Farahani RMZ, Shoja MM:
Amlodipine-induced gingival hyperplasia. Med
Oral Patol Oral Cir Bucal 2006; 11: 480-482
Tavassoli S, Yamalik N, Caglayan F, Caglayan G,
Eratalay K: The clinical effects of nifedipine on
periodontal status. J Periodontol 1998; 69: 108112.
Camargo PM, Melnick PR, Pirih FQ, Lagos R,
Takei HH. Treatment of drug-induced gingival
enlargement: aesthetic and functional
considerations. Periodontology 2000; 27: 131138
Hallmon WW, Rossmann JA: The role of drugs
in the pathogenesis of gingival overgrowth. A
collevtive review of current concepts. Periodontol
2000; 21: 176-196.
African Health Sciences Vol 12 Issue 4 December 2012
You might also like
- Lymphbuster Version 1.0 PDFDocument395 pagesLymphbuster Version 1.0 PDFDiana MariaNo ratings yet
- Proctor Tobacco History Report Canadian Trial Rapport - Expert - ProctorDocument112 pagesProctor Tobacco History Report Canadian Trial Rapport - Expert - Proctorkirk_hartley_1No ratings yet
- Acne and Whey Protein Supplementation Among Bodybuilders PDFDocument4 pagesAcne and Whey Protein Supplementation Among Bodybuilders PDFHenyta TsuNo ratings yet
- Amlodipine-Induced Gingival HyperplasiaDocument3 pagesAmlodipine-Induced Gingival HyperplasiaMuchlis Fauzi ENo ratings yet
- Case Report: Untypical Amlodipine-Induced Gingival HyperplasiaDocument4 pagesCase Report: Untypical Amlodipine-Induced Gingival HyperplasiakarenafiafiNo ratings yet
- Cap 2012 110026 PDFDocument8 pagesCap 2012 110026 PDFSmitha Kapani gowdaNo ratings yet
- Oral and Maxillofacial Surgery Cases: Janina Golob Deeb, Denver J. Lyons, Daniel M. Laskin, George R. DeebDocument6 pagesOral and Maxillofacial Surgery Cases: Janina Golob Deeb, Denver J. Lyons, Daniel M. Laskin, George R. DeebAgum Nila Sari IINo ratings yet
- Crid2014 741402Document4 pagesCrid2014 741402karenafiafiNo ratings yet
- Conservative Management of Amlodipine Induced Gingival Enlargement: A ClinicalDocument3 pagesConservative Management of Amlodipine Induced Gingival Enlargement: A ClinicalachmadsyobriNo ratings yet
- Fanc. AnemiaDocument8 pagesFanc. AnemiaLujainNo ratings yet
- Case Report Necrotizing Ulcerative Gingivitis, A Rare Manifestation As A Sequel of Drug-Induced Gingival Overgrowth: A Case ReportDocument7 pagesCase Report Necrotizing Ulcerative Gingivitis, A Rare Manifestation As A Sequel of Drug-Induced Gingival Overgrowth: A Case ReportPutri Dwi AnggrainiNo ratings yet
- Conservative Management of Nifedipine Influenced Gingival Enlargement - A Case ReportDocument9 pagesConservative Management of Nifedipine Influenced Gingival Enlargement - A Case ReportAgathaPutriNo ratings yet
- Dent06 p0095Document6 pagesDent06 p0095Insan KamilNo ratings yet
- Antihistamin in OMEDocument4 pagesAntihistamin in OMERahma NovitasariNo ratings yet
- Oral Litchen PlanusDocument5 pagesOral Litchen PlanusWulan Ambar WatyNo ratings yet
- Oral Care For Children With Leukaemia: SY Cho, AC Cheng, MCK ChengDocument6 pagesOral Care For Children With Leukaemia: SY Cho, AC Cheng, MCK Chengvefiisphepy83312No ratings yet
- Drug Induced Gingival Overgrowth: Corresponding AuthorDocument5 pagesDrug Induced Gingival Overgrowth: Corresponding Authorjorge villamizarNo ratings yet
- Maxillary Ulceration Resulting From Using A Rapid Maxillary Expander in A Diabetic PatientDocument5 pagesMaxillary Ulceration Resulting From Using A Rapid Maxillary Expander in A Diabetic PatientPrabowo BomazdicahyoNo ratings yet
- 12 Sequel To Renal Transplant An Oral Physician's PerspectiveDocument5 pages12 Sequel To Renal Transplant An Oral Physician's PerspectivevinhannyNo ratings yet
- Aloe vera gel effective as adjuvant for oral submucous fibrosisDocument8 pagesAloe vera gel effective as adjuvant for oral submucous fibrosisNidhin ValappilaNo ratings yet
- LEUKAEMIA AND ORTHDONTICS by AlmuzianDocument3 pagesLEUKAEMIA AND ORTHDONTICS by AlmuzianRahulLife'sNo ratings yet
- Amlodipine-Induced Gingival Overgrowth: Case ReportDocument4 pagesAmlodipine-Induced Gingival Overgrowth: Case ReportjulietNo ratings yet
- Drug Induced Gingival OvergrowthDocument4 pagesDrug Induced Gingival OvergrowthAdit VekariaNo ratings yet
- Open Drainage or NotDocument6 pagesOpen Drainage or Notjesuscomingsoon2005_No ratings yet
- Soalan Temuduga Pegawai PergigianDocument8 pagesSoalan Temuduga Pegawai PergigianMuhammad Hafizul Amin Bin JimaainNo ratings yet
- Aggressive Periodontitis and Pituitary AdenomaDocument28 pagesAggressive Periodontitis and Pituitary AdenomaMohit_Gupta_9876No ratings yet
- Nbde IiDocument5 pagesNbde IisoyrubeNo ratings yet
- Jurnal SistemikDocument3 pagesJurnal SistemikMariatun Zahro NasutionNo ratings yet
- Orthodontic Treatment For An Adolescent With A History of AcuteDocument5 pagesOrthodontic Treatment For An Adolescent With A History of Acuteelvin peraltaNo ratings yet
- Cleft Lip & Palate: Doctorate of Clinical Dentistry in Orthodontics (Notes) Orthodontic Dept. University of GlasgowDocument31 pagesCleft Lip & Palate: Doctorate of Clinical Dentistry in Orthodontics (Notes) Orthodontic Dept. University of Glasgowdruzair007No ratings yet
- Lip Abscess From Isotretinoin CheilitisDocument2 pagesLip Abscess From Isotretinoin CheilitisSulistya NingsihNo ratings yet
- Oral Health in Children With Leukemia: Review ArticleDocument8 pagesOral Health in Children With Leukemia: Review ArticleNindy TweelingenNo ratings yet
- Orthodontic Lect 2Document16 pagesOrthodontic Lect 2Said SaidNo ratings yet
- Tongue Injury Healing in Epileptic Teen: Case ReportDocument12 pagesTongue Injury Healing in Epileptic Teen: Case ReportJoão EduardoNo ratings yet
- Podj 4Document6 pagesPodj 4cutchaimahNo ratings yet
- Dental Implants Manage Malocclusion in Acromegaly PatientDocument6 pagesDental Implants Manage Malocclusion in Acromegaly PatientAkanksha MahajanNo ratings yet
- Iis ManisDocument6 pagesIis ManisNurbaetty RochmahNo ratings yet
- Papillon Lefevre Syndrome-A Literature Review and Case ReportDocument7 pagesPapillon Lefevre Syndrome-A Literature Review and Case ReportnadhiracindyNo ratings yet
- A Painful Red EyeDocument2 pagesA Painful Red EyeCristhian Agustin ParedesNo ratings yet
- HepB Foc InfcDocument6 pagesHepB Foc InfcEping St WindahNo ratings yet
- Jurnalku OsccDocument4 pagesJurnalku OsccAndiOctafiantoNo ratings yet
- Lase Report: Infection From An Exfoliating Primary Tooth in A Child With Severe Neutropenia: A CaseDocument4 pagesLase Report: Infection From An Exfoliating Primary Tooth in A Child With Severe Neutropenia: A CaseangelpazcalNo ratings yet
- Physiotherapy Improves Mouth Opening in OSMFDocument9 pagesPhysiotherapy Improves Mouth Opening in OSMFViral ParekhNo ratings yet
- 2.1 Effects of Levetiracetam and Topiramate MonotherapyDocument6 pages2.1 Effects of Levetiracetam and Topiramate MonotherapyRaluca ElenaNo ratings yet
- Reporte de 58 ExtraccionesDocument6 pagesReporte de 58 Extraccionesyisuskakaroto300No ratings yet
- The Management of Herpes Labialis Oral Thrush andDocument5 pagesThe Management of Herpes Labialis Oral Thrush andFebri YolandaNo ratings yet
- Jkimsu, Vol. 4, No. 1, Janom-Mar 2015 Page 148-154Document7 pagesJkimsu, Vol. 4, No. 1, Janom-Mar 2015 Page 148-154Mahendra PrihandanaNo ratings yet
- Case Report: Idiopathic Gingival Hyperplasia: A Case Report With A 17-Year FollowupDocument6 pagesCase Report: Idiopathic Gingival Hyperplasia: A Case Report With A 17-Year Followupade ismailNo ratings yet
- Hairy TongueDocument2 pagesHairy Tonguegodswar3dNo ratings yet
- Effectiveness of Non-Surgical Periodontal Therapy On Amlodipine Induced Gingival Enlargement: A Case ReportDocument3 pagesEffectiveness of Non-Surgical Periodontal Therapy On Amlodipine Induced Gingival Enlargement: A Case ReportAliyah SaraswatiNo ratings yet
- Amlodipine-induced gingival enlargement case reportDocument4 pagesAmlodipine-induced gingival enlargement case reportIlma NassaniaNo ratings yet
- Craniofacial DeformitiesDocument12 pagesCraniofacial DeformitiessarahekmateanNo ratings yet
- Dental Management of Hemophiliac ChildDocument6 pagesDental Management of Hemophiliac ChildMelisa RuthNo ratings yet
- Chemotherapy-Associated Oral Sequelae in Patients With Cancers Outside The Head and Neck RegionDocument10 pagesChemotherapy-Associated Oral Sequelae in Patients With Cancers Outside The Head and Neck RegionNndaydnaNo ratings yet
- Best of Fives For Dentistry 3 ST Ed (2014)Document179 pagesBest of Fives For Dentistry 3 ST Ed (2014)chimedbator100% (3)
- Esclerosis TuberosaDocument4 pagesEsclerosis TuberosaJesus NietoNo ratings yet
- Effects of Oral Contraceptives On The Oral Cavity: ReviewDocument3 pagesEffects of Oral Contraceptives On The Oral Cavity: ReviewAldi JieNo ratings yet
- Manejo Clinico de La AI Hipoplasica NateraDocument15 pagesManejo Clinico de La AI Hipoplasica NateraDanitza GutiérrezNo ratings yet
- Orthodontic Care of Medically Compromised Patient: Department of Orthodontics AND Dentofacial OrthopaedicsDocument48 pagesOrthodontic Care of Medically Compromised Patient: Department of Orthodontics AND Dentofacial Orthopaedicsankita rawatNo ratings yet
- JR DK 3Document8 pagesJR DK 3Amadea EmanuelaNo ratings yet
- Lichen Planus Article PDFDocument3 pagesLichen Planus Article PDFSuresh KumarNo ratings yet
- Ijpi 3 2 75 79Document5 pagesIjpi 3 2 75 79Rabiatul AdawiyahNo ratings yet
- Maurice Nicoll The Mark PDFDocument4 pagesMaurice Nicoll The Mark PDFErwin KroonNo ratings yet
- Clinical Characteristics of Intermittent ExotropiaDocument6 pagesClinical Characteristics of Intermittent ExotropiakarenafiafiNo ratings yet
- LIBRO II Pathogens HandbookDocument84 pagesLIBRO II Pathogens HandbookmelaNo ratings yet
- Maurice Nicoll The Mark PDFDocument4 pagesMaurice Nicoll The Mark PDFErwin KroonNo ratings yet
- Infantile Esotropia Diagnosis and ManagementDocument10 pagesInfantile Esotropia Diagnosis and ManagementkarenafiafiNo ratings yet
- Fisiologi Hati Dan Kandung EmpeduDocument24 pagesFisiologi Hati Dan Kandung EmpeduMusmul AlqorubNo ratings yet
- Presentation 05-06-15 EnglishDocument20 pagesPresentation 05-06-15 EnglishkarenafiafiNo ratings yet
- Visa Request for FranceDocument1 pageVisa Request for FrancekarenafiafiNo ratings yet
- Erika ReportDocument5 pagesErika ReportkarenafiafiNo ratings yet
- CPG 12Document41 pagesCPG 12AMessi YoungNo ratings yet
- Fisiologi Hati Dan Kandung EmpeduDocument37 pagesFisiologi Hati Dan Kandung EmpedukarenafiafiNo ratings yet
- S64 FullDocument3 pagesS64 FullkarenafiafiNo ratings yet
- Fisiologi Hati Dan Kandung EmpeduDocument31 pagesFisiologi Hati Dan Kandung EmpedukarenafiafiNo ratings yet
- Results of Surgery For Congenital Esotropia: Original ResearchDocument6 pagesResults of Surgery For Congenital Esotropia: Original ResearchkarenafiafiNo ratings yet
- Intermittent ExotropiaDocument15 pagesIntermittent ExotropiakarenafiafiNo ratings yet
- Surgery Versus Active Monitoring in Intermittent Exotropia (Samexo) : Study Protocol For A Pilot Randomised Controlled TrialDocument11 pagesSurgery Versus Active Monitoring in Intermittent Exotropia (Samexo) : Study Protocol For A Pilot Randomised Controlled TrialkarenafiafiNo ratings yet
- ASC Non Hodgkin LimfomaDocument63 pagesASC Non Hodgkin LimfomaBambang PriyambodoNo ratings yet
- Calcium Channel Blocker Use and Mortality Among Patients With End-Stage Renal DiseaseDocument8 pagesCalcium Channel Blocker Use and Mortality Among Patients With End-Stage Renal DiseasekarenafiafiNo ratings yet
- 129 Accommodative EsotropiaDocument6 pages129 Accommodative EsotropiakarenafiafiNo ratings yet
- Non-Hodgkin Lymphoma Affecting The Tongue: Unusual Intra-Oral LocationDocument5 pagesNon-Hodgkin Lymphoma Affecting The Tongue: Unusual Intra-Oral LocationkarenafiafiNo ratings yet
- Oral Test PracticeDocument12 pagesOral Test PracticekarenafiafiNo ratings yet
- Update On Otitis Media - Prevention and TreatmentDocument12 pagesUpdate On Otitis Media - Prevention and TreatmentkarenafiafiNo ratings yet
- Update On Otitis MediaDocument28 pagesUpdate On Otitis MediakarenafiafiNo ratings yet
- VP ShuntDocument5 pagesVP ShuntPradeep SharmaNo ratings yet
- Ayurvedic KalpaDocument171 pagesAyurvedic KalparagalwarNo ratings yet
- ANATOMY AND PHYSIOLOGY of RabiesDocument5 pagesANATOMY AND PHYSIOLOGY of RabiesDavid CalaloNo ratings yet
- EEG ArtifactsDocument18 pagesEEG ArtifactsNazwan Hassa100% (1)
- Tilapia 4Document69 pagesTilapia 4Annisa MeilaniNo ratings yet
- Clinical Study ReportDocument5 pagesClinical Study ReportAlexandraCirlanNo ratings yet
- AsepsisDocument6 pagesAsepsisMarcus, RN100% (5)
- AlgorithmACLS Tachycardia 200612Document1 pageAlgorithmACLS Tachycardia 200612YassarNo ratings yet
- Evidence-Based Recommendations For Natural Bodybuilding Contest Preparation: Nutrition and Supplementation by Eric Helms, Alan Aragon, and Peter FitschenDocument40 pagesEvidence-Based Recommendations For Natural Bodybuilding Contest Preparation: Nutrition and Supplementation by Eric Helms, Alan Aragon, and Peter FitschenSixp8ck100% (3)
- Annamalai University Postgraduate Medical Programmes 2020-2021Document12 pagesAnnamalai University Postgraduate Medical Programmes 2020-2021Velmurugan mNo ratings yet
- Massive Haemorrhage: P Donnelly B FergusonDocument18 pagesMassive Haemorrhage: P Donnelly B FergusonRizqiNo ratings yet
- Thumb Exercises ActiveDocument6 pagesThumb Exercises ActiveMichael NelsonNo ratings yet
- Hipraviar ClonDocument2 pagesHipraviar Clonst_richard04100% (2)
- Audit Program Ppi (Mariani, SKM, Mha)Document23 pagesAudit Program Ppi (Mariani, SKM, Mha)Dedy HartantyoNo ratings yet
- Effects of Boiling Time On Mineral and Vitamin C Content of Three Varieties of Hibiscus Sabdriffa Drink in NigeriaDocument6 pagesEffects of Boiling Time On Mineral and Vitamin C Content of Three Varieties of Hibiscus Sabdriffa Drink in NigeriariniyuliasamosirNo ratings yet
- MedAspectsBio PDFDocument633 pagesMedAspectsBio PDFDuy PhúcNo ratings yet
- Drug Control Policy of BangladeshDocument51 pagesDrug Control Policy of BangladeshHedayat Ullah33% (3)
- MRCP PacesDocument243 pagesMRCP PacesTank Tank94% (17)
- Safety of Low-Dose Oral Minoxidil for Hair Loss: A Study of 1404 PatientsDocument8 pagesSafety of Low-Dose Oral Minoxidil for Hair Loss: A Study of 1404 PatientsLuisNo ratings yet
- 6/27/2019 NO NAMA BARANG QTY UOM Batch EDDocument8 pages6/27/2019 NO NAMA BARANG QTY UOM Batch EDRedCazorlaNo ratings yet
- MYMAN Chanchinbu Issue No. 5Document6 pagesMYMAN Chanchinbu Issue No. 5Langa1971No ratings yet
- Genetic Counseling: A Study and SuggestionsDocument10 pagesGenetic Counseling: A Study and SuggestionsraphynjNo ratings yet
- EMTALADocument5 pagesEMTALAJimmy MillerNo ratings yet
- Critical Perspectives - Dissertation OgrDocument15 pagesCritical Perspectives - Dissertation OgrPipNo ratings yet
- Revised Market Potential Estimates for Biopure's Hemopure Based on Competitive LandscapeDocument4 pagesRevised Market Potential Estimates for Biopure's Hemopure Based on Competitive Landscapeargand_xw9097No ratings yet
- Pediatric PerfectionismDocument20 pagesPediatric Perfectionismapi-438212931No ratings yet
- Price Increase 1 January 2020 For Customers VET PDFDocument9 pagesPrice Increase 1 January 2020 For Customers VET PDFI Dewa made SuwardanaNo ratings yet