Professional Documents
Culture Documents
Walker4 Ism Ch30
Uploaded by
Jeykel EspinozaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Walker4 Ism Ch30
Uploaded by
Jeykel EspinozaCopyright:
Available Formats
Chapter 30: Quantum Physics
Answers to Even-Numbered Conceptual Questions
2.
If energy is quantized, as suggested by Planck, the amount of energy for even a single high-frequency
photon can be arbitrarily large. The finite energy in a blackbody simply cant produce such high-frequency
photons, and therefore the infinite energy implied by the ultraviolet catastrophe cannot occur. In classical
physics, any amount of energy can be in the form of high-frequency lightthe energy does not have to be
supplied in discrete, large lumps as in Plancks theory. Therefore, classical physics implies that all
frequencies of light have the same amount of energy, no matter how high the frequency. This is what leads
to the catastrophe.
4.
Plancks theory of blackbody radiation implies a one-to-one relationship between the absolute temperature
of a blackbody and the frequency of light at the peak of its radiated energy spectrum. This relationship is
given by Wiens displacement law (equation 30-1). Therefore, by measuring the peak in the radiated energy
from a star, we can tell its temperature. In broad terms, a blue star is very hot, a red star much less so, and a
yellowish star like our Sun is intermediate in temperature.
6.
A monochromatic source of light meansliterallythat it emits light of a single color. This means that all
the photons emitted by the source have the same frequency, and hence they also have the same energy.
8.
(a) A photon from a green light source always has less energy than a photon from a blue light source. (b) A
photon from a green light source always has more energy than a photon from a red light source. The reason
for these results is that the energy of a photon depends linearly on the frequency of light; that is, E = h.
10.
Classically, it should be possible to eject electrons with light of any frequencyall that is required is to
increase the intensity of the beam of light sufficiently. The fact that this is not the case means that the
classical picture is incorrect. In addition, the fact that there is a lowest frequency that will eject electrons
implies that the energy of the photon is proportional to its frequency, in agreement with E = h.
12.
Yes. An electron and a proton have the same de Broglie wavelength
( = h p)
if they have the same
momentum.
Solutions to Problems and Conceptual Exercises
1.
Picture the Problem: The blackbody spectrum of blackbody A peaks at a longer wavelength than that of blackbody B.
Strategy: Use Wiens Displacement Law (equation 30-1) to answer the conceptual question.
Solution: 1. (a) Because blackbody A has the longer wavelength it also has the lower frequency. We know from
equation 301, however, that the peak frequency of a blackbody spectrum is proportional to the absolute temperature of
the blackbody. Therefore, the temperature of blackbody A is lower than the temperature of blackbody B.
2. (b) The best explanation is II. Blackbody B has the higher temperature because an increase in temperature means an
increase in frequency, which corresponds to a decrease in wavelength. Statement I is false.
Insight: Statement I is false because increasing the temperature of a blackbody shifts the peak emission to greater
frequency, not greater wavelength.
2.
Picture the Problem: The star Betelgeuse emits a peak frequency that is proportional to its surface temperature.
Strategy: Solve Wiens Displacement Law (equation 30-1) for the temperature of Betelgeuse.
f peak = ( 5.88 1010 s 1 K 1 ) T
Solution: Solve equation 30-1 for the temperature:
T=
1.82 1014 Hz
= 3100 K
5.88 1010 s 1 K 1
Insight: Betelgeuse, like all red-giant stars, has a cooler surface temperature than our own Sun. The first printing of the
third edition text had incorrectly listed the peak frequency as 3.091014 Hz, which would correspond to an incorrect
surface temperature of 5260 K, much closer to the 5800 K surface temperature of our Sun.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 1
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
3.
Picture the Problem: The human body can be considered a blackbody radiator with a surface temperature of 95F. The
peak radiation frequency and wavelength are functions of that temperature.
Strategy: Solve Wiens Displacement Law (equation 30-1) for the peak frequency. Remember to convert the Fahrenheit temperature to Kelvin. Use equation 14-1 to calculate the wavelength, where the wave speed is the speed of light.
f peak = ( 5.88 1010 s 1 K 1 ) T
Solution: 1. Calculate the peak frequency:
= ( 5.88 1010 s 1 K 1 ) 95 ( 95 32 ) + 273.15 K
f peak = 1.81 1013 Hz
2. Calculate the wavelength:
3.00 108 m/s
c
=
= 16.6 m
f 1.812 1013 Hz
Insight: This wavelength falls in the infrared portion of the electromagnetic spectrum (see Chapter 25).
4.
Picture the Problem: The initial Big Bang can be considered as a blackbody radiator whose peak frequency is a
function of its temperature.
Strategy: Solve Wiens Displacement Law (equation 30-1) for the peak frequency. Use equation 14-1 to calculate the
wavelength, where the wave speed is the speed of light.
f peak = ( 5.88 1010 s 1 K 1 ) T
Solution: 1. (a) Calculate the peak frequency:
= ( 5.88 1010 s 1 K 1 ) ( 2.7 K ) = 1.6 1011 Hz
2. (b) Calculate the wavelength:
c 3.00 108 m/s
=
= 1.9 mm
f 1.6 1011 Hz
Insight: This wavelength falls in the microwave portion of the electromagnetic spectrum (see Chapter 25).
5.
Picture the Problem: The Sun is a blackbody radiator with a surface temperature of 5800 K.
Strategy: Solve Wiens Displacement Law (equation 30-1) for the peak frequency.
f peak = ( 5.88 1010 s 1 K 1 ) T
Solution: Write the frequency using equation 30-1:
= ( 5.88 1010 s 1 K 1 ) ( 5800 K ) = 3.4 1014 Hz
Insight: This peak corresponds to a wavelength of 880 nm and falls in the infrared portion of the electromagnetic
spectrum (see Chapter 25). We feel this infrared light from the Sun as warmth.
6.
Picture the Problem: A blackbody emits radiation according to Wiens Displacement Law.
Strategy: Use equation 30-1 to calculate the ratio of the peak frequencies for temperatures of 20.0 K and 40.0 K.
Repeat for the Kelvin temperatures that correspond to 20.0C and 40.0C.
Solution: 1. (a) Calculate the ratio of
peak frequencies for 20.0 K and 40.0 K:
2. (b) Calculate the ratio of peak
frequencies for 20.0C and 40.0C:
f peak,40
f peak,20
f peak,40
f peak,20
( 5.88 10
=
( 5.88 10
( 5.88 10
=
( 5.88 10
10
10
10
10
s 1 K 1 ) T20
1
s K
s K
s K
)T
)T
)T
40.0 K
= 2.00
20.0 K
273.15 + 40.0C
= 1.068
273.15 + 20.0C
10
40
20
Insight: Doubling the temperature in Kelvin actually doubles the average kinetic energy of the molecules. However, in
this case doubling the temperature in Celsius only corresponds to a slight (6.8%) increase in the Kelvin temperature.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 2
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
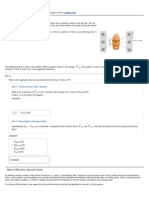
7.
Picture the Problem: A negative image of the double star Albireo is shown at right. When
viewed through a telescope, the upper-left star A is a warm golden color and the lower-right
star B is a brilliant blue.
Strategy: According to Wiens Displacement Law, a hotter star will emit light with a higher
peak frequency. Because blue has a higher frequency than yellow, the blue star will be the
hotter star. Use Wiens Displacement Law (equation 30-1) to calculate the ratio of the peak
frequencies from these two stars.
Solution: 1. (a) Star B is the blue star. A higher temperature corresponds to a higher frequency, and f blue > f yellow .
10 1
1
f A ( 5.88 10 s K ) TA
4700 K
=
=
= 0.36
1
10 1
f B ( 5.88 10 s K ) TB 13, 000 K
2. (b) Calculate the ratio of the peak frequencies:
Insight: Star As peak frequency is in the infrared spectrum (see Chapter 25), so it has a higher intensity in the redyellow part of the visible spectrum and it appears golden. Star Bs peak frequency is in the ultraviolet spectrum, so it has
a higher intensity in the blue end of the spectrum and it appears blue.
8.
Picture the Problem: An incandescent light bulb operates at a cooler temperature than a halogen bulb. Therefore, the
blackbody radiation from the two light bulbs will have different peak frequencies.
Strategy: According to Wiens Displacement Law, a hotter bulb will emit light with a higher peak frequency. Because
the halogen bulb is hotter, it will emit a higher peak frequency than the incandescent bulb. Use Wiens Displacement
Law (equation 30-1) to calculate both of the peak frequencies and calculate their ratio.
Solution: 1. (a) Because f peak T , the halogen bulb has the higher peak frequency.
2. (b) Calculate the ratio of the peak frequencies:
10 1
1
f hal ( 5.88 10 s K ) Thal 3400 K
=
=
= 1.2
f std ( 5.88 1010 s 1 K 1 ) Tstd 2900 K
3. (c) Calculate the peak frequencies of the two bulbs:
f hal = ( 5.88 1010 s 1 K 1 ) ( 3400 K ) = 2.0 1014 Hz
f std = ( 5.88 1010 s 1 K 1 ) ( 2900 K ) = 1.7 1014 Hz
4. The halogen bulb produces a peak frequency that is closer to 5.5 1014 Hz than the standard incandescent bulb.
Insight: Because the halogen bulb produces a peak frequency closer to the sensitive frequencies of the human eye, more
of the radiation from the halogen bulb is visible, making it appear brighter for the same power output.
9.
Picture the Problem: The tungsten filament in a standard light bulb can be considered a blackbody radiator.
Strategy: Use Wiens Displacement Law (equation 30-1) to find the peak frequency of the radiation from the tungsten
filament.
Solution: 1. (a) Solve Eq. 30-1
for the peak frequency:
f peak = ( 5.88 1010 s 1 K 1 ) T
= ( 5.88 1010 s 1 K 1 ) ( 2850 K ) = 1.68 1014 Hz
2. (b) Because the peak frequency is that of infrared electromagnetic radiation, the light bulb radiates more energy in the
infrared than the visible part of the spectrum.
Insight: This radiation in the infrared spectrum is what makes the light from a standard light bulb feel warm. By
contrast, a fluorescent light bulb emits most of its radiation in the ultraviolet and visible spectrum, and therefore the
light from the fluorescent bulb does not feel warm. Fluorescent lamps are also much more efficient at converting
electrical energy into visible light, because most of the light from a standard light bulb is in the infrared.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 3
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
10. Picture the Problem: The image shows two oxygen atoms oscillating
back and forth, similar to a mass on a spring. The image also shows the
evenly spaced energy levels of the oscillation.
Strategy: Use equation 13-11 to calculate the period of oscillation for the
two atoms. Calculate the frequency, using equation 13-1, from the
inverse of the period. Multiply the period by Plancks constant to
calculate the spacing of the energy levels.
Solution: 1. (a) Set the frequency
equal to the inverse of the period:
T = 2
f =
2. (b) Multiply the frequency by Plancks constant:
m
k
1
1
=
T 2
k
1
=
m 2
1215 N/m
= 4.792 1013 Hz
1.340 1026 kg
E = hf = (6.63 1034 J s)(4.792 1013 Hz) = 3.18 1020 J
Insight: Oxygen molecules in the atmosphere absorb light waves with a frequency 4.792 1013 Hz , and the energy from
the light is converted into the oscillations of the atoms.
11. Picture the Problem: A source of red light, a source of green light, and a source of blue light each produce beams of
light with the same power.
Strategy: Use the concepts of wavelength, frequency, and photon energy to answer the conceptual questions. Note that
these sources have the same power; that is, they give off the same amount of energy per time. In addition, note that red
photons have less energy than green photons, and green photons have less energy than blue photons.
Solution: 1. (a) The frequency of light increases as you go from red to green to blue, which means that the wavelength
decreases. Therefore, the ranking in order of increasing wavelength is blue < green < red.
2. (b) The ranking in order of increasing frequency is red < green < blue.
3. (c) More red photons must be emitted to give the same energy as blue photons. Therefore, the ranking in order of
increasing number of photons emitted per second is blue < green < red.
Insight: If each source were to emit the same number of photons per second, the ranking of the power emitted would be
red < green < blue.
12. Picture the Problem: A source of red light has a higher wattage than a source of green light.
Strategy: Use the expression for photon energy (equation 30-4) to answer the conceptual question.
Solution: 1. (a) The energy of photons emitted by the red source is less than the energy of photons emitted by the green
source, regardless of the wattage of the source. This conclusion follows from the relation E = h f and the fact that green
light has a higher frequency than red light.
2. (b) The best explanation is II. The red-source photons have less energy than the green-source photons because they
have a lower frequency. The wattage of the source doesnt matter. Statements I and III are each false.
Insight: A higher wattage source in general produces more photons than does a lower wattage source, but the energy of
each photon is determined by the frequency, not the power of the source.
13. Picture the Problem: A source of yellow light has a higher wattage than a source of blue light.
Strategy: Use the expression for photon energy (equation 30-4) to answer the conceptual question.
Solution: 1. (a) The number of photons emitted per time by the yellow source is greater than the number of photons
emitted per second by the blue source. There are two reasons for this. First, its photons have less energy than the
photons from the blue source, because red light has a lower frequency than blue light and E = h f. Therefore, even if the
two sources had the same power, the yellow source would emit more photons per second. Second, the yellow source
has the greater power, requiring even more photons per second.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 4
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
2. (b) The best explanation is I. The yellow source emits more photons per second, because (i) it emits more energy per
second than the blue source, and (ii) its photons have less energy than those of the blue source. Statements II and III are
each false.
Insight: The number of photons emitted per second might be equal if you compared a low wattage yellow source with a
higher wattage blue source of light.
14. Picture the Problem: Light of a particular wavelength does not eject electrons from the surface of a given metal.
Strategy: Use the concepts that describe the photoelectric effect to answer the question.
Solution: 1. (a) To eject electrons from this metal requires photons with greater energy, which means that the frequency
of the light must be increased. If the frequency of the light is increased, its wavelength must be decreased.
2. (b) The best explanation is II. The energy of a photon is proportional to its frequency; that is, inversely proportional
to its wavelength. To increase the energy of the photons so they can eject electrons, one must decrease their
wavelength. Statement I erroneously asserts that increasing the wavelength will increase the energy.
Insight: Increasing the intensity of the first light source will have no effect, because that would only direct more
photons to the metal surface, none of which have sufficient energy to eject an electron.
15. Picture the Problem: Light of a certain wavelength and intensity does not eject electrons from the surface of a metal.
Strategy: Use the concepts that describe the photoelectric effect to answer the question.
Solution: No. If the intensity of the light is increased there will be more photons striking the surface per second. Each
photon, however, will still have too little energy to eject an electron.
Insight: Electrons could only be ejected if the wavelength of the light were decreased until the photon energies are
larger than the work function of the metal.
16. Picture the Problem: UV photons have an energy of 6.5 10 19 J. This energy corresponds to a specific frequency and
wavelength.
Strategy: Solve equation 30-4 for the frequency of the photon. Insert the frequency into equation 14-1 to calculate the
wavelength.
Solution: 1. Calculate the frequency:
f =
E
6.5 1019 J
=
= 9.8 1014 Hz
h 6.63 1034 J s
2. Calculate the wavelength from the frequency:
c 3.00 108 m/s
=
= 310 nm = 0.31 m
f 9.80 1014 Hz
Insight: As expected, this frequency and wavelength lie in the UV spectrum (see Chapter 25).
17. Picture the Problem: The signal from a radio station can be considered as the transmission of many photons of the
same frequency. The power output is equal to the number of photons emitted per second multiplied by the energy of
each photon.
Strategy: Divide the emitted power by the energy of each photon (equation 30-4) in order to calculate the rate of
photon emission.
Solution: Calculate the rate of photon emission:
P P
270 103 W
=
=
= 4.6 1032 photons/s
E hf ( 6.63 1034 J s )( 880 103 Hz )
Insight: Note that a radio station that broadcasts with the same power output, but at a higher frequency, would emit
fewer photons per second. For example, a station broadcasting 270 kW at 1400 kHz would emit 2.9 1032 photons/s.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 5
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
18. Picture the Problem: A helium atom is ionized when it absorbs the energy from a 50.4-nm photon.
Strategy: Set the energy of the photon (from equations 30-4 and 14-1) equal to the ionization potential of helium.
Solution: Replace the frequency in equation 30-4
with the speed of light divided by the wavelength:
E=
hc
( 6.63 10
34
J s )( 3.00 108 m/s )
50.4 109 m
= 3.95 10 18 J
Insight: Photons with wavelengths longer than 50.4 nm cannot completely ionize the helium atom because they have
too little energy. When photons with wavelengths that are shorter than 50.4 nm interact with the helium atom, they will
both ionize the atom and give extra kinetic energy to the emitted electrons.
19. Picture the Problem: The light from a flashlight can be considered as the emission of many photons of the same
frequency. The power output is equal to the number of photons emitted per second multiplied by the energy of each
photon.
Strategy: Divide the emitted power by the energy of each photon, given by equation 30-4, to calculate the rate of
photon emission.
Solution: Calculate the rate
of photon emission:
P P
2.5 W
=
=
= 7.3 1018 photons/s
34
E hf ( 6.63 10 J s )( 5.2 1014 Hz )
Insight: A flashlight emitting photons of a lower frequency would need to emit more photons per second to achieve the
same power output. For instance, if the frequency were 4.6 1014 Hz , then 8.21018 photons/s would need to be
emitted in order to produce a power of 2.5 W.
20. Picture the Problem: As photons interact with silver, some of the energy of the photon frees an electron from the silver
atom. The remainder of the energy becomes kinetic energy of the electron.
Strategy: Solve equation 30-7 for the work function of silver.
Solution: Calculate the work function:
K max = hf W0
W0 = hf K max = ( 6.63 1034 J s )( 9.95 1014 Hz ) 0.180 10 19 J
= 6.42 1019 J
Insight: In order for a photon to eject an electron from silver, it must have a frequency greater than 9.68 1014 Hz. At
this frequency, all of the energy of the photon goes into the work function and the ejected electron has no kinetic
energy.
21. Picture the Problem: As photons interact with gold, some of the energy of the photon frees an electron from the gold.
The remainder of the energy becomes kinetic energy of the electron.
Strategy: Solve equation 30-7 for the frequency of light. The work function is given in units of electron volts and must
be converted into joules.
Solution: 1. Solve eq. 30-7
for the frequency:
K max = hf W0
2. Insert the given data:
f =
f =
K max + W0
h
( 6.48 10
19
J ) + ( 4.58 eV ) (1.60 10 19 J eV )
6.63 1034 J s
= 2.08 1015 Hz
Insight: If this same photon were to interact with silver (which has a work function of 4.0 eV), the ejected electron
would have a greater kinetic energy.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 6
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
22. Picture the Problem: Each photon contains a quantized amount of energy, determined by the photon wavelength. The
total energy of 2.5 J is obtained by the addition of many photons.
Strategy: Calculate the energy of a single photon from its wavelength using equations 30-4 and 14-1. Divide the total
energy by the energy of the single photon to calculate the number of photons.
Solution: 1. (a) Use equations
30-4 and 14-1 to write the
energy of a single photon:
E = hf = h
2. Divide the total energy
by the energy of a single
350-nm photon:
n=
3. (b) Divide the total energy by
the energy of a 750-nm photon:
n=
( 350 109 m ) ( 2.5 J )
Etotal
Etotal
=
=
= 4.4 1018 photons
34
8
Ephoton
hc
6.63
10
J
s
3.00
10
m/s
(
)(
)
( 750 10
( 6.63 10
34
m ) ( 2.5 J )
J s )( 3.00 108 m/s )
= 9.4 1018 photons
Insight: More red photons than UV photons are required in order to produce the same amount of energy.
23. Picture the Problem: A monochromatic light bulb emits many photons per second. The number of photons per second
multiplied by the energy per photon is the power radiated by the light bulb.
Strategy: Divide the power output by the energy per photon (from equations 30-4 and 14-1) to obtain the rate of photon
emission in all directions. The fraction of these photons that enters your eye is the ratio of the area of your pupil to the
surface area of a sphere with radius 15 m. Use this fraction to find the number of photons entering your eye per second.
Solution: 1. (a) Calculate
the energy per photon:
E = hf = h
2. Divide the power by the
energy per photon to find
the rate of emission:
n=
3. (b) Multiply the photon rate
by the ratio of the areas of your
pupil and the 15-m sphere:
( 650 109 m ) ( 45 W ) = 1.5 1020 photons/s
P P
=
=
E hc ( 6.63 1034 J s )( 3.00 108 m/s )
neye = n
Aeye
Asphere
photons ( 0.0025 m )
= 1.5 1020
= 1.0 1012 photons/s
2
s
4 (15 m )
2
Insight: The minimum photon flux that your eye can detect is about 500 photons/s.
24. Picture the Problem: Two radio stations broadcast at the same power level, but station A broadcasts at a lower
frequency than station B.
Strategy: The rate of photon emission is the power level divided by the energy of a single photon. Note that the energy
of each photon is proportional to its frequency (equation 30-4).
Solution: 1. (a) The rate of emitted photons is determined by the ratio of the emitted power to the energy of a photon.
This ratio is inversely proportional to the frequency of the emitted photons. Therefore, the station with the lower
frequency has the highest rate of photon emission, and station A emits more photons per second than station B.
2. (b) Because the energy per photon is directly proportional to the frequency of the photon, the station that broadcasts
at the higher frequency emits the higher energy photons. So, station B emits higher energy photons than station A.
Insight: In this example, station A emits 9.7 1031 photons/s that each have an energy of 5.9 1028 J. Station B emits
6.2 1031 photons/s that each have an energy of 9.3 1028 J.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 7
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
25. Picture the Problem: The two atoms of a hydrogen molecule are bound together. When a photon with sufficient energy
is absorbed by the molecule it can disassociate the molecule into two hydrogen atoms.
Strategy: Calculate the energy necessary to disassociate a single hydrogen molecule by dividing the given energy by
the number of molecules in a mole. Use equation 30-4 to calculate the frequency of the light and equation 14-1 to
calculate the wavelength. Refer to Figure 25-8 to determine the region of the electromagnetic spectrum of this photon.
(104.2 kcal mol )
Solution: 1. (a) Calculate the energy
to disassociate one molecule:
E=
2. Calculate the photon frequency:
f =
E 7.245 1019 J
=
= 1.09 1015 Hz
h 6.63 1034 J s
3. Calculate the wavelength:
c 3.00 108 m/s
=
= 275 nm
f 1.09 1015 Hz
6.02 1023 molecules/mole
( 4186 J/kcal ) = 7.245 1019
4. (b) The photon lies in the ultraviolet region of the electromagnetic spectrum because UV < 400 nm.
Insight: Photons with wavelengths greater than 275 nm (such as visible light) do not contain sufficient energy to
disassociate the hydrogen molecule.
26. Picture the Problem: The light from the laser can be considered as the emission of many photons of the same
wavelength. The power output is the product of the photon emission rate and the energy of each photon.
Strategy: Divide the emitted power by the energy of each photon (equation 30-4) to calculate the rate of photon
emission, and then find the frequency using equation 14-1.
Solution: 1. (a) Calculate the
rate of photon emission:
n=
P P
P
P
=
=
=
E hf hc / hc
2. Insert the given wavelength and power:
n=
( 632.8 10 m )(1.0 10 W ) = 3.2 10
( 6.63 10 J s )( 3.00 10 m/s )
3. Calculate the frequency from equation 14-1:
f =
15
34
photons/s
3.00 108 ms
= 4.74 1014 Hz
632.8 109 m
Insight: The large number of photons in a laser beam can cause permanent damage to the retina before you can blink,
which is why you should never look into a laser!
27. Picture the Problem: A 150-watt red light bulb emits photons with wavelength of 650 nm, and a 25-watt blue light
bulb emits photons with a wavelength of 460 nm.
Strategy: Combine equations 30-4 and 14-1 to find the energy of the photons for each bulb. Divide the emitted power
by the energy of each photon to calculate the rate at which photons are emitted.
Solution: 1. (a) The energy emitted per photon is inversely related to the wavelength, so the red light bulb must emit
more photons to produce the same power output. The red bulb also produces more power than the blue bulb, so the red
bulb emits more photons per second than does the blue bulb.
2. (b) The energy per photon is inversely proportional to the wavelength. Because the blue bulb has the shorter
wavelength, the blue bulb emits photons of higher energy than the red bulb.
3. (c) Calculate the energy of the red photon:
E = hf = h
4. Divide the power by the energy:
nred =
= ( 6.63 1034 J s )
3.00 108 m/s
= 3.06 10 19 J
650 109 m
P
150 W
=
= 4.9 1020 photons/s
E 3.06 1019 J/photon
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 8
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
5. Calculate the energy of the blue photon:
E = ( 6.63 1034 J s )
6. Divide the power by the energy:
nblue =
3.00 108 m/s
= 4.32 10 19 J
460 109 m
P
25 W
=
= 5.8 1019 photons/s
E 4.32 1019 J/photon
Insight: If the blue bulb were to produce the same number of photons per second as the red bulb, it would emit a power
of 210 W.
28. Picture the Problem: A photon of wavelength 264 nm will eject an electron from a copper surface. The resulting
electron, however, has no kinetic energy.
Strategy: Set the work function of copper equal to the energy of the photon, given by equations 30-4 and 14-1.
Solution: Calculate the work function of copper:
W0 = hf =
hc
( 6.63 10
34
J s )( 3.00 108 m/s )
264 109 m
= 7.53 10 19 J
Insight: Electrons ejected from copper by photons with wavelengths less than 264 nm will have kinetic energy. Photons
larger than 264 nm cannot eject electrons from copper.
29. Picture the Problem: Photons with energies greater than the work function of a metal can eject electrons from that
metal.
Strategy: Use equation 30-6 to calculate the cut-off frequencies for the two metals.
Solution: 1. (a) Because higher-frequency photons have higher energies, and since WAl > WCa , aluminum requires
higher-frequency light to produce photoelectrons.
2. (b) Calculate the cutoff frequency for aluminum:
19
WAl ( 4.28 eV ) (1.60 10 J/eV )
f Al =
=
= 1.03 1015 Hz
h
6.63 1034 J s
3. Calculate the cutoff frequency for calcium:
f Ca =
19
WCa ( 2.87 eV ) (1.60 10 J/eV )
=
= 6.93 1014 Hz
h
6.63 1034 J s
Insight: A photon with frequency 1.031015 Hz will eject electrons from either surface. Such a photon has a
wavelength of 291 nm and is in the ultraviolet region of the electromagnetic spectrum (see Chapter 25).
30. Picture the Problem: When a photon is absorbed by a metal, its energy is split into the energy needed to eject the
electron and the kinetic energy of the electron. The energy of the photon is inversely proportional to its wavelength.
Strategy: Use equations 30-7 and 14-1 to write an expression of the kinetic energy of the electron in terms of the
wavelength of the photon.
Solution: 1. (a) Because K max = hf W0 =
hc
W0 , a shorter wavelength implies a larger kinetic energy. So, the beam
with wavelength B produces photoelectrons with greater kinetic energy than the beam with wavelength A .
2. (b) Calculate the kinetic energy of the
photoelectron when struck by photon A:
K max,A =
( 6.63 10 J s )( 3.00 10 m/s ) 1.9 eV = 0.1 eV
( 620 10 m )(1.60 10 J/eV )
3. Calculate the kinetic energy of the
photoelectron when struck by photon B:
K max,B =
( 6.63 10 J s )( 3.00 10 m/s ) 1.9 eV = 1.1 eV
( 410 10 m )(1.60 10 J/eV )
34
19
34
19
Insight: The cutoff wavelength that will eject an electron from cesium is 654 nm. Because photon A has a wavelength
slightly smaller than the cutoff, the resulting photoelectron has a small kinetic energy.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 9
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
31. Picture the Problem: When a photon is absorbed by a metal, its energy is split into the energy needed to eject the
electron (the work function) and the kinetic energy of the electron. The energy of the photon is inversely proportional to
its wavelength.
Strategy: Calculate the energy of the photon using equations 30-4 and 14-1. Then use equation 30-5 to write an
expression for the maximum kinetic energy of photoelectrons ejected from each metal.
Solution: 1. (a) The difference in energy between the incident photons and the work function of the metal is the
photoelectrons kinetic energy. Therefore, if two different metals are illuminated by photons with the same energy, the
metal with the smaller work function will emit photoelectrons with a greater maximum kinetic energy. In this case,
cadmium has a smaller work function and emits photoelectrons with the greater maximum kinetic energy.
( 6.63 10 J s )( 3.00 10 m/s ) = 4.52 eV
=
( 275 10 m )(1.60 10 J/eV )
34
hc
2. (b) Calculate the energy of the photon:
E = hf =
3. Insert the work function for zinc into equation 30-5:
K max, Zn = E W0 = 4.52 eV 4.33 eV = 0.19 eV
4. Repeat for cadmium:
K max, Cd = 4.52 eV 4.22 eV = 0.30 eV
19
Insight: Because the work function of zinc is closer to the energy of the photon, the electron ejected from zinc has less
kinetic energy than the electron ejected from the cadmium.
32. Picture the Problem: When white light is incident upon the potassium, the photons with energies greater than the work
function of potassium will eject electrons. The greater the photon energy, the greater the kinetic energy of the ejected
electron.
Strategy: Because the photon energy is proportional to the frequency, the photons with the greatest frequency will eject
electrons with the maximum kinetic energy. Insert the maximum frequency into equation 30-7 to calculate the
maximum kinetic energy of the photoelectrons. Then use equation 30-6 to calculate the cutoff frequency. All photons
with smaller frequencies will not eject electrons.
Solution: 1. (a) Use equation 30-7 to find K max :
K max = hf W0
( 6.63 10
=
34
J s )( 7.90 1014 Hz )
1.60 1019 J/eV
2.24 eV
= 1.03 eV
19
W0 ( 2.24 eV ) (1.60 10 J/eV )
=
= 5.41 1014 Hz
h
6.63 1034 J s
2. (b) Calculate the cutoff frequency:
f =
3. Write the frequency range for which
no electrons are emitted:
4.00 1014 Hz f < 5.411014 Hz
Insight: Photons that are incident upon a potassium surface and that have frequencies greater than 5.41 1014 Hz will
emit photoelectrons with kinetic energies ranging from zero to 1.03 eV.
33. Picture the Problem: When the electromagnetic waves are incident upon the aluminum surface, the photons with
energies greater than the work function of aluminum eject electrons. The greater the photon energy, the greater the
kinetic energy of the ejected electron.
Strategy: Because the photon energy is proportional to the frequency, the photons with the greatest frequency will eject
electrons with the maximum kinetic energy. Insert the maximum frequency into equation 30-7 to calculate the
maximum kinetic energy of the photoelectrons. Then use equation 30-6 to calculate the cutoff frequency. All photons
with smaller frequencies will not eject electrons.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 10
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
Solution: 1. (a) Use equation 30-7 to find K max :
K max = hf W0 =
( 6.63 10
34
J s )( 9.00 1016 Hz )
1.60 1019 J/eV
4.28 eV
= 369 eV
19
W0 ( 4.28 eV ) (1.60 10 J/eV )
=
= 1.03 1015 Hz
h
6.63 1034 J s
2. (b) Calculate the cutoff frequency:
f =
3. Write the frequency range for
which no electrons are emitted:
4.00 1014 Hz f < 1.03 1015 Hz
Insight: Photons with frequencies greater than 1.03 1015 Hz emit electrons with kinetic energies ranging from zero to
369 eV. It is a bit odd to call these photons electromagnetic waves as in the problem statement, because the classical
description of light as a wave is inadequate to explain the photoelectric effect.
34. Picture the Problem: When photons of the given frequency are incident on the two metals, some of the energy of the
photon will eject the electron from the surface (work function) and the remainder of the energy will become kinetic
energy of the electron.
Strategy: Use equation 30-4 to calculate the energy of the photon. Then insert that energy together with the work
function of each metal into equation 30-5 to calculate the maximum kinetic energy.
Solution: 1. (a) Because K max = hf W0 , the photoelectrons emitted by the metal with the smaller work function will
have the greater kinetic energy. The electrons ejected from the iron surface will have the greater maximum kinetic
energy because iron has a smaller work function than platinum.
( 6.63 10
=
34
J s )(1.88 1015 Hz )
2. (b) Calculate the photon energy:
E = hf
3. Calculate the maximum kinetic energy
for electrons photoejected from platinum:
K max, Pt = E W0 = 7.79 eV 6.35 eV = 1.44 eV
4. Calculate the maximum kinetic energy
for photoelectrons ejected from iron:
K max, Fe = 7.79 eV 4.50 eV = 3.29 eV
1.60 1019 J/eV
= 7.79 eV
Insight: The cutoff frequency for platinum is 1.53 1015 Hz and for iron is 1.09 1015 Hz . Photons with frequencies
between these two values will eject photoelectrons from the iron, but not from the platinum.
35. Picture the Problem: As photons of two different frequencies are incident upon a metal, they eject photoelectrons with
two different maximum kinetic energies. The maximum kinetic energy of photoelectrons is the difference between the
energy of the photon and the work function of the metal.
Strategy: Solve equation 30-7 for the work function. Because the work function for the metal is constant, set the work
functions for both experiments equal and solve for Plancks constant.
Solution: 1. Solve equation 30-7
for the work function:
K max = hf W0
2. Set the work functions equal
and solve for Plancks constant:
hf1 K max,1 = hf 2 K max,2
W0 = hf K max
h=
K max,1 K max,2
f1 f 2
1.260 1019 J 2.480 1019 J
547.5 1012 Hz 738.8 1012 Hz
= 6.377 1034 J s
Insight: These experiments determine Plancks constant to within 4% of its accepted value of 6.6260693 1034 J s.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 11
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
36. Picture the Problem: The large pupil in an owls eye allows for more photons per second to enter the eye than the
number of photons that would enter the human eye. In addition, the retina of the owl is more sensitive to fewer photons
than the human eye.
Strategy: Multiply the minimum intensity of light by the area of the owls pupil to determine the power absorbed by the
owls eye. Divide this power by the energy per photon to determine the minimum photon flux that the owl can detect.
Solution: 1. Calculate the power absorbed
in the owls eye at minimum intensity:
P = IA = I r 2 = ( 5.0 10 13 W/m 2 ) ( 0.00425 m ) = 2.837 1017 W
2. Calculate the energy per photon:
E = hf = ( 6.63 1034 J s )( 7.0 10 34 Hz ) = 4.64 1019 J
3. Divide the power by the energy:
n=
17
P ( 2.837 10 W )
=
= 61 photons/s
E
4.64 1019 J
Insight: If the human eye were sensitive to 61 photons/s, the smaller pupil of the human eye would require a minimum
light intensity of 7.4 1013 W/m 2 .
37. Picture the Problem: The momentum of a photon is doubled, causing its energy to increase.
Strategy: Use the expression p = E c for a photon to answer the conceptual question.
Solution: The momentum of a photon is linearly proportional to its energy. Therefore, doubling the momentum of a
photon increases its energy by a multiplicative factor of 2.
Insight: The relationship between p and E is a consequence of the fact that both the energy and the momentum of a
photon are linearly proportional to its frequency (equations 30-4 and 30-11).
38. Picture the Problem: The photons in a microwave have a given momentum and wavelength.
Strategy: Use equation 30-11 to calculate the wavelength of the microwaves.
Solution: 1. (a) Solve equation 30-11 for the wavelength:
h
6.63 1034 J s
=
= 13 cm
p 5.1 1033 kg m/s
2. (b) The wavelength is much larger than the size of the holes in the metal screen.
Insight: Because the holes are smaller than the microwaves, the microwaves reflect off the screen and remain inside the
microwave oven. However, visible light, which has a much smaller wavelength, can exit through the holes and allow
you to see the food cooking.
39. Picture the Problem: A photon and an electron have the same momentum. However, because the electron has mass, its
speed will be slower than that of the photon.
Strategy: Use equation 30-11 to calculate the momentum of the photon. Set the momentum of the photon equal to the
momentum of the electron. Assume that the speed of the electron is not relativistic ( v < 0.10c ) and use equation 9-1.
Solution: 1. Calculate the
momentum of the photon:
p=
6.63 1034 J s
= 2.65 10 24 kg m/s
0.25 109 m
p = mv
2. Set the momentum equal to
the momentum of an electron
and solve for the velocity:
v=
p 2.65 1024 kg m/s
=
= 2.9 106 m/s = 0.0097c
m
9.11 1031 kg
Insight: Since the speed of the electron is much less than the speed of light, our assumption of classical momentum is
valid. If the speed had been greater than 0.10c, it would have been better to use a relativistic formula (equation 29-5).
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 12
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
40. Picture the Problem: A photon and an electron have the same momentum. The electrons momentum is determined by
its speed and mass. The photons momentum is determined by its wavelength.
Strategy: Calculate the momentum of the electron using equation 9-1. The relativistic formula (equation 29-5) is not
necessary because the speed is much lower than the speed of light. Set the electron and photon momenta equal and use
equation 30-11 to calculate the photon wavelength.
Solution: 1. Find the momentum of the electron:
p = mv = ( 9.11 1031 kg ) (1200 m/s ) = 1.09 1027 kg m/s
2. Calculate the wavelength of the photon:
h
6.63 1034 J s
=
= 610 nm = 0.61 m
p 1.09 1027 kg m/s
Insight: This wavelength corresponds to visible, green light.
41. Picture the Problem: A photon and a neutron have the same momentum. The neutrons momentum is determined by
its speed and mass. The photons momentum is determined by its wavelength.
Strategy: Calculate the momentum of the neutron using equation 9-1. The relativistic formula (equation 29-5) is not
necessary because the speed is much lower than the speed of light. Set this momentum equal to the momentum of the
photon and use equation 30-11 to calculate the photon frequency.
Solution: 1. Find p for the neutron:
p = mv = (1.675 1027 kg ) (1500 m/s ) = 2.513 1024 kg m/s
2. Calculate the frequency of the photon:
f =
24
8
pc ( 2.513 10 kg m/s )( 3.00 10 m/s )
=
= 1.11018 Hz
h
6.63 1034 J s
Insight: The photon with this frequency is in the X-ray part of the spectrum.
42. Picture the Problem: A hydrogen atom that is initially at rest emits a 122-nm photon. The atom recoils in the direction
opposite to the propagation of the photon.
Strategy: Before the emission the hydrogen atom was at rest. The photon and the hydrogen atom then have equal but
opposite momenta. Use equation 30-11 to calculate the momentum of the photon. Set it equal to the momentum of the
hydrogen atom and use equation 9-1 to calculate the recoil speed of the hydrogen atom.
h
pUV =
2. Solve for the recoil speed:
pUV = pH = mv
v=
6.63 1034 J s
= 5.43 1027 kg m/s
122 109 m
Solution 1: Calculate the momentum of the photon:
pUV 5.43 1027 kg m/s
=
= 3.25 m/s
m
1.674 10 27 kg
Insight: Note that the speed of the hydrogen atom is inversely proportional to the wavelength of the photon. If the atom
had emitted a photon with wavelength = 61 nm, the recoil speed would double to 6.5 m/s.
43. Picture the Problem: A blue-green photon collides with a stationary hydrogen atom. The hydrogen atom absorbs the
photon and moves forward with the same momentum as the initial photon.
Strategy: Calculate the initial momentum of the photon using equation 30-11. Then set that momentum equal to the
momentum of the hydrogen atom after the absorption. Use equation 9-1 to calculate the speed of the hydrogen.
h
pph =
2. Solve for the speed of the hydrogen atom:
pph = pH = mv
v=
pph
m
6.63 1034 J s
= 1.36 1027 kg m/s
486 109 m
Solution: 1. Calculate the momentum of the photon:
1.364 1027 kg m/s
= 0.815 m/s
1.674 1027 kg
Insight: The speed of the hydrogen atom is inversely proportional to the wavelength of the incident photon. Decreasing
the wavelength of the photon will increase its momentum, and thus increase the speed of the hydrogen atom.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 13
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
44. Picture the Problem: A red photon and a blue photon each carry momentum in proportion to their frequencies.
Strategy: Calculate the momenta of the two photons using equation 30-11.
Solution: 1. (a) Because f blue > f red , and since p =
hf
, pblue > pred . A photon of blue light has the greater momentum.
c
2. (b) Calculate pred:
pred =
3. Calculate pblue:
pblue =
34
14
hf 6.63 10 J s ( 4.0 10 Hz )
=
= 8.8 1028 kg m/s
c
3.00 108 m/s
6.63 1034 J s ( 7.9 1014 Hz )
3.00 108 m/s
= 1.7 1027 kg m/s
Insight: Because the frequency of the blue photon is about double the frequency of the red photon, the momentum of
the blue photon is about double the momentum of the red photon.
45. Picture the Problem: Photons A and B carry momentum in inverse proportion to their wavelengths.
Strategy: Set the momentum of photon A equal to twice the momentum of photon B. Write the momenta in terms of
the wavelengths using equation 30-11. Solve the resulting equation for the wavelength of photon B.
Solution: 1. (a) Because a photons momentum is inversely proportional to its wavelength, the photon with the smaller
momentum has the longer wavelength. Therefore, photon B has the longer wavelength.
2. (b) Use equation 30-11 to calculate
the wavelength of photon B:
2 pB = pA 2
B = 2A = 2 ( 333 nm ) = 666 nm
Insight: Because the wavelength is inversely proportional to the momentum, a photon with twice the momentum will
have half the wavelength.
46. Picture the Problem: As a beam of photons is absorbed by a black surface, the momentum of the photons is absorbed
by the surface, and a corresponding force is exerted on the surface.
Strategy: Calculate the rate of photon production by dividing the power of the laser beam by the energy in each photon
(from equations 30-4 and 14-1). Calculate the change in momentum by using equation 30-11 for the photon momentum.
Multiply the photon rate by the momentum change to obtain the net force on the black surface.
( 6.63 10
34
Solution: 1. (a) Calculate
the energy in each photon:
E=
2. Divide the power by the energy per photon:
n=
3. (b) Calculate the momentum change
when each photon is stopped:
p = pf pi = 0 pi
hc
632.8 10 9 m
P
Ephoton
J s )( 3.00 108 m/s )
= 3.143 1019 J
5.00 10 3 W
= 1.59 1016 photons/s
-19
3.143 10 J
6.63 1034 J s
= 1.05 1027 kg m/s
632.8 109 m
F = np = (1.59 1016 photons/s )(1.05 10 27 kg m/s )
4. (c) Multiply the momentum change
by the photon rate:
= 1.67 1011 N
Insight: This force, even though it is small, has applications in fusion research, where it is used to confine hydrogen
pellets. It is also employed by an optical tweezers, a laser device that allows a researcher to manipulate very tiny
objects while looking at them through a microscope.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 14
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
47. Picture the Problem: As each photon in a laser beam is reflected by a mirror, its momentum is reversed, resulting in an
impulse on the mirror. The impulses from many photons produce an applied force on the mirror.
Strategy: Calculate the rate of photon production by dividing the power of the laser beam by the energy in each photon
(from equations 30-4 and 14-1). When the photon is reflected, the magnitude of its change in momentum is equal to
twice its momentum. Calculate this change in momentum by using equation 30-11. Multiply the photon rate by the
momentum change to obtain the net force on the mirror.
( 6.63 10
34
Solution: 1. (a) Calculate the
energy in each photon:
E=
2. Divide the power by the energy per photon:
n=
3. (b) Calculate the momentum change
when each photon is reflected:
p = pf pi = 2 pi
hc
P
Ephoton
2h
J s )( 3.00 108 m/s )
632.8 109 m
=
= 3.143 1019 J
7.50 103 W
= 2.39 1016 photons/s
3.143 10-19 J
2 ( 6.63 1034 J s )
632.8 109 m
= 2.10 10 27 kg m/s
F = np = ( 2.39 1016 photons/s )( 2.10 10 27 kg m/s )
4. (c) Multiply the momentum
change by the photon rate:
= 5.00 1011 N
Insight: The force on a reflecting mirror is twice the force on an absorbing surface (see problem 46) because the change
in momentum is double for the reflecting surface.
48. Picture the Problem: In a Compton scattering experiment, the scattered electron is observed to move in the same
direction as the incident X-ray photon.
Strategy: Use the principles that govern the Compton effect to answer the conceptual question.
Solution: Suppose the initial photon moves in the positive x direction. This means that the initial y component of
momentum is zero. After the collision, we are told that the electron moves in the positive x directionit has no y
component of momentum. Therefore, the scattered photon must also have zero y component of momentum, which
means it will propagate in either the positive x direction or the negative x direction. If it were to propagate in the
positive x direction, the scattering angle would be 0 and the Compton formula (equation 30-15) indicates the photon
will transfer no momentum to the electron. We reject that solution because it implies the electron does not scatter at all,
and we conclude that the scattering angle of the photon is 180.
Insight: Inserting = 180 into equation 30-15 shows that the wavelength of the scattered photon will increase by the
amount = 2h me c = 2 ( 2.426 pm ) = 4.85 pm.
49. Picture the Problem: An X-ray photon scatters off of an electron and transfers part of its energy to the electron.
Strategy: By conservation of energy, the kinetic energy of the electron will equal the change in energy of the X-ray.
Solution: Calculate the change in energy of the X-ray:
K electron = K photon = K i K f = 38.0 keV 33.5 keV = 4.5 keV
Insight: Inserting the maximum change in wavelength ( = 180) into equation 30-15 implies that the energy of the
final X-ray must be at least 33.1 keV, for momentum to be conserved.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 15
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
50. Picture the Problem: The wavelength of a photon increases after it
scatters off an electron and travels at an angle from its initial direction.
Strategy: Use the Compton scattering equation (equation 30-15) to find
for each of the scattering angles. A recurring value in similar
Compton scattering problems is h me c = 2.426 1012 m = 2.426 pm.
h
(1 cos )
me c
Solution: 1. Insert values for
the constants in equation 30-15:
= =
2. (a) Insert = 30 :
= ( 2.426 pm )(1 cos 30.0 ) = 0.325 pm
3. (b) Insert = 90 :
= ( 2.426 pm )(1 cos 90.0 ) = 2.43 pm
4. (c) Insert = 180 :
= ( 2.426 pm )(1 cos180.0 ) = 4.85 pm
= ( 2.426 pm )(1 cos )
Insight: The maximum change in wavelength is 4.85 pm because the maximum scattering angle is 180.
51. Picture the Problem: The wavelength of a photon increases after it
scatters off an electron and travels at an angle from its initial direction.
Strategy: Solve the Compton scattering equation (equation 30-15) for the
scattering angle as a function of the change in wavelength. A recurring
value in similar Compton scattering problems is
h me c = 2.426 1012 m = 2.426 pm.
=
Solution: 1. Solve equation 30-15 for :
h
(1 cos )
me c
= cos 1 1
2. Insert the given wavelength change:
= cos 1 1
h me c
3.13 pm
= 107
2.426 pm
Insight: If the change in wavelength had only one-half of this value (1.565 pm), the scattering angle would be 69,
which is greater than one-half of the scattering angle from this problem. The change in wavelength and scattering angles
are not linearly proportional to each other.
52. Picture the Problem: The smallest change in wavelength in a Compton scattering is zero, when the photon continues to
travel straight forward. The greatest change in wavelength is when the photon is scattered directly backward. A change
in wavelength of one-fourth of the maximum will occur at an angle between the minimum and maximum.
Strategy: Set the scattering angle in equation 30-15 to 180 to find the maximum change in wavelength. Then solve
equation 30-15 for the scattering angle, when the change in wavelength is equal to one-quarter of the maximum. A
recurring value in similar Compton scattering problems is h me c = 2.426 1012 m = 2.426 pm.
Solution: 1. Calculate the maximum change in wavelength:
max =
2. Set the change in wavelength equal to a quarter
of the maximum and solve for the scattering angle:
h
h
2h
1 cos (180 ) =
(1 cos ) =
me c
me c
me c
h
(1 cos ) = max (1 cos )
me c
2
set
max
4
= cos 1 (1 12 ) = 60
Insight: This angle is one-third of the maximum angle. If the angle were two-thirds of the maximum, or 120, the
change in wavelength would be 34 max .
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 16
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
53. Picture the Problem: Two photons of different wavelengths are scattered backward from collisions with free-electrons.
Strategy: Use equation 30-15 to calculate the change in wavelength for a scattering of 180. Calculate the percent
difference by dividing the change in wavelength by the wavelength of each photon. A recurring value in similar
Compton scattering problems is h me c = 2.426 1012 m = 2.426 pm.
Solution: 1. (a) Because the change in wavelength does not depend on the wavelength of the photon, the change in
wavelength is the same for both photons.
2. (b) Because the change in wavelength is the same, the photon with the shorter wavelength, = 0.030 nm, will
experience the greater percent change in wavelength. The X-ray photon thus experiences the greater percent change.
=
3. (c) Use equation 30-15 to find :
h
(1 cos )
me c
= ( 2.426 pm ) 1 cos (180 ) = 4.85 pm = 0.00485 nm
4. Calculate the percent change in
wavelength of the visible photon:
5. Calculate the percent change in
wavelength of the X-ray:
0.00485 nm
100% = 9.3 104 %
520 nm
0.00485 nm
100% = 16%
0.030 nm
Insight: Even though the change in wavelength is constant, the X-ray transfers more energy to the electron in order to
conserve momentum in the collision. As the wavelength of the photon decreases, the transferred energy becomes a
larger portion of the total energy of the incident photon.
54. Picture the Problem: A photon of wavelength 0.240 nm scatters off a free
electron at rest at an angle of 105.
Strategy: Calculate the initial momentum of the photon using equation
30-11. Then use equation 30-15 to calculate the final wavelength of the
photon. Insert the final wavelength into equation 30-11 to calculate the
final momentum. A recurring value in similar Compton scattering
problems is h me c = 2.426 1012 m = 0.002426 nm.
Solution: 1. (a) Calculate
the initial momentum:
pi =
6.63 1034 J s
0.240 109 m
= 2.76 1024 kg m/s
2. (b) Solve equation 30-15
for the final wavelength:
= f i =
f = i +
h
(1 cos )
me c
h
(1 cos )
me c
= 0.240 nm + ( 0.002426 nm ) 1 cos (105 ) = 0.243 nm
3. (c) Calculate the final momentum:
pf =
6.63 10 34 J s
= 2.73 1024 kg m/s
0.243 109 m
Insight: The final momentum of the electron can be calculated from the vector change in the momentum of the photon.
The final electron momentum is 4.36 1024 kg m/s at an angle of = 37.3.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 17
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
55. Picture the Problem: A photon that has scattered off of an electron at an
angle of 175 has a wavelength of 0.320 nm.
Strategy: The incident photon will have a wavelength that is shorter than
0.320 nm. Solve equation 30-15 for the wavelength of the initial photon. For
parts (b) and (c), calculate the initial and final energies of the photons using
equations 30-4 and 14-1. The kinetic energy of the electron is equal to the
difference in energies of the photons. A recurring value in similar Compton
scattering problems is h me c = 2.426 1012 m = 0.002426 nm.
= f i =
Solution: 1. (a) Solve
equation 30-15 for i :
i = f
h
(1 cos )
me c
h
(1 cos )
me c
= 0.320 nm ( 0.002426 nm )(1 cos175 ) = 0.315 nm
2. (b) Calculate the energy
of the incident photon:
E i = hf =
3. Calculate the energy
of the scattered photon:
Ef =
hc
hc
( 6.63 10
( 0.315 10
( 6.63 10
( 0.320 10
34
34
J s )( 3.00 108 m/s )
m )(1.60 1016 J/keV )
J s )( 3.00 108 m/s )
m )(1.60 1016 J/keV )
= 3.94 keV
= 3.88 keV
4. (c) The kinetic energy of the electron
K = Ei Ef = 3940 eV 3880 eV = 60 eV
equals the change in photon energy:
Insight: In this situation only 1.4% of the photon energy is transferred to the electron.
56. Picture the Problem: An X-ray scatters 180 of an electron or off a helium atom. As a result of the scattering, some of
the energy of the X-ray is transferred to the particle, and the wavelength of the X-ray is increased.
Strategy: Use equation 30-15 to calculate the change in wavelength, where = 180. For the scattering off of the
helium atom, replace the mass of the electron with the mass of helium.
Solution: 1. (a) Because
1
and me < mHe , the change in wavelength of the X-ray is greater for the electron.
m
2. (b) Calculate the change in wavelength
for scattering off of the electron:
3. Calculate the change in wavelength
for scattering off of the helium atom:
electron
He
6.63 10 34 J s ) (1 cos180 )
(
h
=
= 4.85 pm
(1 cos ) =
me c
9.11 1031 kg ( 3.00 108 m/s )
6.63 1034 J s ) (1 cos180 )
(
h
=
= 0.666 fm
(1 cos ) =
mHe c
6.64 1027 kg ( 3.00 108 m/s )
Insight: Because the helium atom has much more mass than the electron, the helium atom requires less kinetic energy
to carry away the same momentum, as such the photon does not need to transfer as much energy to the helium atom.
57. Picture the Problem: When a certain photon scatters off of an electron, its wavelength increases by 10% when its
scattering angle is = 135.
Strategy: Set the change in wavelength equal to 0.10 i in equation 30-15 and solve for the initial wavelength. Then use
equation 30-4 and 14-1 to calculate the initial energy of the photon. A recurring value in similar Compton scattering
problems is h me c = 2.426 1012 m = 2.426 pm.
Solution: 1. Solve eq. 30-15
for the initial wavelength:
= 0.100 i =
i =
h
(1 cos )
me c
h (1 cos )
0.100me c
( 2.426 pm )(1 cos135 )
0.100
= 41.4 pm
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 18
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
2. Calculate the initial energy of the photon:
E i = hf =
hc
( 6.63 10
34
J s )( 3.00 108 m/s )
41.4 1012 m
= 4.80 fJ
Insight: The final energy of the photon is 4.36 fJ, because 0.44 fJ of energy is transferred to the electron as a result of
the collision.
58. Picture the Problem: The image shows a photon with an initial
wavelength of 0.525 nm scattering off an electron. The wavelength of the
scattered photon is 3.33 pm longer than the initial wavelength. As a result
of the collision, the electron scatters at an angle from the positive x-axis.
Strategy: Use equation 30-11 to calculate the magnitude of the initial and
final momentum of the photon. Calculate the scattering angle of the photon
using equation 30-15. Use the scattering angle to calculate the horizontal
and vertical components of the momentum of the scattered X-ray photon.
Use conservation of momentum to calculate the components of the
electrons momentum. Finally, use these components to calculate the angle
that the electron scatters. A recurring value in similar Compton scattering
problems is h me c = 2.426 1012 m = 2.426 pm.
h
6.63 1034 J s
= 1.263 1024 kg m/s
0.525 nm
Solution: 1. Calculate the initial
momentum of the photon:
pi =
2. Calculate the final
momentum of the photon:
pf =
h
6.63 1034 J s
=
= 1.255 1024 kg m/s
i + 0.525 nm + 0.00333 nm
3. Calculate the scattering
angle of the photon:
h
(1 cos )
me c
= cos 1 1
4. Calculate the horizontal and
vertical components of the final
photon momentum:
me c
3.33 pm
= cos 1 1
= 111.9
h
2.426
pm
px, f = p f cos = (1.255 1024 kg m/s ) cos (111.9 )
= 4.677 1025 kg m/s
py, f = p f sin = (1.255 1024 kg m/s ) sin (111.9 )
= 1.164 1024 kg m/s
5. Calculate the horizontal
component of the electron
momentum:
pi = px, f + pe,x
pe,x = pi px, f = 1.263 1024 kg m/s ( 4.677 1025 kg m/s )
= 1.73 1024 kg m/s
6. Calculate the vertical component
of the electron momentum:
0 = py, f + pe, y
pe, y = py, f = (1.164 1024 kg m/s ) = 1.164 1024 kg m/s
24
pe,y
kg m/s
1 1.164 10
= tan
= 34
24
m/s
p
1.73
10
kg
e,x
7. Calculate the angle at
which the electron recoils:
= tan 1
Insight: The momentum of the recoiling electron is
pe,2 x + pe,2 y = 2.09 1024 kg m/s and its kinetic energy is 14.9 eV.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 19
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
59. Picture the Problem: Your car gains momentum as it accelerates away from a stoplight.
Strategy: Use the de Broglie wavelength equation (equation 30-16) to answer the conceptual question.
Solution: 1. (a) The de Broglie wavelength = h p is inversely proportional to the momentum. Therefore, as the
momentum of your car increases, the de Broglie wavelength will decrease.
2. The best explanation is II. The momentum of the car increases. It follows that the de Broglie wavelength will
decrease, because it is inversely proportional to the wavelength. Statement I erroneously assumes the de Broglie
wavelength is proportional to the momentum, and statement III is false.
Insight: Why doesnt the de Broglie wavelength of your car increase to infinity when you come to a stop? While this
would be the case for a single particle at rest, you car is composed of many billions of particles that are all moving due
to their thermal energy. The de Broglie wavelength of each atom is too small to measure unless the atom is nearly at rest
(which requires the temperature to be nearly absolute zero).
60. Picture the Problem: The de Broglie wavelength of a particle changes when either its momentum or its kinetic energy
is doubled.
Strategy: Use the de Broglie wavelength equation (equation 30-16) to answer the conceptual question.
Solution: 1. (a) Because the de Broglie wavelength is = h p , it follows that doubling the momentum halves the
wavelength. Therefore, the wavelength changes by a multiplicative factor of 1 2 .
2. Doubling the kinetic energy means that the momentum increases by a factor of
wavelength changes by a multiplicative factor of 1
2 . As a result, the de Broglie
2.
Insight: In each case the de Broglie wavelength of the particle decreases as the speed increases.
61. Picture the Problem: The de Broglie wavelength of a particle is inversely proportional to its momentum.
Strategy: Replace the momentum in the de Broglie wavelength equation (equation 30-16) with equation 9-1. Solve the
resulting expression for the particles speed.
Solution: 1. Combine equations 30-16 and 9-1:
h
h
=
p mv
2. Solve for the particle velocity:
v=
6.63 1034 J s
h
=
= 13.7 km/s
m ( 7.22 1012 m )( 6.69 1027 kg )
Insight: It is appropriate to use the classical equation for the momentum because the particle speed is much less than
the speed of light.
62. Picture the Problem: The de Broglie wavelength of a neutron is inversely proportional to its momentum.
Strategy: Replace the momentum in the de Broglie wavelength equation (equation 30-16) with equation 9-1. Solve the
resulting equation for the neutron velocity.
Solution: 1. Combine equations 30-16 and 9-1:
h
h
=
p mv
2. Solve for the neutron velocity:
v=
h
6.63 1034 J s
=
= 1.40 km/s
m ( 0.282 10 9 m )(1.675 1027 kg )
Insight: When the wavelength of the neutron is equal to the interatomic spacing, the neutron beam will diffract and
produce a maximum at an angle of 60 from the normal.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 20
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
63. Picture the Problem: If we consider a 79-kg jogger to be a particle we can determine her de Broglie wavelength.
Strategy: Use equation 30-16 to calculate the wavelength of the jogger, where the joggers momentum is given by the
classical momentum equation (equation 9-1).
Solution: Calculate the de Broglie wavelength:
h
h
6.63 10 34 J s
=
=
= 2.0 1036 m
p mv ( 79 kg )( 4.2 m/s )
Insight: This wavelength is a billion trillion times smaller than the nucleus of a single atom, much too small to
measure! Furthermore, the jogger is really a collection of particles (atoms), each of which is moving rapidly in thermal
motion and has a de Broglie wavelength that is smaller than an atom.
64. Picture the Problem: The de Broglie wavelength of an electron is related to both the momentum and the kinetic energy
of an electron.
Strategy: Write the kinetic energy (equation 7-6) of the electron in terms of the momentum (equation 9-1). Then use
equation 30-16 to write the momentum in terms of the de Broglie wavelength.
1 2 m2v 2
p2
h2
=
=
mv =
2
2m
2m 2m 2
Solution: 1. Write the kinetic energy in
terms of the de Broglie wavelength:
K=
2. Insert the wavelength:
( 6.63 10 J s )
K=
2 ( 9.11 10 kg )(1.5 10
34
31
10
m)
= 1.1 1017 J
Insight: In this problem we used the classical equations for the kinetic energy and the momentum. This assumption can
be checked solving equation 30-16 for the speed. The resulting ve = 4.9 106 m/s = 0.016c is small enough that
relativistic effects can be neglected.
65. Picture the Problem: A beam of neutrons diffracts off of a crystal due to the de Broglie wavelength of the neutrons.
Strategy: Set the momentum in equation 30-16 equal to the mass times velocity and solve for the velocity. To calculate
the second-order diffraction angle, solve equation 30-17 for the angle , where m = 2.
6.63 1034 J s
h
h
h
=
v=
=
= 1.58 km/s
m ( 0.250 109 m )(1.675 1027 kg )
p mv
Solution: 1. (a) Solve
equation 30-16 for v:
2. (b) Calculate the secondorder diffraction angle:
2d sin = m
= sin 1
2 ( 0.250 nm )
m
= sin 1
= 62.4
2d
2 ( 0.282 nm )
Insight: The neutron beam will exhibit both a first- and a second-order diffraction maximum. However, there can be no
higher-order maxima, because for m = 3, m 2d = 1.33 is outside the acceptable range of the inverse sine function.
66. Picture the Problem: Because the proton is significantly more massive than an electron, it will have a greater
momentum when the proton and electron have the same speed. The de Broglie wavelength is inversely proportional to
the particle momentum.
Strategy: Calculate the ratio of the de Broglie wavelengths using equation 30-16, where the momentum is the mass
times velocity.
Solution: 1. (a) Because = h mv and me < mp , for identical speeds, an electron has a longer de Broglie wavelength
than a proton.
2. (b) Calculate the ratio of wavelengths:
e h me v mp 1.673 1027 kg
=
=
=
= 1836
p h mp v me 9.109 1031 kg
Insight: In this problem we assumed a classical speed so that we could use the classical momentum equation. However,
because the relativistic factor depends only upon the speed, the ratio of the wavelengths would still be 1840 at
relativistic speeds.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 21
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
67. Picture the Problem: An electron and proton have the same de Broglie wavelength, which means that they must also
have the same momentum. However, their kinetic energies will differ because they have different masses.
Strategy: Use equation 30-16 to write the kinetic energy in terms of the de Broglie wavelength. Then divide the kinetic
energy of the proton by the kinetic energy of the electron to calculate their ratio.
Solution: 1. (a) The proton and electron have the same momentum because they have the same de Broglie wavelength.
The kinetic energy, K = p 2 2m , is inversely proportional to the mass, and me < mp . For identical momenta, an
electron has a greater kinetic energy than a proton.
2. (b) Write the kinetic energy in terms of the wavelength:
K = p 2 2m = h 2 2m 2
3. Calculate the ratio of the kinetic energies:
K e h 2 2me 2 mp 1.673 1027 kg
=
=
=
= 1836
K p h 2 2mp 2 me 9.109 1031 kg
Insight: In this problem we used the classical equation for the kinetic energy. As the speeds approach the speed of light,
the ratio of kinetic energies decreases due to relativistic effects.
68. Picture the Problem: When a person walks through a doorway, we typically do not see the person diffract. However, if
the persons de Broglie wavelength were on the order of the door width, the diffraction would be observable.
Strategy: Solve equation 30-16 for the speed of a person whose de Broglie wavelength equals the width of the door.
For part (b), divide one millimeter by the velocity to determine the time elapsed.
Solution: 1. (a) Solve eq. 30-16
for the persons speed:
2. (b) Calculate the time to travel 1.0 mm:
h
h
=
p mv
v=
h
6.63 1034 J s
=
= 1.3 1035 m/s
m 0.76 m ( 65 kg )
t=
x
0.0010 m
=
= 7.5 1031 s
v 1.342 1035 m/s
Insight: This time is about 2.0 1014 times longer than the age of the universe. The doorway would not exist long
enough to observe the diffraction! In reality, the person is really a collection of many particles (atoms), each of which is
moving rapidly in thermal motion and has a de Broglie wavelength that is smaller than an atom. It would be impossible
to slow down every atom in the persons body to a speed as small as that found in part (a).
69. Picture the Problem: A particle of mass m and charge q gains momentum when it is accelerated through a potential
difference V. The particles momentum is inversely proportional to its de Broglie wavelength.
Strategy: Write the kinetic energy of the particle ( K = p 2 2m ) in terms of the de Broglie wavelength using equation
30-16. Then set the kinetic energy equal to the change in electrostatic potential energy ( U = qV ) and solve for the
wavelength.
Solution: 1. Write the kinetic energy in
terms of the de Broglie wavelength:
K=
p2
h2
=
2m 2m 2
2. Set K = U and solve for the wavelength:
K=
h2
= qV
2m 2
h
2mqV
Insight: The de Broglie wavelength is inversely proportional to the square root of the potential difference. Quadrupling
the potential difference will cut the wavelength in half.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 22
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
70. Picture the Problem: According to the Heisenberg uncertainty principle, the product of the uncertainty in position and
uncertainty in momentum must be greater than Plancks constant divided by 2.
Strategy: The 5.0% uncertainty in speed means that the magnitude of the momentum (mass times speed) of the electron
and the baseball are also uncertain by 5.0%. The minimum uncertainty in position is given by equation 30-19.
h
2 p y
Solution: 1. Solve the uncertainty principle
for the uncertainty in position:
2. Set p y = 0.050mv :
3. Calculate the uncertainty for the baseball:
ybaseball
4. Calculate the uncertainty for the electron:
yelectron
2 ( 0.050mv y )
6.63 1034 J s
= 3.4 1034 m
2 ( 0.050 )( 0.15 kg )( 41 m/s )
6.63 1034 J s
2 ( 0.050 ) ( 9.11 1031 kg ) ( 41 m/s )
= 57 m
Insight: The minimum uncertainty in the position of the baseball is smaller than an atom by a factor of 1024. However,
the minimum uncertainty in the position of the electron is measurable, roughly the thickness of a human hair.
71. Picture the Problem: Because the proton is confined within the nucleus, the maximum uncertainty in its position is the
diameter of the nucleus. This maximum uncertainty in position corresponds to a minimum uncertainty in the momentum
of the proton because of the Heisenberg uncertainty principle.
Strategy: Solve equation 30-19 for the uncertainty in the protons momentum.
Solution: Solve the uncertainty principle
for the uncertainty in momentum:
p y
h
6.63 1034 J s
=
2y 2 ( 7.5 1015 m )
p y 1.4 10 20 kg m/s
Insight: If we set this uncertainty equal to the protons minimum momentum, we conclude that the proton must have a
kinetic energy of at least 0.37 MeV inside the nucleus.
72. Picture the Problem: When the position of a cart is measured with a specific accuracy, the Heisenberg uncertainty
principle requires that the momentum must also have a minimum uncertainty.
Strategy: Write the momentum in equation 30-19 in terms of the mass and uncertainty in velocity and solve the
inequality for the uncertainty in velocity.
Solution: 1. Write equation 30-19 with p y = mv y :
p y y
2. Solve for the uncertainty in the velocity:
v y
h
2
mv y y
h
2
h
6.63 1034 J s
=
2 my 2 ( 0.26 kg )( 0.0022 m )
= 1.8 1031 m/s
Insight: At this speed it would take the cart over a billion years to move the diameter of a proton! The uncertainty
principle is not significant for large objects.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 23
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
73. Picture the Problem: The amount of time required to measure the energy is equal to the maximum uncertainty in the
time measurement. According to the uncertainty principle, this corresponds to a minimum uncertainty in the energy.
Strategy: Divide the inequality in equation 30-20 by the measurement time tmax to solve for the minimum uncertainty
in the energy measurement.
h
2
h
6.63 1034 J s
=
=
= 1.1 1026 J
2 t 2 (1.0 108 s )
E t
Solution: Calculate the minimum uncertainty in energy:
Emin
Insight: If an electron does not remain in an excited level of an atom for at least 108 seconds, the energy cannot be
measured (or known) to an accuracy greater than 1026 J.
74. Picture the Problem: When the energy is measured to within a given uncertainty, the time cannot be known to within a
greater uncertainty than is stipulated by the Heisenberg uncertainty principle.
Strategy: Solve equation 30-20 for the minimum uncertainty in the time.
Solution: Calculate the minimum
uncertainty in time:
E t
tmin =
h
2
h
6.63 1034 J s
=
= 6.6 1013 s
2 Emax 2 ( 0.0010 eV ) (1.60 1019 J/eV )
Insight: As the energy is measured with greater precision, the time at which the particle had that given energy is known
with less certainty. It is impossible to simultaneously measure both energy and time with arbitrary certainty.
75. Picture the Problem: The lifetime of an excited state limits the time over which an energy measurement can be taken.
The Heisenberg uncertainty principle correlates this maximum uncertainty in the time measurement with a minimum
uncertainty in the energy measurement.
Strategy: Solve equation 30-20 for the minimum uncertainty in the energy.
Solution: Calculate the minimum
uncertainty in energy:
E t
h
2
Emin =
6.63 1034 J s
h
=
= 1.8 1025 J
2 tmax 2 ( 0.60 109 s )
Insight: Because the minimum uncertainty in energy is inversely proportional to the mean lifetime of the energy level,
energy levels with smaller lifetimes will necessarily have greater uncertainties in energy.
76. Picture the Problem: The lifetime of the particle limits the time over which an energy measurement can be taken. The
Heisenberg uncertainty principle correlates this maximum uncertainty in the time measurement with a minimum
uncertainty in the energy measurement.
Strategy: Solve equation 30-20 for the minimum uncertainty in the particles energy.
Solution: Calculate the minimum
uncertainty in energy:
E t
h
2
Emin =
6.63 1034 J s
h
=
= 4.2 1025 J
2 tmax 2 ( 2.5 1010 s )
Insight: Because the minimum uncertainty in energy is inversely proportional to the mean lifetime of the particle,
particles with smaller lifetimes will necessarily have greater uncertainties in energy.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 24
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
77. Picture the Problem: When an electrons location is known to within an uncertainty of 0.15 nm, it will have a
minimum uncertainty in momentum as required by the uncertainty principle. That minimum uncertainty requires the
electron to have a nonzero kinetic energy.
Strategy: Use equation 30-19 to calculate the minimum uncertainty p in the momentum, and then use p = p to find
the kinetic energy of the electron according to the kinetic energy equation ( K = p 2 2m).
Solution: 1. (a) Solve equation 30-19 for
the minimum uncertainty in momentum:
h
2
6.63 1034 J s
h
=
=
= 7.0 1025 kg m/s
2 x 2 ( 0.15 109 m )
p x
pmin
( 7.0 1025 kg m/s )
p2
=
= 1.7 eV
K=
2m 2 ( 9.11 1031 kg )(1.60 1019 J/eV )
2
2. (b) Calculate the minimum kinetic energy:
Insight: Decreasing the uncertainty in the position will proportionately increase the uncertainty in the momentum and
increase the kinetic energy by the square of the momentum. For example, if the uncertainty were decreased by a factor
of three (to 0.05 nm), the momentum would triple and the kinetic energy would increase by a factor of nine.
78. Picture the Problem: When a protons location is known to within an uncertainty of 0.15 nm, it will have a minimum
uncertainty in momentum as required by the uncertainty principle. That minimum uncertainty requires the proton to
have a nonzero kinetic energy.
Strategy: Use equation 30-19 to calculate the minimum uncertainty p in the momentum, and then use p = p to find
the kinetic energy of the proton according to the kinetic energy equation ( K = p 2 2m).
Solution: 1. (a) Solve equation 30-19 for
the minimum uncertainty in momentum:
h
2
6.63 1034 J s
h
=
=
= 7.0 1025 kg m/s
2 x 2 ( 0.15 109 m )
p x
pmin
( 7.0 1025 kg m/s )
p2
=
= 0.92 meV
K=
2m 2 (1.673 1027 kg )(1.60 1019 J/eV )
2
2. (b) Calculate the minimum kinetic energy:
Insight: When we compare the results of this problem with problem 77 (which concerned an electron confined with the
same x), we see that the uncertainty in momentum for the electron and proton are the same. The minimum kinetic
energy of the electron, however, is 1840 times greater than the minimum kinetic energy of the proton.
79. Picture the Problem: As the position of an electron becomes more certain (it has less uncertainty), the uncertainty of
the momentum increases.
Strategy: Calculate the maximum uncertainty in the momentum by multiplying the momentum by 1.0%. Then use
equation 30-19 to calculate the minimum uncertainty in the position.
Solution: 1. Calculate the maximum p:
p = 0.010 p = 0.010 (1.7 1025 kg m/s ) = 1.7 1027 kg m/s
2. Solve equation 30-19 for x:
p x
h
2
xmin =
6.63 1034 J s
h
=
= 62 nm
2 pmax 2 (1.7 1027 kg m/s )
Insight: The minimum uncertainty in the position could be decreased to 12 nm and still keep the relative uncertainty of
the momentum less than 5%.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 25
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
80. Picture the Problem: You perform an experiment on the photoelectric effect using light with a frequency high enough
to eject electrons. The intensity of the light is increased while the frequency is held constant.
Strategy: Recall the principles that govern the photoelectric effect when answering the conceptual questions. Note that
increasing the intensity while holding the frequency constant simply means that more photons (each with the same
original energy) strike the metal surface per second.
Solution: 1. (a) Because the energies of the photons stay the same when the frequency is held constant, the maximum
kinetic energy of an ejected electron will stay the same.
2. (b) Because the energies of the photons stay the same when the frequency is held constant, the maximum kinetic
energy of an ejected electron and therefore its minimum de Broglie wavelength will stay the same.
3. (c) Because the number of photons will increase when the intensity of the light is increased, the number of electrons
ejected per second will increase.
4. (d) Because the number of photons will increase when the intensity of the light is increased, the number of electrons
ejected per second and therefore the current in the phototube will increase.
Insight: The maximum kinetic energy of an ejected electron will increase only if the frequency of the light is increased.
81. Picture the Problem: You perform an experiment on the photoelectric effect using light with a frequency high enough
to eject electrons. The frequency of the light is increased while the intensity is held constant.
Strategy: Recall the principles that govern the photoelectric effect when answering the conceptual questions. Note that
increasing the frequency of the light means that the energy carried by each photon will increase.
Solution: 1. (a) Because the energies of the photons increase when the frequency is increased, the maximum kinetic
energy of an ejected electron will increase.
2. (b) Because the energies of the photons increase when the frequency is increased, the maximum kinetic energy of an
ejected electron will increase. Because the de Broglie wavelength of an electron is inversely proportional to its
momentum (equation 30-16), we conclude that the minimum de Broglie wavelength of ejected electrons will decrease.
3. (c) Because the intensity (energy per area per time) is unchanged, there must be fewer of these higher-energy photons
reaching the surface each second. As a result, the number of electrons ejected per second will decrease.
4. (d) Fewer electrons ejected per second means that the current in the phototube will decrease.
Insight: If the number of photons per second were held constant, the intensity of the light would increase if the
frequency of the light were increased.
82. Picture the Problem: An electron that is accelerated from rest through a potential difference V0 has a de Broglie
wavelength 0 .
Strategy: Use the expressions = h p (equation 30-16), K = p 2 2m (Conceptual Checkpoint 9-3), and
K = U = qV (equation 20-2) to answer the conceptual question.
Solution: To double the electrons wavelength we must halve its momentum. This means, in turn, that we must
decrease its kinetic energy by a factor of four (recall that K = p2/2m). The kinetic energy is linearly proportional to the
potential difference. Therefore, the electron should be accelerated from rest through the potential difference V0/4.
Insight: We can also come to this conclusion using a ratio:
2
V
V
K q
K
p 2 2m p h 0 0
1
=
=
= 2
= =
V= 0
= =
=
V0 K 0 q K 0 p0 2m p0 h 0 20
4
4
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 26
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
83. Picture the Problem: A beam of particles diffracts from a crystal, producing an interference maximum at the angle .
Strategy: Use the expressions = h p (equation 30-16) and 2 d sin = m (equation 30-17) to answer the conceptual
question.
Solution: 1. (a) Increasing the mass of the particles implies that their momentum increases as well. It follows from
= h p that their de Broglie wavelength decreases. Therefore, from 2d sin = m we see that the angle of the
interference maximum will decrease.
2. (b) Increasing the energy of the particles also implies that their momentum increases. Therefore, we again expect that
the angle of the interference maximum will decrease.
Insight: We can also come to this conclusion using a ratio:
new sin new mnew 2d new h pnew
p
=
=
=
=
= old so that if pnew > pold , then new < old .
mold 2d
h pold
pnew
old sin old
old
84. Picture the Problem: In order to construct a photocell that works on visible light, the energy from a photon in the
visible spectrum must be sufficient to eject an electron from the metal.
Strategy: Use equations 30-6 and 14-1 to calculate the maximum wavelength that a photon can have so that it is able to
eject electrons from each metal. Compare the results with the shortest wavelength of visible light (400 nm).
Solution: 1. Determine the max required
to eject electrons from each metal surface:
max
34
8
c
hc ( 6.63 10 J s )( 3.00 10 m/s ) 1240 eV nm
=
=
=
=
f 0 W0
W0
W0 (1.60 1019 J/eV )
2. Calculate the max for aluminum:
max =
1240 eV nm
= 290 nm
4.28 eV
3. Calculate the max for lead:
max =
1240 eV nm
= 292 nm
4.25 eV
4. Calculate the max for cesium:
max =
1240 eV nm
= 579 nm
2.14 eV
5. Of the three materials, cesium is the only material that will eject electrons from visible light.
Insight: Red light will not eject electrons from the surface because the maximum wavelength for cesium is 579 nm,
which is in the yellow portion of the spectrum. The photocell will work for visible light of wavelengths between
579 nm (yellow) and 400 nm (violet).
85. Picture the Problem: The eye must absorb at least 100 photons per second in order to detect green light.
Strategy: Use equations 30-4 and 14-1 to calculate the energy per photon for green light with a wavelength of 545 nm.
Multiply this by the rate of 100 photons per second in order to determine the power absorbed by the eye.
c
= 6.63 1034 J s
3.00 108 m/s
= 3.65 1019 J
545 109 m
Solution: 1. Calculate the photon energy:
E = hf = h
2. Multiply the energy of the photon
by the rate of photon absorption:
P = ( N t ) E = (100 photons/s ) ( 3.65 1019 J ) = 3.65 1017 W
Insight: If the eye were to absorb the same power from blue light of wavelength 484 nm, the eye would only need to
receive 89 photons per second from the source. However, the eye is more sensitive to green light than to blue, and so
this amount of power of blue light would not be visible to the eye.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 27
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
86. Picture the Problem: The length of a pendulum determines the frequency at which it oscillates. The energy of a typical
pendulum oscillation can be considered as a multiple of the quantum oscillation.
Strategy: Calculate the frequency of the oscillation using equations 13-1 and 13-20. Multiply the frequency by Plancks
constant and the quantum number n to calculate the energy of the oscillation. Set the energy equal to the kinetic energy
and solve for the velocity.
Solution: 1. (a) Calculate
the oscillation frequency:
2. (b) Set the oscillation energy
equal to the kinetic energy and
solve for the speed:
f =
1
1
=
T 2
g
1
=
L 2
9.81 m/s 2
= 0.56 Hz
0.78 m
E = nhf = 12 mv 2
2 (1.0 1033 )( 6.63 1034 J s ) ( 0.56 Hz )
2nh f
=
m
v=
0.15 kg
= 2.2 m/s
Insight: The n = 1 quantum state corresponds to the minimum energy state for this pendulum oscillator. The maximum
speed of the pendulum bob in this state would be 7.11017 m/s.
87. Picture the Problem: A radio antenna receives power by absorbing many photons per second, where each photon is a
quantum of energy determined by the frequency of the station. The received photons exert a force on the antenna when
they are absorbed.
Strategy: Divide the power by the energy per photon (from equation 30-4) to calculate the number of photons absorbed
per second. Set the power equal to the force times the speed of the wave (equation 7-13) to calculate the force on the
antenna.
Solution: 1. (a) Calculate the number
of photons absorbed per second:
2. (b) Divide the power by the speed of the
wave to calculate the force on the antenna:
P
Ephoton
1.0 1010 W
P
=
= 1.6 1015 photons/s
hf ( 6.63 1034 J s )( 96 106 Hz )
P = Fv = Fc
F=
P 1.0 1010 W
=
= 3.3 1019 N
c 3.00 108 m/s
Insight: The force on the antenna is negligibly small when compared with other forces such as wind and gravity.
88. Picture the Problem: As ice absorbs photons of light, the energy is converted into heat and melts the ice.
Strategy: Use equation 30-4 to calculate the energy of one photon. Divide the energy needed to melt 1.0-kg of ice
( Q = mL ) by the energy of one photon to calculate the number of photons needed. To calculate the number of ice
molecules that one photon will melt, divide the number of ice molecules in one kilogram by the number of photons to
melt the kilogram of ice. The number of water molecules is equal to Avogadros number times 1 kilogram divided by
the atomic mass of water (18 g/mole).
Solution: 1. (a) Calculate
the energy in one photon:
Ephoton = h f = ( 6.63 1034 J s )( 6.0 1014 Hz ) = 3.978 1019 J
2. Divide the heat by the photon energy:
N=
mL f
Ephoton
(1.0 kg )(80.0 kcal/kg )( 4186 J/kcal )
3.978 1019 J
= 8.4 1023 photons
3. (b) Divide the number of molecules
by the number of photons:
23
N A m M N A m ( 6.022 10 molecules/mole ) (1.0 kg )
=
=
N
NM
(8.42 1023 photons ) ( 0.018 kg/mole )
= 40 molecules/photon
Insight: When a photon is absorbed by a water molecule, the intermolecular bonds that attach the molecule to the other
molecules in the lattice can be broken. The molecule will then have sufficient remaining kinetic energy that it can
collide with 39 other molecules and break their bonds as well.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 28
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
89. Picture the Problem: As water absorbs photons of light, the energy is converted into heat and raises the temperature of
the water.
Strategy: Use equations 30-4 and 14-1 to calculate the energy of one photon. Find the energy required to heat
1.0 gram of water by one Celsius degree (equation 16-13), and divide this energy by the energy of one photon in order
to calculate the number of photons needed to heat the water.
Solution: 1. Find the energy in one photon:
Ephoton = h f =
2. Divide the heat by the photon energy:
N=
hc
( 6.63 10
34
J s )( 3.0 108 m/s )
550 109 m
= 3.6 1019 J
m cw T (1.0 g )( 4.186 J/g C )(1 C )
=
= 1.2 1019 photons
Ephoton
3.6 1019 J
Insight: Each photon has enough energy to increase the temperature of 3000 molecules by 1 C. Note the difference in
the two c variables; one represents the speed of light in vacuum and the other represents specific heat.
90. Picture the Problem: As water absorbs microwave photons, the energy is converted into heat and raises the
temperature of the water.
Strategy: Use equations 30-4 and 14-1 to calculate the energy of each photon. Use equation 16-13 to find the energy
required to heat the water, and divide this energy by the energy of one photon in order to calculate the number of
photons needed to heat the water.
hc
34
J s )( 3.0 108 m/s )
= 1.630 1024 J
Ephoton = hf =
2. Calculate the heat added to the water:
Q = mC T = ( 0.205 kg )( 4186 J/kg C )( 90.0C 20.0C )
( 6.63 10
Solution: 1. Calculate the photon energy:
0.122 m
= 6.007 104 J
3. Divide the heat by the energy per photon:
N=
Q
Ephoton
6.007 104 J
= 3.68 1028 photons
1.630 1024 J
Insight: If a magnifying glass that is 3.00 inches in diameter captures the entire solar spectrum on a day in which the
solar intensity is 1000 W/m2, it would take 3.66 hours to change the temperature of the same mass of water by the same
amount.
91. Picture the Problem: When light ejects electrons from a lead surface, part of the photon energy is used to overcome the
work function. The remainder becomes kinetic energy of the photoelectron. The kinetic energy is related to the
momentum of the electron, and thus is related to the de Broglie wavelength of the electron.
Strategy: Write the kinetic energy of the electron (from equation 30-7) in terms of the momentum K = p 2 2m and
solve for the momentum. Then use equation 30-16 to calculate the wavelength from the momentum.
Solution: 1. Use equation 30-7
to calculate the kinetic energy:
2. Calculate the electron
momentum:
3. Calculate the electron
wavelength:
K max = hf W0 = ( 6.63 1034 J s )( 2.11 1015 Hz ) 4.25 eV (1.6 10 19 J/eV
= 7.2 1019 J
K max = p 2 2m
p = 2mK max = 2 ( 9.11 1031 kg )( 7.2 1019 J ) = 1.15 1024 kg m/s
= h p = ( 6.63 1034 J s ) (1.15 1024 kg m/s ) = 0.58 nm
Insight: Increasing the photon frequency will decrease the electron wavelength. For example, if the photon frequency
were increased to 4.22 1015 Hz, the wavelength drops to 0.34 nm. Note that doubling the photon frequency does not
cut the electrons de Broglie wavelength in half because of the effect of the work function.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 29
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
92. Picture the Problem: The de Broglie wavelength of the electron and the wavelength of the photon are both inversely
proportional to their momenta.
Strategy: Use equation 30-16 to calculate the de Broglie wavelength of the electron, where the momentum is given by
equation 9-1. Calculate the wavelength of the photon using equation 30-11, setting the momentum of the photon equal
to the momentum of the electron.
Solution: 1. (a) Find the de Broglie wavelength:
h
h
6.63 1034 J s
=
=
= 0.27 nm
pe me v ( 9.11 1031 kg )( 2.7 106 m/s )
2. (b) Calculate the wavelength of the photon:
h
h
=
= e = 0.27 nm
p pe
Insight: Any object (electron, photon, proton, person, etc.) with the same momentum will have the same wavelength.
93. Picture the Problem: The spectrum of light emitted by a firefly peaks at a frequency of 5.4 1014 Hz, which
corresponds to visible, yellow-green light.
Strategy: Use equation 30-1 to calculate the temperature of a blackbody that has a peak frequency at 5.4 1014 Hz.
Compare this temperature with the probable temperature of a fireflys abdomen.
T=
Solution: 1. Solve equation 30-1 for T:
f peak
5.88 1010 s 1 K 1
5.4 1014 Hz
= 9200 K
5.88 1010 s 1 K 1
2. Firefly radiation is definitely not well approximated by blackbody radiation. A temperature of 9200 K is hotter than
the surface of the Sun, and is sufficient to completely destroy the firefly.
Insight: The light from the firefly is caused by a low-temperature chemical reaction that produces a specific wavelength
of light (characteristic of the species of firefly).
94. Picture the Problem: Light of a given frequency and energy will eject electrons from the surface of the metal. Part of
the energy from the photon overcomes the work function of the metal. The remainder of the energy goes into the kinetic
energy of the electron.
Strategy: (a) It is not necessary to use relativistic mechanics because v << c. Substitute
1
2
2
mvmax
for K max in the
equation K max = hc W0 and solve for W0 . Then calculate the cutoff frequency using f 0 = W0 h .
Solution: 1. (b) Solve eq.30-7
for the work function:
K max =
W0 =
hc
hc
W0
K max =
1 2
mvmax
2
hc
( 6.63 1034 J s )( 3.00 108 m/s ) 1
2
5
31
9.11
10
kg
3.10
10
m/s
=
(
)(
)
2
545 109 m
W0 = ( 3.212 1019 J )(1 eV 1.60 1019 J ) = 2.01 eV
2. (b) Use equation 30-6 to
calculate the cutoff frequency:
f0 =
W0 3.212 1019 J
=
= 4.84 1014 Hz
h 6.63 1034 J s
Insight: This cutoff frequency falls in the orange part of the visible spectrum. Therefore, essentially the entire visible
spectrum (except red light) will eject electrons from the metal.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 30
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
95. Picture the Problem: The image shows an atom absorbing a photon of
wavelength 486.2 nm and later emitting a photon of wavelength 97.23 nm.
Strategy: Use equations 30-4 and 14-1 to calculate the energy of the
absorbed photon and the energy of the emitted photon. Set the change in
energy of the atom equal to the absorbed energy minus the emitted energy.
Solution: 1. (a) Photon energy is inversely proportional to wavelength, so
the absorbed photon has less energy than the emitted photons energy.
Therefore, the net energy of the atom has decreased.
Eabs = hf = hc = ( 6.63 1034 J s ) ( 3.00 10 m/s
2. (b) Calculate the energy
of the absorbed photon:
486.2 109 m
= ( 4.091 1019 J ) (1.60 1019 J/eV ) = 2.56 eV
3. Calculate the energy
of the emitted photon:
Eemit = hc = ( 6.63 10 34 J s )( 3.00 108 m/s
4. Calculate E for the atom:
E = Eabs Eemit = 2.56 eV 12.8 eV = 10.2 eV
97.23 10 9 m
= ( 2.046 10 18 J ) (1.60 10 19 J/eV ) = 12.8 eV
Insight: It is more common for an atom to absorb a photon of short wavelength and then emit two (or more) photons of
longer wavelength, such that the energy of the absorbed photon is equal to the sum of the energies of the emitted
photons.
96. Picture the Problem: As the atoms emerge from an oven, their most probable speed is proportional to their most
probable momentum, which is inversely proportional to their most probable wavelength.
Strategy: Write the most probable wavelength of the atoms in terms of the momentum using equation 30-16 and
equation 9-1. Solve the resulting equation for the most probable speed.
Solution: 1. Write equation 30-16
in terms of the velocity:
mp =
2. Solve for the most probable speed:
vmp =
h
h
h
=
=
pmp mvmp
5mkT
5 (1.38 1023 J/K ) ( 450 K )
5kT
=
= 4.31 km/s
m
1.677 1027 kg
Insight: The most probable speed increases in proportion to the square root of the Kelvin temperature.
97. Picture the Problem: The de Broglie wavelength is inversely proportional to the momentum of the electron. In
classical mechanics, the kinetic energy of the electron is proportional to the square of the momentum.
Strategy: Write the kinetic energy of the electron in terms of the momentum, and then replace the momentum with
h according to equation 30-16. Solve the resulting equation for the wavelength, writing the kinetic energy K in units
of eV.
Solution: 1. (a) The de Broglie wavelength is inversely proportional to momentum, and the momentum increases when
the kinetic energy increases. Therefore, the de Broglie wavelength will decrease as the kinetic energy increases.
2. (b) Write K in terms of
the de Broglie wavelength:
( hc )
p2 ( h )
h2
=
=
=
K=
2
2m
2m
2m
2mc 2 2
3. Solve for the wavelength:
34
5
( 6.63 10 J s )( 3.00 10 m/s ) 1.23 nm eV
=
=
2
K
2mc K 2 ( 5.11 105 eV ) K (1.60 1019 J/eV )
hc
Insight: This equation indicates that the wavelength is inversely proportional to the square root of the kinetic energy.
For example, an electron with a kinetic energy of 16.3 eV will have a de Broglie wavelength of 0.305 nm.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 31
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
98. Picture the Problem: Helium atoms in a jar at room temperature and atmospheric pressure are often considered to
behave like point particles. Quantum effects between the particles are generally ignored. This problem tests the
assumption that quantum effects are small for the interaction between helium atoms.
Strategy: Use equation 17-12 to calculate the kinetic energy of the helium atoms. Write the kinetic energy in terms of
the momentum. Then using equation 30-16 write the momentum in terms of the wavelength. Solve the resulting
equation for the average de Broglie wavelength of the atoms. Solve the ideal gas law (equation 17-2) for the volume per
atom, and then take the cube root of the volume per atom to calculate the average separation distance.
Solution: 1. (a) Write the kinetic energy
in terms of and set it equal to 32 kT :
p2 ( h )
h2
K av =
=
=
2m
2m
2m 2
2. Solve for the wavelength
in terms of the temperature:
av =
3mkT
set
3
kT
2
6.63 1034 J s
3 ( 6.65 1027 kg )(1.38 10 23 J/K ) ( 295.15 K )
= 0.0732 nm
3. (b) Use the ideal gas law to calculate
the volume occupied per atom:
PV = NkT
4. Take the cube root of the volume to
calculate the average separation:
d avg =
23
V kT (1.38 10 J/K ) ( 295.15 K )
=
=
= 4.033 1026 m3
1.01 105 Pa
N
P
V
= 3 4.033 1026 m3 = 3.44 nm
N
Insight: The de Broglie wavelength of the helium atom is 47 times smaller than the separation distance between atoms.
Therefore, quantum effects are not a significant factor in determining the behavior of the atoms in this helium gas.
99. Picture the Problem: The coefficient of the Compton scattering equation is called the Compton wavelength and is
inversely proportional to the mass of the particle.
Strategy: Use the equation C = h mc to calculate the Compton wavelength of a proton. Then use equations 30-4 and
14-1 to write the energy of a photon with the same wavelength. Insert the equation for the Compton wavelength into the
energy equation of the photon to show that the photon energy is equal to the rest energy of the particle.
Solution: 1. (a) Calculate the Compton
wavelength of a proton:
C =
2. (b) Calculate the energy of a
photon with the same wavelength:
E=
3. (c) Insert the Compton wavelength into
the equation for the photon energy:
E=
6.63 1034 J s
h
=
= 1.32 fm
mp c (1.673 1027 kg )( 3.00 108 m/s )
hc
hc
( 6.63 10
=
=
34
J s )( 3.00 108 m/s )
1.32 1015 m
= 1.51 1010 J
hc
= mc 2 = E0
h mc
Insight: When a photon Compton scatters after colliding with a free particle, its wavelength will decrease by up to
twice the Compton wavelength of the scattered particle. Note that the Compton wavelength of an electron is 2426 fm or
2.426 pm, a value that is often involved in the solutions to the problems from Section 30-4.
100. Picture the Problem: When a photon is absorbed by the surface of a metal it will eject an electron. The kinetic energy
of the ejected electron will equal the difference between the photon energy and the work function of the metal. When
photons of the same frequency are incident on two metals of different work functions, the ejected electrons will have
different maximum kinetic energies.
Strategy: Solve equation 30-7 for the work function. Then subtract the two work functions to show that the difference
in kinetic energies of the ejected electrons is equal to the difference in work functions for any incident frequency.
Solution: 1. (a) The difference in maximum kinetic energy observed from the two surfaces is due to the difference in
the work functions of the two materials. Because the maximum kinetic energy depends linearly upon the work function,
and because the work function does not depend upon the frequency of the light, the difference in maximum kinetic
energy will stay the same.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 32
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
K max = hf W0
2. (b) Solve equation 30-8
for the work function:
W0 = hf K max
W0B W0A = ( hf K max,B ) ( hf K max,A )
3. Subtract the work functions
of the two metals:
2.00 1019 J
= 1.25 eV
1.60 1019 J/eV
Insight: As long as both work functions are below 3.41 eV, the light will eject electrons from both surfaces whose
kinetic energies differ by1.25 eV. If one of the work functions were greater than 3.41 eV, that surface would not eject
photoelectrons.
= K max,A K max,B =
101. Picture the Problem: When photons of different wavelengths shine on a lithium surface, they eject electrons with
kinetic energies equal to the difference between the energy of the photon and the work function of lithium.
Strategy: Solve equation 30-7 (writing the frequency in terms of the wavelength) for the constants hc. Set the resulting
equations for the two experiments equal to each other and solve for the work function.
Solution: 1. Solve equation 30-7 for hc:
K max = hc W0
hc = (W0 + K max )
2. Set the two expressions equal
and solve for the work function:
1 (W0 + K max, 1 ) = 2 (W0 + K max, 2 )
W0 =
=
2 K max, 2 1 K max, 1
1 2
( 253.5 nm )( 2.57 eV ) ( 433.9 nm )( 0.550 eV )
433.9 nm 253.5 nm
W0 = 2.29 eV
Insight: The above procedure essentially completes a linear regression between the two ( f, Kmax) data points and finds
the x-intercept, which corresponds to the minimum frequency (maximum wavelength) at which photoelectrons are
produced. The energy of a photon with that frequency equals the work function of the lithium.
102. Picture the Problem: When photons of different wavelengths shine on a lithium surface, they eject electrons with
kinetic energies equal to the difference between the energy of the photon and the work function of lithium.
Strategy: Insert the data from one of the experiments (and the calculated work function from the previous question)
into the equation for hc and solve for Plancks constant.
Solution: Solve for Plancks constant:
h=
(W0 + K max ) =
433.9 109 m
( 2.289 eV + 0.550 eV )
3.00 108 m/s
= ( 4.106 1015 eV s )(1.60 1019 J/eV ) = 6.57 10 34 J s
Insight: Millikan was able to calculate Plancks constant to within 1% of its presently accepted value using these two
experiments.
103. Picture the Problem: When photons of different wavelengths shine on a lithium surface, they eject electrons with
kinetic energies equal to the difference between the energy of the photon and the work function of lithium.
Strategy: Use equation 30-7 together with the work function and Plancks constant (see the previous two questions) to
calculate the maximum kinetic energy of electrons that are ejected when 365-nm light shines on the lithium surface.
Solution: Calculate the maximum K:
K max =
( 4.106 10
15
eV s )( 3.00 108 m/s )
365.0 109 m
2.29 eV = 1.08 eV
Insight: Millikan was able to calculate Plancks constant to within 1% of its presently accepted value using these two
experiments.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 33
James S. Walker, Physics, 4th Edition
Chapter 30: Quantum Physics
104. Picture the Problem: The image shows a 0.6500-nm photon
scattering off of an electron. After the collision, the electron has a
kinetic energy of 7.750 eV and the photon scatters at an angle .
Strategy: Use equations 20-4 and 14-1 to calculate the initial
energy of the photon. Subtract the kinetic energy of the electron
from the photons energy to calculate the final energy of the
photon, and then use equation 20-4 to find the final wavelength.
Then use equation 30-15 to calculate the scattering angle. A
recurring value in similar Compton scattering problems is
h me c = 2.426 1012 m = 0.002426 nm.
Solution: 1. (a) In this problem the photon has transferred less energy to the electron than it did in Example 30-4, so the
scattering angle will be less than 152.
( 6.63 10
34
J s )( 3.0 108 m/s )
2. (b) Calculate the initial energy of the photon:
E = hc =
3. Calculate the final energy of the photon:
E = E K = 1912.5 eV 7.750 eV = 1904.75 eV
4. Calculate the final wavelength of the photon:
= hc E =
5. Solve equation 30-15 for the scattering angle:
= cos 1 1
0.6500 nm (1.6 1019 J/eV )
( 6.63 10
34
= 1912.5 eV
J s )( 3.0 108 m/s )
1904.75 eV (1.6 10 19 J/eV )
= 0.6526 nm
h me c
( 0.6526 nm 0.6500 nm )
= cos 1 1
= 94
0.002426 nm
Insight: The scattering angle of the photon increases as the momentum of the electron increases. Therefore, if the
kinetic energy of the electron decreases, the momentum of the electron also decreases, and the scattering angle of the
photon also decreases.
105. Picture the Problem: The image shows a 0.6500-nm photon
scattering off of an electron. After the collision, the photon has a
wavelength of 0.6510 nm and scatters at an angle .
Strategy: Use equation 30-15 to calculate the scattering angle of
the photon. A recurring value in similar Compton scattering
problems is h me c = 2.426 1012 m = 0.002426 nm.
Solution: 1. (a) The photon will scatter at a smaller angle because the change in wavelength in this problem is less than
the change in wavelength of Example 30-4, which means that less energy has been transferred to the electron.
Therefore, the scattering angle will be less than 152.
2. (b) Solve equation 30-15 for the scattering angle:
= cos 1 1
h me c
0.6510 nm 0.6500 nm
= cos 1 1
= 54
0.002426 nm
Insight: Scattering angles greater than 152 will occur when the final wavelength of the scattered photon is between
0.6546 nm and 0.6549 nm.
Copyright 2010 Pearson Education, Inc. All rights reserved. This material is protected under all copyright laws as they currently exist. No
portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.
30 34
You might also like
- Walker4 ISM Ch31Document36 pagesWalker4 ISM Ch31Alejandro Romero MejiaNo ratings yet
- Walker4 Ism Ch23Document32 pagesWalker4 Ism Ch23mariomato100% (1)
- Walker4 Ism Ch18Document36 pagesWalker4 Ism Ch18Walter Ruby100% (2)
- ch19 Physics SolutionsDocument30 pagesch19 Physics Solutionsalkalkindin119284849No ratings yet
- Homework 3 PDFDocument31 pagesHomework 3 PDFnaveena kumariNo ratings yet
- Chapter 15 Electric FieldsDocument17 pagesChapter 15 Electric FieldsdrewNo ratings yet
- Solutions) Mastering Physics HW11Document7 pagesSolutions) Mastering Physics HW11jamie_heineckeNo ratings yet
- XX MP4Document19 pagesXX MP4abeck171383% (6)
- Mastering Physics 3Document14 pagesMastering Physics 3ozilla_26_me0% (4)
- MP2 DDDocument30 pagesMP2 DDabeck171344% (9)
- Physics 2 Chapter 13 SHM problemsDocument8 pagesPhysics 2 Chapter 13 SHM problemsrmachado70% (1)
- MasteringPhysics - Assignment 4 - Motion in 2-DDocument4 pagesMasteringPhysics - Assignment 4 - Motion in 2-DStrange Sht100% (1)
- Chapter 32 - Electromagnetic WavesDocument19 pagesChapter 32 - Electromagnetic WavesKarla PereraNo ratings yet
- Chapter 9 Static Equilibrium Elasticity and Fracture: Physics: Principle and Applications, 7e (Giancoli)Document24 pagesChapter 9 Static Equilibrium Elasticity and Fracture: Physics: Principle and Applications, 7e (Giancoli)KarissaNo ratings yet
- Laws of Motion ExplainedDocument52 pagesLaws of Motion ExplainedAmeen ShaikNo ratings yet
- Mastering Physics Wk2-1Document3 pagesMastering Physics Wk2-1Livardy Wufianto0% (1)
- Ch06 ISMDocument67 pagesCh06 ISMThai-Son LeNo ratings yet
- Sph4uc Unit 4 Lesson 14Document18 pagesSph4uc Unit 4 Lesson 14Akif Prasla0% (1)
- Mastering PhysicsDocument10 pagesMastering Physicsozilla_26_me100% (1)
- Geometric Optics Multiple Choice QuestionsDocument14 pagesGeometric Optics Multiple Choice QuestionsqvrlenarasegtNo ratings yet
- Wolfson Eup3 Ch14 Test BankDocument29 pagesWolfson Eup3 Ch14 Test Bankifghelpdesk100% (1)
- Ch04 Homework SolutionsDocument88 pagesCh04 Homework SolutionsOsama Maher100% (1)
- 24Document19 pages24anon_33083814No ratings yet
- HW1 Mastering PhysicsDocument16 pagesHW1 Mastering Physicszmontgom183% (6)
- HW 12Document23 pagesHW 12Siva RamNo ratings yet
- Kinetic Theory of Gases Multiple Choice PracticeDocument13 pagesKinetic Theory of Gases Multiple Choice PracticeMuhammad Tayyab MadniNo ratings yet
- HW1 Mastering PhysicsDocument16 pagesHW1 Mastering PhysicsdominicNo ratings yet
- HW 6 - Phys - AnswersDocument17 pagesHW 6 - Phys - AnswersJohn GamboaNo ratings yet
- Mastering Physics CH 02 HW College Physics I LCCCDocument33 pagesMastering Physics CH 02 HW College Physics I LCCCSamuel80% (15)
- MasteringPhysics - Assignment 10 - Motion in 1-DDocument5 pagesMasteringPhysics - Assignment 10 - Motion in 1-DStrange ShtNo ratings yet
- Version 058 – Test 2 Questions and Answers10. Vsub /V = 5/6Document11 pagesVersion 058 – Test 2 Questions and Answers10. Vsub /V = 5/6brunosipodNo ratings yet
- Chapter 9 RotationDocument7 pagesChapter 9 RotationHana Lee Jorel ZekeNo ratings yet
- DUAL NATURE - PHOTOELECTRIC EFFECTDocument6 pagesDUAL NATURE - PHOTOELECTRIC EFFECTjagatdhatriNo ratings yet
- AC Circuits Multiple Choice QuestionsDocument18 pagesAC Circuits Multiple Choice QuestionsqvrlenarasegtNo ratings yet
- Mastering Physics CH 11 HW College Physics I LCCCDocument50 pagesMastering Physics CH 11 HW College Physics I LCCCSamuel90% (10)
- Physics Chapter 9 AnswersDocument40 pagesPhysics Chapter 9 AnswersAbovethesystem94% (17)
- DP Physics Questions from Chapter 2Document5 pagesDP Physics Questions from Chapter 2Enock KamugishaNo ratings yet
- Magnetic Forces and Fields ProblemsDocument5 pagesMagnetic Forces and Fields Problemsshihabsultan100% (1)
- Multiple Choice Questions For Physics 1Document55 pagesMultiple Choice Questions For Physics 1OsamaHeshamNo ratings yet
- MasteringPhysics - Assignment 4 - Forces Part OneDocument7 pagesMasteringPhysics - Assignment 4 - Forces Part OneStrange Sht100% (2)
- PHYS 212-071 HW-2 Solution (1) PhysicsDocument3 pagesPHYS 212-071 HW-2 Solution (1) PhysicsBrenda Michelle ReyesNo ratings yet
- Conservation of Energy Problems and SolutionsDocument22 pagesConservation of Energy Problems and Solutionsmukesh3021No ratings yet
- Physics 40a Final Exam ReviewDocument4 pagesPhysics 40a Final Exam ReviewRexRu100% (1)
- Newton's Laws of Motion - Solutions PDFDocument6 pagesNewton's Laws of Motion - Solutions PDFwolfretonmathsNo ratings yet
- Chapter 23Document26 pagesChapter 23Carlos Arturo AlanizNo ratings yet
- Mastering Physics - Energy LabDocument9 pagesMastering Physics - Energy Labpalparas100% (1)
- Potential Energy Mastering PhysicsDocument23 pagesPotential Energy Mastering PhysicsLea Dominique Mangubat Fariola0% (1)
- Physics For Scientists and Engineers 6th Edition: CHAPTER 12Document22 pagesPhysics For Scientists and Engineers 6th Edition: CHAPTER 12Fredy Arana Rodríguez100% (1)
- Homework6 Chap5 PDFDocument47 pagesHomework6 Chap5 PDFFedaNahawi100% (30)
- PSE 9e CH 24 CH 25 26 Combined SerwayDocument60 pagesPSE 9e CH 24 CH 25 26 Combined SerwayYaas BahNo ratings yet
- Homework1 Answer Key Quantum ChemistryDocument5 pagesHomework1 Answer Key Quantum ChemistryLuther James Langston IINo ratings yet
- Black Body Radiation A: Course Number:Quantum Mechanics-1 Course Title:Phy-3201Document26 pagesBlack Body Radiation A: Course Number:Quantum Mechanics-1 Course Title:Phy-3201Sheikh Emon HossainNo ratings yet
- Black Body RadiationDocument33 pagesBlack Body RadiationSafnas Kariapper100% (1)
- Lab 6 Stellar Temperatures and LuminosityDocument9 pagesLab 6 Stellar Temperatures and Luminositymei sya100% (1)
- Temu 1Document36 pagesTemu 1Farida UtamiNo ratings yet
- Blackbody radiation spectrumDocument23 pagesBlackbody radiation spectrumkowletNo ratings yet
- Assgn 1Document4 pagesAssgn 1Spandana PrustyNo ratings yet
- 60 Surface Temperature & LuminosityDocument2 pages60 Surface Temperature & Luminosityvalsamis8334No ratings yet
- Electromagnetic Radiation Behaving As ParticlesDocument48 pagesElectromagnetic Radiation Behaving As ParticlesLeo YipNo ratings yet
- Interactions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsFrom EverandInteractions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsNo ratings yet
- In My Opinion An International Experience Help Me To Consider The Good AspectsDocument2 pagesIn My Opinion An International Experience Help Me To Consider The Good AspectsJeykel EspinozaNo ratings yet
- Chang Overby CH-5 HW PDFDocument38 pagesChang Overby CH-5 HW PDFRalph Evidente0% (1)
- 11 - Lorenzo's Oil WorksheetDocument7 pages11 - Lorenzo's Oil WorksheetJeykel Espinoza100% (1)
- When It RainsDocument1 pageWhen It RainsJeykel EspinozaNo ratings yet
- InglesDocument24 pagesInglesJeykel EspinozaNo ratings yet
- Microstructural Characterization, Strengthening and Toughening Mechanisms of A Quenched and Tempered Steel Effect of Heat Treatment ParametersDocument22 pagesMicrostructural Characterization, Strengthening and Toughening Mechanisms of A Quenched and Tempered Steel Effect of Heat Treatment ParametersAlmerindo JuniorNo ratings yet
- Separating Acetic Acid and Water by DistillationDocument8 pagesSeparating Acetic Acid and Water by DistillationSuzanne Clariz M. BaltazarNo ratings yet
- Solex Adj ProcedureDocument6 pagesSolex Adj Procedureprivate 2No ratings yet
- محطات الطاقةDocument22 pagesمحطات الطاقةJoe LewisNo ratings yet
- Gas Chromatograph OptimizationDocument18 pagesGas Chromatograph OptimizationUmair KazmiNo ratings yet
- Exhaust ManifoldDocument5 pagesExhaust ManifoldDeepak Chachra100% (1)
- Operation Manual - WSRF-50Document175 pagesOperation Manual - WSRF-50eduardo100% (2)
- Damper mechanism details for Atlas Copco rock drills under 40 charactersDocument27 pagesDamper mechanism details for Atlas Copco rock drills under 40 characterssalvador341100% (2)
- B23 B24 User Manual PDFDocument168 pagesB23 B24 User Manual PDFAurel BodenmannNo ratings yet
- Kinetic and Potential Energy PracticeDocument1 pageKinetic and Potential Energy Practicealchemist2000No ratings yet
- Repair and RehabilitationDocument22 pagesRepair and RehabilitationConstro FacilitatorNo ratings yet
- Pump Mechanical Seals GuideDocument41 pagesPump Mechanical Seals GuideArief Hidayat100% (1)
- 50 Straw Bale House PlansDocument9 pages50 Straw Bale House Plansdarius-matuiza-81990% (1)
- SHD30 and SHD30-45 Models: (Standard)Document2 pagesSHD30 and SHD30-45 Models: (Standard)Roger TorrejonNo ratings yet
- Ed Current DynamometerDocument3 pagesEd Current DynamometerOM MUNGELWARNo ratings yet
- P6112 Alstom PG9171E PowerPlant SpecificationsDocument1 pageP6112 Alstom PG9171E PowerPlant SpecificationsSunario YapNo ratings yet
- Chapter 2 Structure of AtomsDocument16 pagesChapter 2 Structure of AtomsCherry T CYNo ratings yet
- Germany and Japan's Remarkable Post-WWII Economic ComebackDocument5 pagesGermany and Japan's Remarkable Post-WWII Economic ComebackHANNALEENo ratings yet
- With Refanned Jt8D Engines: CR-1348s Report Mdcj4519Document238 pagesWith Refanned Jt8D Engines: CR-1348s Report Mdcj4519FernandoNo ratings yet
- Delta Ia-Mds Vfd-Ed Um en 20150910-1Document280 pagesDelta Ia-Mds Vfd-Ed Um en 20150910-1FahadNo ratings yet
- Design and Generating Energy As A Car Alternator TDocument7 pagesDesign and Generating Energy As A Car Alternator TJohnny TestNo ratings yet
- Introduction to Concrete Components and ClassificationDocument29 pagesIntroduction to Concrete Components and ClassificationUsama AliNo ratings yet
- Pepsin Enzyme Activity LabDocument4 pagesPepsin Enzyme Activity LabDebrah DebbieNo ratings yet
- ZhangDocument21 pagesZhangjajajaja21No ratings yet
- EE 004A DC and AC Machinery ExperimentDocument9 pagesEE 004A DC and AC Machinery ExperimentJerome NuevoNo ratings yet
- High Efficiency Battery Charger Using DC-DC ConverterDocument4 pagesHigh Efficiency Battery Charger Using DC-DC ConvertersanilNo ratings yet
- Wa500-6 Sen00236-04d PDFDocument1,705 pagesWa500-6 Sen00236-04d PDFanggie100% (4)
- 240-56063867 Transformer and Reactor Rapid Pressure Rise RelayDocument6 pages240-56063867 Transformer and Reactor Rapid Pressure Rise RelayMichael NgubaneNo ratings yet
- Air Cooler LeafletDocument2 pagesAir Cooler LeafletSaad zubayr MNo ratings yet
- JP For RadiographyDocument7 pagesJP For Radiographytaparia_piyushNo ratings yet