Professional Documents
Culture Documents
2015 Sec3Exp SA1 P1
Uploaded by
Jason TanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2015 Sec3Exp SA1 P1
Uploaded by
Jason TanCopyright:
Available Formats
21
Which diagram below represents an incomplete reaction between the elements
nitrogen gas and hydrogen gas?
A
22
23
Phosphonium carbonate is a compound with the chemical formula, (PH4)2CO3.
How many different elements are present in phosphonium carbonate?
A
Nippon paint for wood consists of resins, thinner (a volatile liquid that does not mix with
water) and dyes. Once applied, wood paint hardens as the thinner in it evaporates.
Which of the following statements is true?
24
Wood paint boils over a range of temperatures because it is a mixture.
In wood paint, the thinner, resins and dyes are always combined in a fixed
proportion as it is a mixture.
Wood paint is an element.
Wood paint is a compound because its components can be separated by
the process of evaporation to dryness.
Emily accidently tripped and dropped his bag of table salt onto a sandy beach. The bag
is made of paper and the table salt is firmly sealed inside the paper bag with aluminium
staples. The fall causes the paper bag to burst and the spilled table salt is mixed with
sand.
Which of the following is an element?
25
A aluminium staples
paper bag
B table salt
sand along the beach
A colourless substance, W has a fixed melting point and a fixed boiling point.
W can be classified as
1
2
3
an element
a pure compound
a mixture
A 1 only
3 only
B 2 only
1 and 2
________________________________________________________________________________________________
Canberra Secondary School
2015 Semestral Assessment 1
5076 Science (Physics/Chemistry) Paper 1
Secondary 3 Express
26
27
28
In the nucleus of an atom, which sub-atomic particles can be found?
A electrons and protons
protons and neutrons
B electrons and neutrons
protons, neutrons and electrons
42
How many neutrons are there in an atom of calcium,
A 20
42
B 22
62
20
Ca ?
The graph below shows how a number changes for the elements from oxygen to
magnesium.
What is this number represented along the Y axis?
A the nucleon number of an atom
B the number of neutrons in an atom
C the number of electrons in an atom
D the number of valence electrons
29
77
An ion of Z contains 3 protons, 4 neutrons and 2 electrons. Which symbol correctly
represents the atom of Z?
A
3
37
B
30
The electronic structure of atoms X and Y are shown.
X reacts with Y to form an ionic compound with the formula
A
XY2 .
X2Y2 .
X2Y .
XY .
________________________________________________________________________________________________
Canberra Secondary School
2015 Semestral Assessment 1
5076 Science (Physics/Chemistry) Paper 1
Secondary 3 Express
31
Which process changes a sodium atom, Na into a sodium ion, Na+?
A
B
32
the gain of one electron
the loss of one electron
C
D
the gain of one proton
the loss of one proton
The table compares the properties of ammonia gas, NH3 and magnesium oxide, MgO.
Which property is matched wrongly?
ammonia gas
33
34
35
magnesium oxide
It does not conduct electricity.
It conducts electricity when molten.
It is a gas.
It is a solid.
It has a low melting point.
It has a high melting point.
It is an ionic compound.
It is a simple covalent molecule.
The Avogadros constant is the number of
A
atoms in 1 g of nitrogen gas.
molecules in 16 g of oxygen gas.
atoms in exactly 2 g of helium.
atoms in exactly 12 g of carbon-12.
How many moles are there in 15.5 g of sodium oxide, Na2O?
A 0.125 mol
0.5 mol
B 0.25 mol
1.0 mol
The equation for the complete combustion of propene is
2C3H6(g) + 9O2(g) 6CO2(g) + 6H2O(g)
This equation shows that
36
two molecules of propene combine with nine moles of oxygen.
six molecules of steam can be obtained from two moles of propene.
six moles of carbon dioxide are produced from nine moles of oxygen gas.
42 g of propene combine with 32 g of oxygen.
What is the mass of 1200 cm3 of carbon dioxide at r.t.p.?
A 1.2 g
24 g
B 2.2 g
52.8 g
________________________________________________________________________________________________
Canberra Secondary School
2015 Semestral Assessment 1
5076 Science (Physics/Chemistry) Paper 1
Secondary 3 Express
37
38
39
40
Which statement is true for alkalis?
A
They produce hydroxide ions when dissolved in water.
They react with zinc to produce carbon dioxide.
They react with acids to give hydrogen gas.
They react with carbonates to give a salt, carbon dioxide and water.
Which of the following statements is true for dilute hydrochloric acid and nitric acid?
A
They react with calcium carbonate to give carbon dioxide.
They react with copper to give hydrogen gas.
They turn red litmus paper blue.
Salt and water are formed when they react together.
An oxide, M, reacts with both dilute sulfuric acid and potassium hydroxide solution to
form a salt and water. What type of oxide is M?
A
acidic
Basic
amphoteric
neutral
Which substance is not used to prepare magnesium nitrate by reaction with nitric acid?
A magnesium carbonate
magnesium chloride
B magnesium hydroxide
magnesium oxide
End of Paper
________________________________________________________________________________________________
Canberra Secondary School
2015 Semestral Assessment 1
5076 Science (Physics/Chemistry) Paper 1
Secondary 3 Express
You might also like
- IT Chem F5 SPM Model Paper (E)Document10 pagesIT Chem F5 SPM Model Paper (E)Norzawati NoordinNo ratings yet
- Sec 4 Chem Prelim Paper 1Document16 pagesSec 4 Chem Prelim Paper 1TeckluckyNo ratings yet
- Yr 12 Chemistry PP1Document11 pagesYr 12 Chemistry PP1NjoroNo ratings yet
- Learning Style QuestionnaireDocument17 pagesLearning Style QuestionnairekrisnuNo ratings yet
- Extra Questions (With Answers)Document5 pagesExtra Questions (With Answers)dineshkumar_subramanNo ratings yet
- 3EChem PRACTICE PAPER 2Document17 pages3EChem PRACTICE PAPER 2Alley EioNo ratings yet
- Mole McqsDocument8 pagesMole McqsShoaib Aslam DhakkuNo ratings yet
- Pei Hwa Chem Prelim P1 2012 QNDocument16 pagesPei Hwa Chem Prelim P1 2012 QNWonderfullyANo ratings yet
- 4024q1 Specimen PaperdocxDocument12 pages4024q1 Specimen PaperdocxLeses MayNo ratings yet
- Prelim Sec4 p1 & p2Document30 pagesPrelim Sec4 p1 & p2dimpledblissNo ratings yet
- MC Practice 2Document10 pagesMC Practice 2jackson wongNo ratings yet
- 4 Stoichiometry PDFDocument8 pages4 Stoichiometry PDFHakim Abbas Ali PhalasiyaNo ratings yet
- Chemistry Exam Form 5Document35 pagesChemistry Exam Form 5Tan Phei LingNo ratings yet
- Kimia XDocument4 pagesKimia Xtri hastutiNo ratings yet
- Chemistry PDFDocument38 pagesChemistry PDFAddict- ionNo ratings yet
- Cambridge IGCSE: Chemistry 0620/23Document16 pagesCambridge IGCSE: Chemistry 0620/23shabanaNo ratings yet
- Pahang 2008 STPM Chem - p1 QuestDocument12 pagesPahang 2008 STPM Chem - p1 QuestLooi Chui YeanNo ratings yet
- Kimia Module 1 5 Diagnostik f4 PDFDocument70 pagesKimia Module 1 5 Diagnostik f4 PDFJuan DavisNo ratings yet
- KTESP SEM 1 TRIAL 2017 With AnswerDocument7 pagesKTESP SEM 1 TRIAL 2017 With AnswerShima SenseiiNo ratings yet
- Grade 10 O Level Chemistry - Mock Test 1 (7-04-2021)Document29 pagesGrade 10 O Level Chemistry - Mock Test 1 (7-04-2021)Roselyn TrixieNo ratings yet
- Aqueous Hydrogen Peroxide: Ó UCLES 2004 5070/01/M/J/04Document14 pagesAqueous Hydrogen Peroxide: Ó UCLES 2004 5070/01/M/J/04thc8477No ratings yet
- Chem Paper 1Document12 pagesChem Paper 1Victoria Petrus100% (1)
- 1 2 3hhDocument9 pages1 2 3hhHasan DöşemeciNo ratings yet
- Chemistry 1995 Paper 2Document14 pagesChemistry 1995 Paper 2api-3824003No ratings yet
- Paper 1 Duplicate - 2007 Year End TestDocument11 pagesPaper 1 Duplicate - 2007 Year End Testsherry_christyNo ratings yet
- OL Moles WS 1 PDFDocument8 pagesOL Moles WS 1 PDFFatima ShahNo ratings yet
- 2014 H2 Chem Promo (DHS) - PKDocument37 pages2014 H2 Chem Promo (DHS) - PKdragon slayerNo ratings yet
- H2 - Prelim 2009 Paper1Document16 pagesH2 - Prelim 2009 Paper1Augustine NgNo ratings yet
- Chemistry Mock Exam ss3Document3 pagesChemistry Mock Exam ss3chrizyboyziNo ratings yet
- CHEMISTRYDocument41 pagesCHEMISTRYLindsayyNo ratings yet
- 5070 w12 QP 11Document16 pages5070 w12 QP 11mstudy123456No ratings yet
- Soalan Kimia Pertengahan Tahun Form 4Document11 pagesSoalan Kimia Pertengahan Tahun Form 4Ridzuan Mohd AliNo ratings yet
- MCQ - WS! June 2023Document7 pagesMCQ - WS! June 2023Prakriti DhakalNo ratings yet
- 1997 Paper 2Document14 pages1997 Paper 2api-3826629No ratings yet
- 2018 Chemistry Standardised Test For Science Stream (SPM)Document7 pages2018 Chemistry Standardised Test For Science Stream (SPM)carnationNo ratings yet
- HCI Chem H2 Paper 1 Question PaperDocument17 pagesHCI Chem H2 Paper 1 Question PaperonnoezNo ratings yet
- Cie QDocument17 pagesCie Qinternationalmakkhayar100% (1)
- 5070 s15 QP 12Document13 pages5070 s15 QP 12Saima SohailNo ratings yet
- Test Topic: Atomic Structure, Formula Writing and Balancing Equation, Bonding and Structure, Redox, ElectrolysisDocument11 pagesTest Topic: Atomic Structure, Formula Writing and Balancing Equation, Bonding and Structure, Redox, ElectrolysisArham Tamim100% (1)
- Chemistry AS Term 1 Exam 2023Document16 pagesChemistry AS Term 1 Exam 2023sugoshi12No ratings yet
- Chemistry 11 PDFDocument25 pagesChemistry 11 PDFChanda S MwambaNo ratings yet
- Chemistry SS IiDocument7 pagesChemistry SS IiAbba YakubuNo ratings yet
- Chemistry-2-2nd-Quarter-Final-Exam 2019 - 2020-PMDocument9 pagesChemistry-2-2nd-Quarter-Final-Exam 2019 - 2020-PMAnalynAsuncionAtaydeNo ratings yet
- SPM Practice Chap3 F4Document7 pagesSPM Practice Chap3 F4Shervin Fernandez0% (1)
- Chem-CGS P1Document17 pagesChem-CGS P1dimpledblissNo ratings yet
- Set VDocument5 pagesSet VChew Gee LanNo ratings yet
- 50 Obj Quest Chem f4Document10 pages50 Obj Quest Chem f4arifahsanimanNo ratings yet
- NL MCQ Timed Practice 13 (R09)Document4 pagesNL MCQ Timed Practice 13 (R09)Alvin LeeNo ratings yet
- 5070 w05 QP 1Document16 pages5070 w05 QP 1mstudy123456No ratings yet
- Section - A: Holiday Homework For Grade XiDocument7 pagesSection - A: Holiday Homework For Grade XiGM Ali KawsarNo ratings yet
- Assignment 1a ChemistryDocument7 pagesAssignment 1a ChemistryJoshua HongNo ratings yet
- CHEM101 172 Final SolvedDocument12 pagesCHEM101 172 Final SolvedTorong VNo ratings yet
- 5070 w12 QP 12Document16 pages5070 w12 QP 12mstudy123456No ratings yet
- PeriodicTrends WS2 MCQsDocument5 pagesPeriodicTrends WS2 MCQsTalal Iqbal Khan100% (1)
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- WB CalculationDocument14 pagesWB CalculationJason TanNo ratings yet
- WB Bonding 2Document16 pagesWB Bonding 2Jason TanNo ratings yet
- Sec 3E Science Chemistry CA 2 Test 1 2014: Suggested Marking Scheme Chemical Bonding Section A (5%) : BDocument2 pagesSec 3E Science Chemistry CA 2 Test 1 2014: Suggested Marking Scheme Chemical Bonding Section A (5%) : BJason TanNo ratings yet
- You Should Have Checked The PT. Neon Has 10 Electrons: in So 3 2 in (SO)Document2 pagesYou Should Have Checked The PT. Neon Has 10 Electrons: in So 3 2 in (SO)Jason TanNo ratings yet
- Secondary Three Normal Academic Mock Paper 1 /2009: Science (Chemistry)Document6 pagesSecondary Three Normal Academic Mock Paper 1 /2009: Science (Chemistry)Jason TanNo ratings yet
- Sec 3E Science Chemistry CA 2 Test 1 2014: Suggested Marking Scheme Chemical Bonding Section A (5%) : BDocument2 pagesSec 3E Science Chemistry CA 2 Test 1 2014: Suggested Marking Scheme Chemical Bonding Section A (5%) : BJason TanNo ratings yet
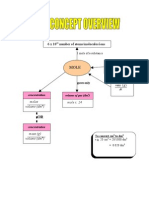
- Overview FormulaDocument1 pageOverview FormulaJason TanNo ratings yet
- Sec 4e1 Revision Paper2Document5 pagesSec 4e1 Revision Paper2Jason TanNo ratings yet
- Prelim 4E 2009 P1+P2 Pure AnsDocument6 pagesPrelim 4E 2009 P1+P2 Pure AnsJason TanNo ratings yet
- Prof Ed QuestionsDocument35 pagesProf Ed QuestionsKatrina BautistaNo ratings yet
- Road Check List Borrow ExcavationDocument2 pagesRoad Check List Borrow Excavationklp_kedarpNo ratings yet
- Silver Iodide MSDS: Section 1: Chemical Product and Company IdentificationDocument5 pagesSilver Iodide MSDS: Section 1: Chemical Product and Company IdentificationelsadwihermiatiNo ratings yet
- Chapter 2-Life TablesDocument18 pagesChapter 2-Life TablesBoby ZooxNo ratings yet
- 2023 - PPS Prelim02Document7 pages2023 - PPS Prelim02Mrdark WarriorNo ratings yet
- Tinnitus Assessment Thi ScoringDocument4 pagesTinnitus Assessment Thi ScoringbssNo ratings yet
- EC8093-DIP - Model Exam QPDocument2 pagesEC8093-DIP - Model Exam QPSanthosh PaNo ratings yet
- Nursing Philosophy PaperDocument6 pagesNursing Philosophy PaperDanielle AalderinkNo ratings yet
- Data Sheet 65hdDocument3 pagesData Sheet 65hdoniferNo ratings yet
- Fibonacci The Numbers of NatureDocument5 pagesFibonacci The Numbers of NatureCindy ValdozNo ratings yet
- Chapter 5 Discrete Probalitity Dsitributions - Jaggia4e - PPTDocument66 pagesChapter 5 Discrete Probalitity Dsitributions - Jaggia4e - PPTpeter shlomoNo ratings yet
- Bs - Civil Engineering 2023 24Document1 pageBs - Civil Engineering 2023 24DawnNo ratings yet
- W5-Comprehensive Local Juvenile Intervention PlanDocument5 pagesW5-Comprehensive Local Juvenile Intervention PlanSammy Codera100% (1)
- Zamak-3 XometryDocument1 pageZamak-3 XometryFrancisco BocanegraNo ratings yet
- 2022 01 MED21A T1-Assessment QestionPaperDocument4 pages2022 01 MED21A T1-Assessment QestionPaperIshmael MvunyiswaNo ratings yet
- ICETEMS-18 Abstract Book PDFDocument152 pagesICETEMS-18 Abstract Book PDFJAMILNo ratings yet
- COMTEC LeafletDocument4 pagesCOMTEC Leafletariksyaiful82No ratings yet
- Introduction To LogicDocument4 pagesIntroduction To LogicLykwhat UwantNo ratings yet
- (Short Term) (Explain The Nursing Diagnosis)Document1 page(Short Term) (Explain The Nursing Diagnosis)Angel MayNo ratings yet
- امتحان وطني نماذج محلولة PDFDocument6 pagesامتحان وطني نماذج محلولة PDFApdel Rahman RazzoukNo ratings yet
- Nursing Care Plan of The MotherDocument20 pagesNursing Care Plan of The Motherbuang2390% (382)
- EN116 WebDocument44 pagesEN116 WebX 6No ratings yet
- Qualtrics Survey Software PostDocument13 pagesQualtrics Survey Software Postapi-283622630No ratings yet
- Listening TestDocument3 pagesListening TestNatalia García GarcíaNo ratings yet
- Scholarship Essay Samples Financial NeedDocument6 pagesScholarship Essay Samples Financial Needflrzcpaeg100% (2)
- Thesis Technology ManagementDocument7 pagesThesis Technology Managementzidhelvcf100% (2)
- FLS OM KPI Handbook Work 2012 Version 7 PDFDocument37 pagesFLS OM KPI Handbook Work 2012 Version 7 PDFTARNo ratings yet
- 1577078761Document54 pages1577078761Jyotishman SharmaNo ratings yet
- R G M College of Engineering & R G M College of Engineering &technology TechnologyDocument1 pageR G M College of Engineering & R G M College of Engineering &technology TechnologyAyanwale-cole pelumi AkeemNo ratings yet
- Lipid Maps Leipzig 2018Document72 pagesLipid Maps Leipzig 2018first name last nameNo ratings yet