Professional Documents
Culture Documents
Assignment I
Uploaded by
ChocolaMeilleurOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Assignment I
Uploaded by
ChocolaMeilleurCopyright:
Available Formats
ASSIGNMENT
COURSE
ORGANIC CHEMISTRY
COURSE CODE
BSB/BSP1133
STUDENTS NAME
MATERIC NO.
ASSIGNMENT
COURSE COORDINATOR :
PROGRAMME CODE :
DUK
FACULTY OF INDUSTRIAL SCIENCES AND TECHNOLOGY
CONFIDENTIAL
BSB/BSP/1415II/BSB/BSP1133
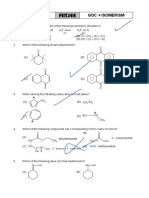
(OBJECTIVE QUESTIONS)
1.
Identify the most electronegative atom.
(A)
(B)
(C)
(D)
2.
Which of the compounds below have bonds that are predominantly ionic?
(A)
(B)
(C)
(D)
3.
4.
I.
II.
III.
IV.
Ethers
Amines
Phenols
Alcohols
(A)
(B)
(C)
(D)
I and II
II and III
II, III and IV
All of them
A molecule of acetone (CH3COCH3) contains ________ sigma bonds and
________ pi bonds.
8, 2
7, 1
9, 1
10, 2
Propose a structure for NBr3 that is consistent with this information.
(A)
(B)
(C)
(D)
6.
NaBr
NH3
CF4
Both A and C
Which of the following molecules can form hydrogen bonds?
(A)
(B)
(C)
(D)
5.
N
Mg
I
S
Tetrahedral
Trigonal planar
Trigonal Pyramid
Bent
Which one of the following is most soluble in hexane (C6H14)?
(A)
(B)
(C)
(D)
CH3OH
CH3CH2CH2OH
CH3CH2CH2CH2OH
CH3CH2CH2CH2CH2OH
CONFIDENTIAL
7.
Give the IUPAC name for the following structure:
(A)
(B)
(C)
(D)
8.
CH3OCH3
CH3CH2OH
CH3CH2Cl
CH3CH2CH3
Give the number of nonbonding pairs of electrons in HOCONH2.
(A)
(B)
(C)
(D)
12.
CH3CH2CH2CH2CH2CH3
CH3CH2CH2CH(CH3)2
(CH3)2CHCH(CH3)2
CH3CH2CH(CH3)CH2CH3
Which of the following is the least soluble in H2O?
(A)
(B)
(C)
(D)
11.
CH3CH2OCH2CH3
CH3CH2CH2CH2OH
CH3CH2CH2CH2NH2
CH3CH2CH2CH2Cl
Which of the following has the highest boiling point?
(A)
(B)
(C)
(D)
10.
8-methyl-4-(2,3-dimethylpropyl)octane
2-methyl-5-(1,2-dimethylpropyl)nonane
6-methyl-5-(1,2-dimethylpropyl)nonane
2-methyl-5-(2,3-dimethylpropyl)decane
Identify the compound with the lowest boiling point.
(A)
(B)
(C)
(D)
9.
BSB/BSP/1415II/BSB/BSP1133
3
5
6
10
What is the weakest intermolecular force present in liquid ethanol?
(A)
(B)
(C)
(D)
ion-ion
dipole-dipole, specifically hydrogen bonding
dipole-dipole, but not hydrogen bonding
Van der Waal forces
3
CONFIDENTIAL
13.
Aliphatic alkane (C16H34) undergoes cracking to produce (C3H8) and what is
the other compound?
(A)
(B)
(C)
(D)
14.
BSB/BSP/1415II/BSB/BSP1133
C13H28
C13H26
propene
C13H24
Use the Cahn-Ingold-Prelog (CIP) rules to arrange the following groups in
order of increasing priority (lowest priority at the left):
CH2OH
CH=O
CH(OMe)2
CO2H
(II)
(III)
(IV)
(I)
(A)
(B)
(C)
(D)
15.
I > II > III>IV
IV>III>II > I
I > III > II > IV
IV > II > III > I
Assign the configuration of molecule as below:
HO
(A)
(B)
(C)
(D)
16.
OH
R - Configuration
S - Configuration
not chiral
meso compound
The following two molecules are:
Br
Br
H
C2H5
C2H5
Br
(A)
(B)
(C)
(D)
C2H5
C2H5
H
Br
identical
structural isomers
enantiomers
diastereoisomers
CONFIDENTIAL
17.
How are the
following
CH3CH2CH2CH2CH2I related?
(A)
(B)
(C)
(D)
18.
BSB/BSP/1415II/BSB/BSP1133
H3C
CH3
(I)
(II)
H
CH3
CH3
H
CH3
H3C
CH3
(III)
H
H
(IV)
I and II
II and III
III and IV
All of the above
Which of the following represent the same compound?
(I)
(II)
(III)
(IV)
CH3CH2CH2=CH2CHCH2
CH3CH2CH2CH=CHCH3
CH2=CHCH2CH2CH2CH3
CH3CH=CHCH2CH2CH3
(A)
(B)
(C)
(D)
I and II
I and III
II and IV
I and IV
How many structural isomers are there for heptane, C7H16?
(A)
(B)
(C)
(D)
21.
and
Pick out the staggered conformation(s) for butane:
(A)
(B)
(C)
(D)
20.
CH3CH2CH2CHICH3
Enantiomers
non-superimposable mirror images
Diastereoisomers
Constitutional isomers
CH3
19.
molecules,
5
7
9
11
Which one of the following substances would be the most soluble in CCl4?
(A)
(B)
(C)
(D)
C10H22
CH3CH2OH
NaCl
H2O
CONFIDENTIAL
22.
BSB/BSP/1415II/BSB/BSP1133
Nitroethane exhibits
O
O
H3CH2C
H3CH2C
N
O
(A)
(B)
(C)
(D)
23.
2 hydrogens
2 carbons
3 carbons
4 carbons
R-configuration
S-configuration
R, S both
meso compound
Br
Br
H3C
CH3
Which of these elements is chemically similar to oxygen?
(A)
(B)
(C)
(D)
27.
The proton and the neutron have identical masses.
An atomic nucleus contains equal numbers of protons and neutrons.
A neutral atom contains equal numbers of protons and electrons.
The neutrons mass is equal to that of a proton plus an electron.
Which configuration is shown by the compound below?
(A)
(B)
(C)
(D)
26.
equilibrium
resonance
constitutional isomer
none of these
A secondary carbon is bonded directly to:
(A)
(B)
(C)
(D)
25.
Which of the following statement about atoms and subatomic particles is
correct?
(A)
(B)
(C)
(D)
24.
Iron
Nickel
Sulpher
Potassium
Give the number of protons (p), electrons (e), and neutron (n) in 37Cl.
(A)
(B)
(C)
(D)
17 p, 17 e, 20 n
17 p, 37 e, 17 n
17 p, 17 e, 37 n
37 p, 17 e, 20 n
6
CONFIDENTIAL
28.
Metal and metal atoms combine to form
(A)
(B)
(C)
(D)
29.
d>s>f>p
s>p>d>f
p>s>f>d
f >d>p>f
The geometry and bond angle of the ether (CH3COCH3) are best described as
(A)
(B)
(C)
(D)
33.
highly electronegative
a cation
an anion
inert
What is the order of decreasing energy of the orbitals within a single energy
level?
(A)
(B)
(C)
(D)
32.
one bonding electron and 3 non-bonding pairs
two bonding and 6 non-bonding pairs
three bonding and one nonbonding pairs
None of all above
When an atom neither gains and nor loses an electron, it is called
(A)
(B)
(C)
(D)
31.
ionic compound
metalloid compound
covalent compound
non-polar compound
In the Lewis dot structure of nitrogen
(A)
(B)
(C)
(D)
30.
BSB/BSP/1415II/BSB/BSP1133
Trigonal pyramidal, 107
Tetrahedral, 109.5
Bent, 111
Trigonal planar, 120
Which of the following hydrocarbons does not have isomers?
(A)
(B)
(C)
(D)
C2H4
C3H8
C3H6
none of all above
CONFIDENTIAL
34.
35.
For which of the compounds below are Cis-Trans isomers possible?
(i)
(ii)
(iii)
CH2=CH-CH2-CH3
CH3-CH2-CH=CH-CH2-CH3
CH3-CH=CH- CH2-CH3
(A)
(B)
(C)
(D)
(i) and (ii)
(ii) and (iii)
All three
None of all above
Which of the following molecules has the shortest carbon-carbon bond length?
(A)
(B)
(C)
(D)
36.
1s2, 2s2, 2p2
N+1
B-1
All of the above
What is the orbital combination in bromine gas?
(A)
(B)
(C)
(D)
39.
Two electrons of atom
Core electron of atom
Valence electron of atom
None of all above
Electronic configuration of 126C is...
(A)
(B)
(C)
(D)
38.
H-CC-H
H2C=CH2
CH3-CH3
All the same
In the periodic table vertical line indicates a group, so it showing
(A)
(B)
(C)
(D)
37.
BSB/BSP/1415II/BSB/BSP1133
2s-3s orbital
3s-3p orbital
3p-3p orbital
2sp3 orbital
In sp2 hybridization of carbon atom consist of
(A)
(B)
(C)
(D)
1s-2p orbital and one pz unhybridized orbital
1s-2p orbital and one py unhybridized orbital
2s-2p orbital and one py unhybridized orbital
1s-3p orbital and one py unhybridized orbital
8
CONFIDENTIAL
40.
How many neutrons are there in an atom of lead, Pb, whose mass number is
208?
(A)
(B)
(C)
(D)
41.
polar solvents dissolve nonpolar solutes and vice versa
solvents can only dissolve solutes of similar molar mass
condensed phases can only dissolve other condensed phases
polar solvents dissolve polar solutes and nonpolar solvents dissolve
nonpolar solutes
repulsion between like-charged water and octane molecules
hydrogen bonding between water molecules
van der Waal forces between octane molecules
dipole-dipole attraction between octane molecules
The p-orbital of a methyl carbocation +CH3 contains _____ electrons.
(A)
(B)
(C)
(D)
45.
NO2
O2
CO
none of above
The dissolution of water in octane (C8H18) is prevented by __________.
(A)
(B)
(C)
(D)
44.
The phrase like dissolves like refers to the fact that __________.
(A)
(B)
(C)
(D)
43.
82
208
126
290
Which structure has delocalized electrons?
(A)
(B)
(C)
(D)
42.
BSB/BSP/1415II/BSB/BSP1133
1
3
4
none of the above
Which of the following does not play a role in the determination of bond
length?
(A)
(B)
(C)
(D)
nuclear charge
the potential energy of electrostatic charges
mass of neutrons
electron charge
CONFIDENTIAL
46.
The hybridization state of an alkyne carbon is:
(A)
(B)
(C)
(D)
47.
An alkyl chloride
A quaternary ammonium salt
An alcohol
An ether
Identify the compound that does not have hydrogen bonding.
(A)
(B)
(C)
(D)
52.
All their chemical reactions are identical
Their physical properties are different
A racemic mixture will rotate the plane polarized light
They will rotate the plane of polarized light in opposite directions
Assuming roughly equivalent molecular weights, which of the following
would have the lowest boiling point?
(A)
(B)
(C)
(D)
51.
4
6
8
10
Which statement is correct about the enantiomers of a chiral compound?
(A)
(B)
(C)
(D)
50.
104
109.5
120
180
How many sigma bonds are there in CH2=C=C=C=CH2
(A)
(B)
(C)
(D)
49.
p
sp
sp2
sp3
The angle ____ is commonly associated with linear molecules.
(A)
(B)
(C)
(D)
48.
BSB/BSP/1415II/BSB/BSP1133
CH3CH2CH2NHCH3
CH3CH2CH2OCH3
CH3CH2CH2CH2NH2
CH3CH2CH2CH2OH
Which of the following has the shortest van der Waal's interaction between
molecules of the same kind?
(A)
(B)
(C)
(D)
CH3(CH3)CHCH2CH3
CH3CH2CH2CH3
(CH3)4C
CH3CH2CH2CH2CH3
10
CONFIDENTIAL
53.
Which of these molecules have the lowest heat of combustion?
H
H
H
C
(I)
(A)
(B)
(C)
(D)
54.
BSB/BSP/1415II/BSB/BSP1133
CH3
(III)
(II)
(IV)
(II) and (III)
(I) and (IV)
(III)
All have the same value
Put the following molecules in the order of decreasing carbon-carbon bond
length:
H
C
H
H
(A)
(B)
(C)
(D)
H
C
a, b, c
c, a, b
b, a, c
They are all the same bond length
In the following 3-Dimention methane molecule, all structure is correct
EXCEPT one which is not
(A)
(B)
(C)
(D)
56.
55.
I and II
II only
III and IV
None of them
When the compounds below are listed in order of increasing boiling point
(lowest to highest) what is the correct order?
1. Chloropropane
(A)
(B)
(C)
(D)
2. Propane
3. Propanoic acid
4. Propanol
4, 3, 1, 2
3, 4, 1, 2
4, 1, 2, 3
2, 1, 4, 3
11
CONFIDENTIAL
57.
Which of the following molecules NOT contain conjugation?
(A)
(B)
(C)
(D)
58.
1
2
3
4
Identify the number of tertiary carbons in the following structure.
(A)
(B)
(C)
(D)
60.
I and IV
I, II and III
I, III and IV
II, III and IV
How many carbon-carbon pi-bonds are in the molecule shown?
(A)
(B)
(C)
(D)
59.
BSB/BSP/1415II/BSB/BSP1133
2
3
4
5
Determine the type of hydrogens in limonene.
Limonene
(A)
(B)
(C)
(D)
2CH3, 4CH2, 1CH, 2C
3CH3, 4CH2, 2CH, 3C
1CH3, 3CH2, 2CH, 2C
None of above
12
CONFIDENTIAL
61.
How many sigma-bonds are there in:
(A)
(B)
(C)
(D)
62.
all biomolecules
identical molecules
all polymers
chiral molecules
Which of the following has the lowest solubility in CH3CH2CH2CH3?
(A)
(B)
(C)
(D)
65.
1, 4 and 5
2, 4 and 5
2, 3 and 5
None of above
The plane polarize light is affected by
(A)
(B)
(C)
(D)
64.
3
4
5
6
Which carbon(s) in the following molecule is (are) sp2 hybridized?
(A)
(B)
(C)
(D)
63.
BSB/BSP/1415II/BSB/BSP1133
CH3OH
CH3NH2
CH3OCH3
(CH3)3CH
Which Lewis-dot structure represents an atom of fluorine in the ground state?
13
CONFIDENTIAL
66.
Give the IUPAC name for the following structure:
(A)
(B)
(C)
(D)
67.
69.
R - Configuration
S - Configuration
Racemic mixture
No R and S Configuration
Which is the least stable conformation of cyclohexane?
(I)
(II)
(III)
(IV)
Staggered
Boat
Chair
All of above
(A)
(B)
(C)
(D)
I
II
III
IV
Pick out the molecules which exist as (E)- and (Z)- configuration:
(A)
(B)
(C)
(D)
70.
bicyclo[2.2.0]hexane
bicyclo[2.2.2]octane
bicyclo[2.2.3]nonane
bicyclo[4.3.1]octane
Assign the configuration of molecule as below:
(A)
(B)
(C)
(D)
68.
BSB/BSP/1415II/BSB/BSP1133
BrFC=CClI
CH3CH2-IC=CFCH3
CH3BrC=CHCl
All of above
Which the following statement is FALSE about butane?
(A)
(B)
(C)
(D)
All bond angles are 109.5
Each carbon is sp3 hybridized
The compound is combustible
The compound undergoes polymerisation to give polybutylene
14
CONFIDENTIAL
71.
The tert-butyl carbocation is stabilised by
(A)
(B)
(C)
(D)
72.
75.
Cyclopropane
Cyclobutane
Cyclopentane
Cyclohexane
Pick out the physical property that is different for a pair of enantiomers:
(I)
(II)
(III)
(IV)
Density
Melting point
Refractive index
Optical
(A)
(B)
(C)
(D)
I
II
III
IV
The number of 2nd shell (n = 2) the maximum number of electrons will be
(A)
(B)
(C)
(D)
76.
1s2, 2s2, 2p6,3s2, 3p2
1s2, 2s2, 2p6,3s2, 4s2 , 3p6
1s2, 2s2, 2p6,3s2, 3p4
1s2, 2s2, 2p6,3s2, 3p6
Which of the following cycloalkanes has the least strain energy?
(A)
(B)
(C)
(D)
74.
resonance
hyperconjugation
steric hindrence
none of above
What is the electronic configuration of 40 20Ca+2.
(A)
(B)
(C)
(D)
73.
BSB/BSP/1415II/BSB/BSP1133
2
8
18
32
Horizontally rows in the periodic table are celled
(A)
(B)
(C)
(D)
groups
columns
period
none of them
15
CONFIDENTIAL
77.
How many hydrogen atoms would combine a 4-carbon chain if it were an
alkane, alkene and alkyne, RESPECTIVELY?
(A)
(B)
(C)
(D)
78.
cyclohexane
cyclohexene
O=C=O
H2C=CH-C=CH2
What is the name used to represent the number of protons in the nucleus of
each atom of an element?
(A)
(B)
(C)
(D)
82.
HHe
Li+
All of above
Compound in which carbon use only sp hybrid orbitals for bond formation is
(A)
(B)
(C)
(D)
81.
hydrolysis
esterification
hydrogenation
neutralization
Which of the following have the electron configuration of 1s2?
(A)
(B)
(C)
(D)
80.
6, 8, 10
8, 10, 6
10, 8, 6
6, 10, 8
A reaction in which a carboxylic acid reacts with a base to form a salt and
water is called
(A)
(B)
(C)
(D)
79.
BSB/BSP/1415II/BSB/BSP1133
Atomic mass units
Isotope number
Atomic number
Mass number
Which molecule has the longest carbon-carbon bond(s)?
(A)
(B)
(C)
(D)
Ethylene
Propene
Acetylene
Ethane
16
CONFIDENTIAL
83.
Organic compounds containing a carbon-oxygen double bond include all of
the following EXCEPT
(A)
(B)
(C)
(D)
84.
C5H10O
C6H12O
C7H10O
C7H12O
If an element (X) has 80 protons and 120 neutrons, the number of electrons
will be
(A)
(B)
(C)
(D)
88.
Cl2
Na2SO4
HCN
KCl
Give the molecular formula of the compound represented by
(A)
(B)
(C)
(D)
87.
1-hexene
Cyclohexane
3-hexanone
3-hexene
Which one of the following compounds involves both ionic and covalent bonding?
(A)
(B)
(C)
(D)
86.
ester
amide
aldehyde
ethers
Which of the following compounds may exist as cis-trans isomers?
(A)
(B)
(C)
(D)
85.
BSB/BSP/1415II/BSB/BSP1133
40
80
200
120
The hybridisation state and shape of a carboanion is:
(A)
(B)
(C)
(D)
sp2 and linear
sp3 and trigonal pyramidal
sp2 and trigonal planar
sp3 and tetrahedral
17
CONFIDENTIAL
89.
Optically active molecules which rotate plane-polarized light in a counter
clockwise direction are said to be
(A)
(B)
(C)
(D)
90.
dextrorotary
of R configuration
levorotary
of S configuration
A correct orbital diagram for the ground state of the O+ ion is
1s
1s
1s
1s
(A)
(B)
(C)
(D)
91.
2s
2s
2s
2s
2p
2p
2p _
2p
Which of the following Newman projections is the least stable conformation
of 2-Iodoethanol?
(A)
(B)
(C)
(D)
92.
BSB/BSP/1415II/BSB/BSP1133
II
III
II and IV
None of the above
Put the following molecules in order of decreasing carbon-carbon bond length:
H
H
H
C
(A)
(B)
(C)
(D)
II
III
I > II > III
II > I > III
III > I > II
I > III > II
18
CONFIDENTIAL
93.
Which of the following molecules contains sp2 hybridisation?
(A)
(B)
(C)
(D)
94.
I and II
I and III
II and III
All of them
Which of the following molecules have approximately a molecular weight of
186?
(A)
(B)
(C)
(D)
95.
BSB/BSP/1415II/BSB/BSP1133
I and II
II and III
I and III
All of them
Identify the hybridization of carbon atoms in below molecule
(A)
(B)
(C)
(D)
1
sp3
sp2
sp3
sp2
2
sp2
sp2
sp
sp2
3
sp2
sp2
sp2
sp2
4
sp2
sp3
sp
sp
19
CONFIDENTIAL
96.
The following two molecules are:
(A)
(B)
(C)
(D)
97.
identical
constitutional isomers
stereoisomers
not isomer
Draw the structure of bicycle [3.2.0] heptane:
(A)
(B)
(C)
(D)
98.
BSB/BSP/1415II/BSB/BSP1133
I
II
III
IV
Which of the following molecule(s) is an ester?
(A)
(B)
(C)
(D)
I, II and III
II, III and IV
III and IV
None of them
20
CONFIDENTIAL
99.
Which is the most stable conformation of cyclohexane?
(A)
(B)
(C)
(D)
100.
BSB/BSP/1415II/BSB/BSP1133
I
II
III
IV
Which statement about the water molecule is incorrect?
(A)
(B)
(C)
(D)
The decrease in bond angle from the tetrahedral angle (109.5) is
greater for H2O than for NH3.
The bond angle for H2O is smaller than for NH3 because of the lone
pair-lone pair repulsion in H2O.
The effect of the very polar bonds in water is opposed by the effect of
the 2 lone pairs of electrons.
The O atom is sp3 hybridized.
(END OF ASSIGNMENT)
21
You might also like
- OCHEM Practice FinalsDocument13 pagesOCHEM Practice FinalsNoleNo ratings yet
- Organic Problems1Document9 pagesOrganic Problems1Sung-Eun KimNo ratings yet
- Alkanes, Alkenes, Alkynes and Their Alicyclic Couterparts: 1. What Is The IUPAC Name For CHDocument17 pagesAlkanes, Alkenes, Alkynes and Their Alicyclic Couterparts: 1. What Is The IUPAC Name For CHEllaŠtrbac100% (1)
- Fall 2008 Old - Exam - 4Document12 pagesFall 2008 Old - Exam - 4alfredNo ratings yet
- CH 331/1 Midterm Examination/Summer 2013/Dr. Daniel J. T. MylesDocument8 pagesCH 331/1 Midterm Examination/Summer 2013/Dr. Daniel J. T. MylesAnita MarLaNo ratings yet
- 235practice Exam 3 AnswerDocument4 pages235practice Exam 3 Answersowmmiya karuppiahNo ratings yet
- Chapter 6 Properties of HaloalkaneDocument5 pagesChapter 6 Properties of HaloalkaneRen Liew Jia QingNo ratings yet
- Instructions:: Part-A I. Answer ALL The Questions (Each Question Carries One Mark) 10x1 10Document3 pagesInstructions:: Part-A I. Answer ALL The Questions (Each Question Carries One Mark) 10x1 10anon_850201470No ratings yet
- Aldehydes and Ketones For IitjeeDocument65 pagesAldehydes and Ketones For Iitjeevarundhall1994No ratings yet
- Organic Chemistry Help! Practice Exam Window For Xula-O1e2Document7 pagesOrganic Chemistry Help! Practice Exam Window For Xula-O1e2Kristia Stephanie BejeranoNo ratings yet
- Ap Chemistry Acid-Base Exam Part I Multiple Choice: K (Hco) (Co) (H O) K (Co) (Co) (OH)Document8 pagesAp Chemistry Acid-Base Exam Part I Multiple Choice: K (Hco) (Co) (H O) K (Co) (Co) (OH)Max SaubermanNo ratings yet
- Solution-Aldehydes Ketones and Carboxylic AcidsDocument5 pagesSolution-Aldehydes Ketones and Carboxylic AcidsAnindya AcharyaNo ratings yet
- Chem52 Su13 PracticeExam1ADocument11 pagesChem52 Su13 PracticeExam1Aamarka01No ratings yet
- Tutorial 3 & 4 - Equilibria & Application of Rates and EquilibriumDocument5 pagesTutorial 3 & 4 - Equilibria & Application of Rates and EquilibriumAhmad Taufiq Mohd ZaidNo ratings yet
- Inter Ipe Isomerism & Bond PolarizationDocument9 pagesInter Ipe Isomerism & Bond PolarizationNalla Umapathi ReddyNo ratings yet
- Lecture 3.1 - Introduction To The Synthesis of Nanomaterials - Molecular SpectrosDocument88 pagesLecture 3.1 - Introduction To The Synthesis of Nanomaterials - Molecular SpectrosGian BanaresNo ratings yet
- Che 232 Test 1 Sptember 2007Document16 pagesChe 232 Test 1 Sptember 2007BONOLO RANKONo ratings yet
- Alkyl Halide PDFDocument6 pagesAlkyl Halide PDFTvisha ViraniNo ratings yet
- ADV. I 57 - 64 (Exercise 3)Document8 pagesADV. I 57 - 64 (Exercise 3)Aditya ShahNo ratings yet
- Alcohols TestDocument2 pagesAlcohols TestAboahmed AliNo ratings yet
- Hyperconjugation, The Anomeric Effect, and More: Chem 206 D. A. EvansDocument12 pagesHyperconjugation, The Anomeric Effect, and More: Chem 206 D. A. Evansomkar9996767No ratings yet
- Chem 1040 Final Exam ReviewDocument8 pagesChem 1040 Final Exam ReviewUzair AliNo ratings yet
- APCHEM Review Practice Test 1Document16 pagesAPCHEM Review Practice Test 1M. JosephNo ratings yet
- Answers To ROH Tutorial PDFDocument12 pagesAnswers To ROH Tutorial PDFCorvo Haosen Al-Han0% (1)
- Alkyl Halide-Jeemain - Guru PDFDocument37 pagesAlkyl Halide-Jeemain - Guru PDFUma JadounNo ratings yet
- Chapter 12: Reactions of Arenes - Electrophilic Aromatic SubstitutionDocument29 pagesChapter 12: Reactions of Arenes - Electrophilic Aromatic SubstitutionAnonymous Ngsu7C4aNo ratings yet
- Chapter 8 - Alkene ReactivityDocument23 pagesChapter 8 - Alkene ReactivitySimran DhunnaNo ratings yet
- Houston Community College System: Organic Chemistry II 2425Document19 pagesHouston Community College System: Organic Chemistry II 2425Banele Ezma LambathaNo ratings yet
- Hand-Out: Chemistry Chapter 4: Haloalkanes & HaloarenesDocument11 pagesHand-Out: Chemistry Chapter 4: Haloalkanes & HaloarenesLuisgarciaBerlangaNo ratings yet
- ACS Review 9 AlkynesDocument9 pagesACS Review 9 AlkynesMohamad HabbabaNo ratings yet
- Aldehydes, Ketones, and Carboxylic Acids PDFDocument5 pagesAldehydes, Ketones, and Carboxylic Acids PDFmadhurima maityNo ratings yet
- Haloalkanes & HaloarenesDocument10 pagesHaloalkanes & Haloarenesakshatshukla2021No ratings yet
- Class-XII (Chemistry) Chapter: Alcohols, Phenols and Ethers Objective Type QuestionsDocument9 pagesClass-XII (Chemistry) Chapter: Alcohols, Phenols and Ethers Objective Type QuestionsPranav DhimanNo ratings yet
- Haloalkanes and Haloarenes, Alcohols, Phenols and Ethers-31-OctDocument7 pagesHaloalkanes and Haloarenes, Alcohols, Phenols and Ethers-31-Octolivia.benson9331No ratings yet
- Lecture Notes Chapter-12-Aldehydes, Ketones & Carboxylic AcidsDocument26 pagesLecture Notes Chapter-12-Aldehydes, Ketones & Carboxylic AcidsSHUBHAMNo ratings yet
- Practice Test 1 CHM2210C CH 1-3 PDFDocument13 pagesPractice Test 1 CHM2210C CH 1-3 PDFkara lane100% (1)
- Alkenes SeatworkDocument5 pagesAlkenes SeatworkJhefNo ratings yet
- Midterm II Key Chem 2312-003 F '12Document7 pagesMidterm II Key Chem 2312-003 F '12acb4039No ratings yet
- 314 Stereochem ProbsDocument14 pages314 Stereochem ProbsAtul SinghNo ratings yet
- 3.AcidBases FinalDocument35 pages3.AcidBases FinalSoham RaneNo ratings yet
- Substitution - EliminationDocument36 pagesSubstitution - EliminationSachin SinghalNo ratings yet
- ACS Review 15 Alcohols Diols and ThiolsDocument10 pagesACS Review 15 Alcohols Diols and ThiolsJana BazziNo ratings yet
- Welcome To Chem 206: Fall Term, 2005, David A. EvansDocument22 pagesWelcome To Chem 206: Fall Term, 2005, David A. EvanseraborNo ratings yet
- Organic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The SDocument9 pagesOrganic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The Ssweta KushwahaNo ratings yet
- Chapter 8 Nucleophilic Substitution: Answers Prof. Sivaguru JayaramanDocument16 pagesChapter 8 Nucleophilic Substitution: Answers Prof. Sivaguru JayaramanRahma AshrafNo ratings yet
- Question Bank Class Xii (Chemistry) Unit 5: Alcohols, Phenols & Ethers Multiple Choice QuestionsDocument21 pagesQuestion Bank Class Xii (Chemistry) Unit 5: Alcohols, Phenols & Ethers Multiple Choice QuestionsSahilNo ratings yet
- Alcohol, Ether & Phenol - QuestionDocument3 pagesAlcohol, Ether & Phenol - Questionbest badmintonNo ratings yet
- Cls Jeead-18-19 Xii Che Target-7 Set-2 Chapter-12Document47 pagesCls Jeead-18-19 Xii Che Target-7 Set-2 Chapter-12DxNo ratings yet
- (02-12-14) AlkenesDocument4 pages(02-12-14) Alkenessasi.curieNo ratings yet
- Directions: This Examination Contains A Total of 80 Multiple ChoiceDocument12 pagesDirections: This Examination Contains A Total of 80 Multiple ChoiceLemi NegesoNo ratings yet
- Practices Exam - Organic Chemistry To 2nd PartialDocument10 pagesPractices Exam - Organic Chemistry To 2nd PartialShary MosqueraNo ratings yet
- Organic Chemistry 231 Final ExamDocument19 pagesOrganic Chemistry 231 Final ExamAlex Rose100% (1)
- Goc + IsomerismDocument5 pagesGoc + IsomerismRohail HussainNo ratings yet
- My Faculty Is Downloading Question Paper Alkyl HalideDocument4 pagesMy Faculty Is Downloading Question Paper Alkyl HalidesanskritiNo ratings yet
- 70 Practice Problems For CH 7Document10 pages70 Practice Problems For CH 7ULFA TUFFAHATINo ratings yet
- Advanced Placement Chemistry TestDocument15 pagesAdvanced Placement Chemistry TestBobNo ratings yet
- Aromatic CompoundsDocument16 pagesAromatic CompoundsadityaNo ratings yet
- 235 Practice Exam 1Document11 pages235 Practice Exam 1bamforNo ratings yet
- ORGANIC20CHEMISTRY20POST20TESTDocument13 pagesORGANIC20CHEMISTRY20POST20TESTJan Mill100% (1)
- KPS Academy Chakwal: Encircle The Correct OptionDocument3 pagesKPS Academy Chakwal: Encircle The Correct Optionali raza chughtaiNo ratings yet
- Costco Vitamins USA 50Document3 pagesCostco Vitamins USA 50ankit3sharmaNo ratings yet
- Chemistry List of ExperimentDocument3 pagesChemistry List of ExperimentKaiswan GanNo ratings yet
- 01 Water Characteristics, Quality, and StandardsDocument28 pages01 Water Characteristics, Quality, and Standardsnihayatun nimahNo ratings yet
- Nowadays, Pesticide Are Not Only Expensive But Also Not Guaranteed To Be Safe. With TheDocument12 pagesNowadays, Pesticide Are Not Only Expensive But Also Not Guaranteed To Be Safe. With TheFery AnnNo ratings yet
- UOP Aromatics Paraxylene Capture Paper1Document16 pagesUOP Aromatics Paraxylene Capture Paper1thanga1981100% (1)
- Procedures For The Analysis of Explosives EvidenceDocument15 pagesProcedures For The Analysis of Explosives EvidenceGerman CarleNo ratings yet
- June 2010 MS - Unit 1 Edexcel Biology A-LevelDocument20 pagesJune 2010 MS - Unit 1 Edexcel Biology A-LevelAyse KerimNo ratings yet
- Basic Requirement For Dyes:: Department of Chemistry A.M.U., AligarhDocument14 pagesBasic Requirement For Dyes:: Department of Chemistry A.M.U., AligarhHarshita MayalNo ratings yet
- Ionic Liquids. J.D. Holbrey, K.R. Seddon PDFDocument14 pagesIonic Liquids. J.D. Holbrey, K.R. Seddon PDFMenelao ZubiriNo ratings yet
- LP CHY Fall 2010-2011Document4 pagesLP CHY Fall 2010-2011Mahmud ShaadNo ratings yet
- Metabolism 1Document41 pagesMetabolism 1mukesh100% (1)
- Shanto Mariam University of Creative Technology: Submitted ToDocument14 pagesShanto Mariam University of Creative Technology: Submitted ToMd Raim razzakNo ratings yet
- GENBIO Packet MELC 3 Q2Document3 pagesGENBIO Packet MELC 3 Q2Kenjie SobrevegaNo ratings yet
- AdditivsDocument40 pagesAdditivsMohsin MalikNo ratings yet
- Heterocyclic Compounds PDFDocument32 pagesHeterocyclic Compounds PDFUrugonda Venumadhav100% (6)
- Kosswell EN Low PDFDocument56 pagesKosswell EN Low PDFChaNo ratings yet
- Thermochemical Conversion of Sugarcane Bagasse by Fast Pyrolysis Highyield of Levoglucosan ProductionDocument8 pagesThermochemical Conversion of Sugarcane Bagasse by Fast Pyrolysis Highyield of Levoglucosan ProductionAlbert LimNo ratings yet
- Organic Pesticide With The Use of Hot Pepper and GarlicDocument6 pagesOrganic Pesticide With The Use of Hot Pepper and GarlicJen Julianna100% (1)
- Masruah - Biotek PanganDocument19 pagesMasruah - Biotek PangandindaNo ratings yet
- Synthesis and Identification of Some New Isoxazolidine Compounds by 1,3 - Dipolar Cycloaddition Reaction of Nitrones and Olefin by Faeza A. AlmashalDocument10 pagesSynthesis and Identification of Some New Isoxazolidine Compounds by 1,3 - Dipolar Cycloaddition Reaction of Nitrones and Olefin by Faeza A. AlmashalTropidinoNo ratings yet
- Organometallicco00wrenrich PDFDocument120 pagesOrganometallicco00wrenrich PDFSrinivas MadalaNo ratings yet
- 1 s2.0 S2590048X22001030 MainDocument17 pages1 s2.0 S2590048X22001030 MainJeff DatinguinooNo ratings yet
- AssignmentDocument4 pagesAssignmentSatyam SachanNo ratings yet
- Acquisition of Ipcl by RelianceDocument18 pagesAcquisition of Ipcl by RelianceHasmukh JadejaNo ratings yet
- Master GeneralDocument440 pagesMaster GeneralSovit PatnaikNo ratings yet
- The Arabinose Operon: Structure and MechanismDocument3 pagesThe Arabinose Operon: Structure and MechanismFildzah AdanyNo ratings yet
- PPR Pipes Fittings CatalogueDocument12 pagesPPR Pipes Fittings CatalogueGMak RealtyNo ratings yet
- Dowfax Muy BuenoDocument20 pagesDowfax Muy Buenohugogauna100% (1)
- Seqel Fe ReactionDocument2 pagesSeqel Fe ReactionAbhijit FarakteNo ratings yet
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Water-Based Paint Formulations, Vol. 3From EverandWater-Based Paint Formulations, Vol. 3Rating: 4.5 out of 5 stars4.5/5 (6)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Guidelines for Integrating Process Safety into Engineering ProjectsFrom EverandGuidelines for Integrating Process Safety into Engineering ProjectsNo ratings yet
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet