Professional Documents
Culture Documents
Pentadienyl Cation
Uploaded by
Abhishek SardaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pentadienyl Cation
Uploaded by
Abhishek SardaCopyright:
Available Formats
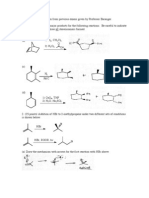
CHE 322 Spring 2008
Exam 1
Form 1: Page 1
Consider the following phenols.
OH
H3C
OH
OH
OH
OH
CH3
O 2N
CH3
CH3
a.
b.
c.
1.
Which of phenols is the strongest acid? e
2.
Which of phenols is the weakest acid? a
NO2
NO2
NO2
d.
e.
Consider the following monosubstituted benzenes.
NO2
(a)
CH3
(b)
(c)
CH3
O
(d)
(e)
3.
Which is the most activated with respect to bromination? e
4.
Which is the most deactivated with respect to bromonation? b
Consider the following addition reaction.
HCl
two products
Here are some possible products. (Chiral products would include their enantiomer.)
Cl
Cl
Cl
Cl
(a)
(b)
Cl
(c)
(d)
(e)
5.
Which compound would be the predicted kinetic product? c
6.
Which compound would be the predicted thermodynamic product? e
CHE 322 Spring 2008
Exam 1
Form 1: Page 2
Consider the following isomeric alkenes.
(a)
(b)
(d)
(e)
(c)
7.
Which compound would have an absorption band at the longest wavelength? b
8.
Which compound would release the most heat upon hydrogenation? (Which would have
the most negative H?) c
Consider the molecular orbitals of the pentadienyl cation.
pentadienyl cation
(a)
9.
(b)
(c)
(d)
Which one of these orbitals is the HOMO of the pentadienyl cation? d
10. Which one of these orbitals is the LUMO of the pentadienyl cation? a
11, Choose the correct name for the following compound.
a. (2Z,5Z)-hepta-2,5-diene
b. (2E,4E)-hepta-2,4-diene
c. (2Z,4E)-hepta-2,4-diene
d. (2E,5Z)-hepta-2,5-diene
e. (2Z,4Z)-hepta-2,4-diene
(e)
CHE 322 Spring 2008
Exam 1
Form 1: Page 3
12. Which one of the following cations is antiaromatic?
H
N
N
H
N
H
(a)
(b)
(c)
(d)
(e)
13. Which one of the following anions is antiaromatic?
(a)
(b)
(c)
(d)
(e)
14. Which of the following reaction sequences would be the best for synthesizing the
compound, 1-bromo-3-propylbenzene.
Br
1-Bromo-3-propylbenzene
O
a)
Br2
FeBr3
Zn(Hg)
Cl
AlCl3
HCl
O
b)
Cl
AlCl3
d)
Br2
Zn(Hg)
FeBr3
HCl
FeBr3
e)
Zn(Hg)
Br2
HCl
FeBr3
c)
AlCl3
Cl
Cl
AlCl3
Br2
AlCl3
O
Cl
Br2
FeBr3
CHE 322 Spring 2008
Exam 1
Form 1: Page 4
15. Predict the product of the following Diels Alder reaction.
O
H3C
H3C
O
C
H 3C
O
C
(a)
CH3
H3C
O
C
(b)
O
C
(c)
Equal
mixture
of all
four
(d)
(e)
16. Predict the monobromination product obtained from the following reaction.
O
(a)
Br2
N
H
Br
Br
N
H
O
O
FeBr3
(b)
(d)
Br
N
H
N
H
Br
O
(c)
N
H
(e)
N
H
Br
17. Draw a curved arrow mechanism for the following reaction.
H-F
F
H
CHE 322 Spring 2008
Exam 1
Form 1: Page 5
18. Complete the following synthetic roadmap. Give structures for compounds (a), (b) and (c).
Be sure to show all stereochemistry clearly.
O
O
TMS
OH
TMS
HF
TMS
2. H2O
a.
19.
OH
1. CH3Li
O
b.
c.
Propose a synthesis of the compound shown below. Your starting materials can include
benzene and any other compounds containing no more than four carbon atoms.
O
Cl
O
Cl2
Cl
AlCl3
AlCl3
Zn(Hg)
HCl
O
Cl
AlCl3
20. The Diels Alder reaction is an example of a cycloaddition reaction. Another possible
cycloaddition reaction is the reaction of an alkene with a allyl anion. Complete the MO
energy level interaction diagram shown below and determine if the reaction is allowed or
forbidden.
Complete the following steps:
a. Draw the MOs, ethene on the left, the allyl anion on the right.
b. Indicate which levels are occupied with electrons.
c. Label each MO as symmetric or antisymmetric by circling (S) or (A) for each MO.
d. Indicate if the reaction is allowed or forbidden.
CHE 322 Spring 2008
Exam 1
Form 1: Page 6
A or S
A or S
A or S
MOs
MOs
A or S
A or S
Energy levels
Check one:
Allowed _X__
Forbidden ___
Challenge Problem. This question is not part of the regular test. Do not take time to work it
until you have completed the rest of your exam.
The compound -bisabolene is a terpene which has been identified as a trail pheromone of
wood termites, reticulitermes lucifugus.
-bisabolene
reticulitermes lucifugus
Propose a synthesis of racemic -bisabolene. Your starting materials can have no more than
five (5) carbon atoms.
H
Cl
Cl
OH
1.
1. CH2=O
Li
2. H3O
HO
1. SOCl2
Li
2. H3O
2. Li
CHE 322 Spring 2008
Exam 1
Form 1: Page 7
You might also like
- EM Chem 2007Document8 pagesEM Chem 2007commonsensec88No ratings yet
- Aldehyde KetoneDocument5 pagesAldehyde Ketonehareharanbt22No ratings yet
- Organic Chemistry Practice Questions on Alkenes and HalidesDocument4 pagesOrganic Chemistry Practice Questions on Alkenes and Halidessowmmiya karuppiahNo ratings yet
- 12thchemistrysamplepaper1 291223044313Document9 pages12thchemistrysamplepaper1 291223044313aditikharb2020No ratings yet
- AldehydesDocument5 pagesAldehydeslove.mansijhaNo ratings yet
- CH1O3 Questions PDFDocument52 pagesCH1O3 Questions PDFPrince T MashandaNo ratings yet
- Previous Hse Questions and Answers of The Chapter "Hydrocarbons"Document10 pagesPrevious Hse Questions and Answers of The Chapter "Hydrocarbons"Muhammed SadiqNo ratings yet
- Introduction To Organic Chemistry Ii CHEM 224: Answer KeyDocument8 pagesIntroduction To Organic Chemistry Ii CHEM 224: Answer Keygautamtajesh1983No ratings yet
- A. 50 B. 30 C. 15 D. 5: 5.12 Practice Exam #4 PAGE 1 Short Questions 1-8 3 Points Each)Document7 pagesA. 50 B. 30 C. 15 D. 5: 5.12 Practice Exam #4 PAGE 1 Short Questions 1-8 3 Points Each)Armando Shehi SayhellotogoodbyeNo ratings yet
- CL CL: Hex-1-En-4-Yne or 1-Hexen-4-YneDocument4 pagesCL CL: Hex-1-En-4-Yne or 1-Hexen-4-YneSamuel Espinoza GarciaNo ratings yet
- Xii MotivationalDocument5 pagesXii MotivationalroobanNo ratings yet
- QUESTION BANK - CHEMISTRY XII - Checked 3Document5 pagesQUESTION BANK - CHEMISTRY XII - Checked 3JijendarNo ratings yet
- Class 12 R - 5 Set - 2Document4 pagesClass 12 R - 5 Set - 2santhosNo ratings yet
- Phy CheDocument11 pagesPhy CheVineeta MishraNo ratings yet
- 2008 Promo 1Document15 pages2008 Promo 1shinkir0No ratings yet
- 2nd Pre Board 2023Document8 pages2nd Pre Board 2023chiragNo ratings yet
- Final Exam (Sample Question Paper)Document5 pagesFinal Exam (Sample Question Paper)Salim MoniNo ratings yet
- Organic Chemistry I - Practice Exercise: Alkene Reactions and MechanismsDocument9 pagesOrganic Chemistry I - Practice Exercise: Alkene Reactions and MechanismsElliot JamesNo ratings yet
- SXHS XII (CHEM) P.T-2 Imp Questions 2023Document7 pagesSXHS XII (CHEM) P.T-2 Imp Questions 2023sampritmodiNo ratings yet
- SET PAPER 5 - CHEM Eklavya (XII-CBSE) 01.02.2024 FULL (WM)Document5 pagesSET PAPER 5 - CHEM Eklavya (XII-CBSE) 01.02.2024 FULL (WM)Rahul YadavNo ratings yet
- Chem Olympiad 2019 Exam Paper AnswersDocument9 pagesChem Olympiad 2019 Exam Paper AnswersPaulette LaurenteNo ratings yet
- CHEMISTRY Questions - 2019-20 - SET2Document7 pagesCHEMISTRY Questions - 2019-20 - SET2-Uddipan BagchiNo ratings yet
- Chem52 Su13 PracticeExam1ADocument11 pagesChem52 Su13 PracticeExam1Aamarka01No ratings yet
- Question Paper AnalysisDocument8 pagesQuestion Paper AnalysismjdNo ratings yet
- C331 S02 Test 1Document7 pagesC331 S02 Test 1api-19827675No ratings yet
- Revision Worksheet For Half Yearly Exam Chemistry From Basic Principles of Organic ChemistryDocument2 pagesRevision Worksheet For Half Yearly Exam Chemistry From Basic Principles of Organic ChemistryVrisanNo ratings yet
- Chemistry MQP Ii Puc 2023-24Document4 pagesChemistry MQP Ii Puc 2023-24Shruthi A R RamNo ratings yet
- Multiple Choice Questions on Organic Chemistry Functional Groups and Intermolecular ForcesDocument9 pagesMultiple Choice Questions on Organic Chemistry Functional Groups and Intermolecular ForcesVictor HuangNo ratings yet
- CHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsDocument15 pagesCHM 1321 Assignment 5 Answers: 1) Name The Following CompoundsSara Yuen100% (1)
- STPM Trials 2009 Chemistry Paper 2 (SMJK Sam Tet Ipoh)Document11 pagesSTPM Trials 2009 Chemistry Paper 2 (SMJK Sam Tet Ipoh)sherry_christyNo ratings yet
- CBSE Sample Paper Class 12 Chemistry Set 4Document5 pagesCBSE Sample Paper Class 12 Chemistry Set 4Sidharth SabharwalNo ratings yet
- Organic Chemistry II Problem Set #1Document4 pagesOrganic Chemistry II Problem Set #1OmarBilbeisiNo ratings yet
- 2nd PU Chemistry Model QP 2Document8 pages2nd PU Chemistry Model QP 2Prasad C M100% (1)
- Chemistry Theory (043) MM: 70 Time: 3hours: A) B) C) D)Document9 pagesChemistry Theory (043) MM: 70 Time: 3hours: A) B) C) D)Arun GuptaNo ratings yet
- Organic Chemistry Additional Problems Final Exam Part2Document6 pagesOrganic Chemistry Additional Problems Final Exam Part2John SmithNo ratings yet
- Chemistry SQP XII PDFDocument14 pagesChemistry SQP XII PDFIshikaGuptaNo ratings yet
- M. Phil Admission Test Organic Chemistry Sample PaperDocument11 pagesM. Phil Admission Test Organic Chemistry Sample PaperRabiaNo ratings yet
- Chemistry SQP PDFDocument8 pagesChemistry SQP PDFÀĺťhàf AnsariNo ratings yet
- Code:SP/LV-2 Sample Paper: General InstructionsDocument3 pagesCode:SP/LV-2 Sample Paper: General InstructionsKhogen MairembamNo ratings yet
- Additional Problems Final Exam Part 2 AnswersDocument10 pagesAdditional Problems Final Exam Part 2 AnswersJohn SmithNo ratings yet
- Chemistry 25481Document6 pagesChemistry 25481rojaramanibkNo ratings yet
- Chemsitry 09.12.2022Document4 pagesChemsitry 09.12.2022santhosNo ratings yet
- Chem106 Final Exam KeyDocument7 pagesChem106 Final Exam KeylavenchiNo ratings yet
- Chemistry Notes For Town BoysDocument5 pagesChemistry Notes For Town BoysArnabNo ratings yet
- 11th - Chemistry MaterialDocument12 pages11th - Chemistry Materialprathiksha6660No ratings yet
- 12 Revision TestDocument5 pages12 Revision TestHeartykingnkNo ratings yet
- ORGANIC CHEMISTRY TESTDocument4 pagesORGANIC CHEMISTRY TESTpritam neogiNo ratings yet
- CU-2020 B.Sc. (Honours) Chemistry Semester-III Paper-CC-7 QPDocument4 pagesCU-2020 B.Sc. (Honours) Chemistry Semester-III Paper-CC-7 QPbuntyckbtNo ratings yet
- Haloalkanes and Haloarenes Class 12 Chemistry MCQs PDFDocument33 pagesHaloalkanes and Haloarenes Class 12 Chemistry MCQs PDFSanjana Sanjay100% (1)
- Practise Paper - Chemistry - Class XI 2023-24Document6 pagesPractise Paper - Chemistry - Class XI 2023-24mysixthidisNo ratings yet
- Candidates Identification Number: Chemistry Olympiad QuestionsDocument9 pagesCandidates Identification Number: Chemistry Olympiad QuestionsEmma BongNo ratings yet
- Chemistry - Concepts and Multiple ChoiceDocument5 pagesChemistry - Concepts and Multiple ChoiceGeorge Isaac McQuiles100% (1)
- XI Mid Term QPDocument3 pagesXI Mid Term QPtechnical SiteNo ratings yet
- Organic Chemistry (Some Basic Principles and TechniquesDocument30 pagesOrganic Chemistry (Some Basic Principles and TechniquesNaveen SharmaNo ratings yet
- STPM Trials 2009 Chemistry Paper 1 (SMJK Sam Tet Ipoh)Document9 pagesSTPM Trials 2009 Chemistry Paper 1 (SMJK Sam Tet Ipoh)sherry_christyNo ratings yet
- Chemistry SET A QPDocument8 pagesChemistry SET A QPdahaka7609No ratings yet
- Plasma Chemistry: International Symposium on Plasma ChemistryFrom EverandPlasma Chemistry: International Symposium on Plasma ChemistryD. E. JensenNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- CL 306: Chemical Processes Spring Semester, 2016-17: Madhu@che - Iitb.ac - in Noronha@che - Iitb.ac - inDocument2 pagesCL 306: Chemical Processes Spring Semester, 2016-17: Madhu@che - Iitb.ac - in Noronha@che - Iitb.ac - inAbhishek SardaNo ratings yet
- DiameterDocument3 pagesDiameterAbhishek SardaNo ratings yet
- Tut2 SolnsDocument2 pagesTut2 SolnsAbhishek SardaNo ratings yet
- Experiment HT - 305: Plate Heat Exchanger: BackgroundDocument10 pagesExperiment HT - 305: Plate Heat Exchanger: BackgroundAbhishek SardaNo ratings yet
- Multi Linear Regression Handout 2x1Document67 pagesMulti Linear Regression Handout 2x1Abhishek SardaNo ratings yet
- Tut1 2016 QDocument5 pagesTut1 2016 QAbhishek SardaNo ratings yet
- Course Content CL305 2016 S1Document2 pagesCourse Content CL305 2016 S1Abhishek SardaNo ratings yet
- Distillation Separation TechniquesDocument12 pagesDistillation Separation TechniquesAbhishek SardaNo ratings yet
- Convective Mass Transfer PDFDocument14 pagesConvective Mass Transfer PDFAnonymous 4XZYsImTW5100% (1)
- Chemical Engineering Thermodynamics (CL 253) Mid-Semester Examination (2012)Document1 pageChemical Engineering Thermodynamics (CL 253) Mid-Semester Examination (2012)Abhishek SardaNo ratings yet
- Chemical Engineering Thermodynamics (CL 253) Mid-Semester Examination (2012)Document1 pageChemical Engineering Thermodynamics (CL 253) Mid-Semester Examination (2012)Abhishek SardaNo ratings yet
- Emulsion Coplymerization of Mma and BaDocument5 pagesEmulsion Coplymerization of Mma and BaAbhishek SardaNo ratings yet
- Apping GuideDocument29 pagesApping GuideAnirudh SubramanyamNo ratings yet
- Issue 1 January 2015 BMW Models Price List NewDocument1 pageIssue 1 January 2015 BMW Models Price List NewAbhishek SardaNo ratings yet
- Critical Diameter of InsulationDocument25 pagesCritical Diameter of InsulationSemihBaşkaleNo ratings yet
- ThermodynamicsDocument19 pagesThermodynamicsAbhishek SardaNo ratings yet
- Apping GuideDocument29 pagesApping GuideAnirudh SubramanyamNo ratings yet
- Culture IQ SternbergDocument8 pagesCulture IQ SternbergAbhishek SardaNo ratings yet
- PsychoDocument3 pagesPsychoAbhishek SardaNo ratings yet
- Transient Flow AnalysisDocument7 pagesTransient Flow AnalysisAbhishek SardaNo ratings yet
- Solution. 1: Homework 1Document14 pagesSolution. 1: Homework 1Abhishek SardaNo ratings yet
- Determination of The Molar Mass of A Volatile Liquid by Vapor DensityDocument5 pagesDetermination of The Molar Mass of A Volatile Liquid by Vapor DensityAbhishek SardaNo ratings yet
- R3000Document4 pagesR3000Abhishek SardaNo ratings yet
- The Golden HarvestDocument3 pagesThe Golden HarvestMark Angelo DiazNo ratings yet
- Technical Bro A4 UK LR NEW v2Document45 pagesTechnical Bro A4 UK LR NEW v2Roxana NegoitaNo ratings yet
- About Topsøe - and What We DoDocument20 pagesAbout Topsøe - and What We DoAbhishek ChaudharyNo ratings yet
- JA Ip42 Creating Maintenance PlansDocument8 pagesJA Ip42 Creating Maintenance PlansvikasbumcaNo ratings yet
- Explore Spanish Lesson Plan - AnimalsDocument8 pagesExplore Spanish Lesson Plan - Animalsapi-257582917No ratings yet
- PoiconverterDocument2 pagesPoiconvertertaco6541No ratings yet
- Eports: India's Defiance of Religious Freedom: A Briefing On Anti-Conversion' LawsDocument16 pagesEports: India's Defiance of Religious Freedom: A Briefing On Anti-Conversion' LawsGabriela StevensNo ratings yet
- Tender Evaluation Template GuideDocument15 pagesTender Evaluation Template GuideKhalid NaeemNo ratings yet
- Questions 32 - 34: Sunny English MqaDocument9 pagesQuestions 32 - 34: Sunny English MqaHạnh NguyễnNo ratings yet
- TR-Pharmacy Services NC IIIDocument135 pagesTR-Pharmacy Services NC IIIAljon Fortaleza Balanag100% (2)
- Ra 11223 PDFDocument34 pagesRa 11223 PDFNica SalazarNo ratings yet
- Description MicroscopeDocument4 pagesDescription MicroscopeRanma SaotomeNo ratings yet
- Topographic Map of Blooming GroveDocument1 pageTopographic Map of Blooming GroveHistoricalMapsNo ratings yet
- Thank you for purchasing your remap from HDI Tuning LtdDocument2 pagesThank you for purchasing your remap from HDI Tuning LtdMaks LebanNo ratings yet
- 1 API 653 Exam Mar 2015 MemoryDocument12 pages1 API 653 Exam Mar 2015 MemorymajidNo ratings yet
- FD-BF-001 Foxboro FieldDevices 010715 LowRes PDFDocument24 pagesFD-BF-001 Foxboro FieldDevices 010715 LowRes PDFThiago FernandesNo ratings yet
- Haier's Performance Management in Other CulturesDocument8 pagesHaier's Performance Management in Other CulturesSubhransu SahooNo ratings yet
- Solution Manual For Illustrated Guide To The National Electrical Code 7th Edition Charles R MillerDocument24 pagesSolution Manual For Illustrated Guide To The National Electrical Code 7th Edition Charles R MillerHenryJohnsonaswek97% (39)
- Anti-Anginal DrugsDocument39 pagesAnti-Anginal Drugspoonam rana100% (1)
- 2009 GCSE PE SpecificationsDocument225 pages2009 GCSE PE SpecificationsAdstasticNo ratings yet
- Morpho Full Fix 2Document9 pagesMorpho Full Fix 2Dayu AnaNo ratings yet
- SID-2AF User Manual English V3.04Document39 pagesSID-2AF User Manual English V3.04om_zahidNo ratings yet
- Tipolo WH Gantt ChartDocument15 pagesTipolo WH Gantt ChartMayeterisk RNo ratings yet
- 1F4 Catalog0808Document12 pages1F4 Catalog0808Edwin Ng0% (1)
- PanimulaDocument4 pagesPanimulaCharmayne DatorNo ratings yet
- Hmdu - EnglishDocument20 pagesHmdu - EnglishAbdulaziz SeikoNo ratings yet
- Metal Oxides Semiconductor CeramicsDocument14 pagesMetal Oxides Semiconductor Ceramicsumarasad1100% (1)
- 8483724Document24 pages8483724ejkiranNo ratings yet
- Marikina Development Corporation vs. FiojoDocument8 pagesMarikina Development Corporation vs. FiojoJoshua CuentoNo ratings yet
- MATH6113 - PPT5 - W5 - R0 - Applications of IntegralsDocument58 pagesMATH6113 - PPT5 - W5 - R0 - Applications of IntegralsYudho KusumoNo ratings yet