Professional Documents
Culture Documents
Crude Oil Desalting Process Removes Salts
Uploaded by
hassan zakwanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Crude Oil Desalting Process Removes Salts
Uploaded by
hassan zakwanCopyright:
Available Formats
Petroleum Refinery and Petrochemicals
Lecturer: Saad Nadeem
CH-406
Crude Oil Desalting
The salt content of the crude measured in pounds per thousand barrels (PTB) can be as high as
2000.
The salt content should be lowered to between 5.7 and 14.3 kg/1000 m3 (2and 5 PTB).
Poor desalting results in:
Salts deposit inside the tubes of furnaces and on the tube bundles of heat exchangers
creating fouling, thus reducing the heat transfer efficiency;
Corrosion of overhead equipment; and,
The salts carried with the products act as catalyst poisons in catalytic cracking units.
Types of Salts in Crude Oil:
The salts can also be present in the form of salts crystals suspended in the crude oil.
Mostly magnesium, calcium and sodium chlorides with sodium chloride being the abundant
type. These chlorides, except for NaCl, hydrolyze at high temperatures to hydrogen chloride:
NaCl does not hydrolyze. Hydrogen chloride dissolves in the overhead system water,
producing hydrochloric acid, an extremely corrosive acid.
Desalting Process
Water washing:
Water is mixed with the crude, dissolves salt crystals and the mixing distributes the salts into
the water, uniformly producing very tiny droplets. (added upto 20% by volume of crude).
Demulsifying:
Demulsifiers are basic copolymers with one end being hydrophilic (loves water and attaches to
the surface of the water droplet), and the other end being hydrophobic (loves the oil and is
directed to the oil side).
Demulsifying agents are added at this stage to aide in breaking the emulsion by removing the
asphaltenes from the surface of the droplets. (Added 3-10 ppm of crude).
Heating:
The crude oil temperature should be in the range of 48.954.4 C (120130 F)
Typical desalting temperature can vary between 50 and 150 C (122 and 302 F).
Petroleum Refinery and Petrochemicals
Lecturer: Saad Nadeem
CH-406
Coalescence:
Water droplets in the range of 110 mm, which do not settle by gravity.
Coalescence is accomplished through an electrostatic electric field between two electrodes
which ionizes the water droplets and orients them so that they are attracted to each other.
Agitation is also produced and aides in coalescence.
A typical desalter contains two metal electrodes as shown in Figure1. A high voltage is applied
between these two electrodes. For effective desalting the electric fields are applied as follows:
A high voltage field called the secondary field of about 1000 V/cm between the two
electrodes is applied. The ionization of the water droplets and coalescence takes place
here.
A primary field of about 600 V/cm between the watercrude interface and the lower
electrode is applied. This field helps the water droplets settle faster.
The desalter of this design achieves 90% salt removal.
However 99% salt removal is possible with two-stage desalters as shown in Figure 2 .
Fig. 1
Fig. 2
You might also like
- A Quick Look at DesaltingDocument12 pagesA Quick Look at Desaltingananth2012No ratings yet
- Sample Problem StatementDocument2 pagesSample Problem Statementsunildubey02No ratings yet
- 10.6. Liquid-Liquid Separation: 10.6.1. Decanters (Settlers)Document6 pages10.6. Liquid-Liquid Separation: 10.6.1. Decanters (Settlers)ashishkapoorsrmNo ratings yet
- Heat duty calculationDocument6 pagesHeat duty calculationwahyuriansyahNo ratings yet
- Bielectric DesaalterDocument2 pagesBielectric DesaalterDaniele CirinaNo ratings yet
- Year 4: DR - Mamdouh GadallahDocument14 pagesYear 4: DR - Mamdouh GadallahYasser AshourNo ratings yet
- Desalters 3Document2 pagesDesalters 3vishnumnair1No ratings yet
- Electrostatic Oil - Water Treater & Desalter: Calculated AnswersDocument2 pagesElectrostatic Oil - Water Treater & Desalter: Calculated AnswersLeonardo ChávezNo ratings yet
- Three Phase SeparatorDocument9 pagesThree Phase SeparatorboringNo ratings yet
- CalculationDocument13 pagesCalculationajit kumarNo ratings yet
- Desalter EfficiencyDocument3 pagesDesalter Efficiencykronos39zeusNo ratings yet
- Lecture 04c - Shortcut Exchanger Design ProcedureDocument47 pagesLecture 04c - Shortcut Exchanger Design Proceduresds0% (1)
- Desalting Agar Interface Level Control MIRO KinmanDocument4 pagesDesalting Agar Interface Level Control MIRO KinmanMatthew PhillipsNo ratings yet
- Sparger Calc MotDocument5 pagesSparger Calc MotRajesh NareNo ratings yet
- Module 2 - Oil and Gas Separation - LectDocument62 pagesModule 2 - Oil and Gas Separation - LectmahmoudNo ratings yet
- Understanding Heat Flux Limitations CCTI 2010Document8 pagesUnderstanding Heat Flux Limitations CCTI 2010B rgNo ratings yet
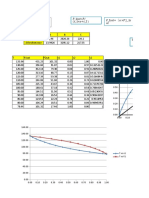
- Conc. vs. Enthalpy Plot.: 2.5 X y X y y XDocument8 pagesConc. vs. Enthalpy Plot.: 2.5 X y X y y XakhilNo ratings yet
- Equilibrium Vapor-Liquid CalculationsDocument8 pagesEquilibrium Vapor-Liquid Calculationsdhea novitaNo ratings yet
- Optimizing Gas/Liquid Separator PerformanceDocument14 pagesOptimizing Gas/Liquid Separator PerformanceWendellReeceFrankNo ratings yet
- Desalter Control PDFDocument2 pagesDesalter Control PDFmotalebyNo ratings yet
- Evaporation NewDocument64 pagesEvaporation NewshashwatNo ratings yet
- Oil/Water Separators: T P: O WDocument4 pagesOil/Water Separators: T P: O Wvgogulakrishnan100% (1)
- Vapor Pressure of A Liquid SolutionDocument40 pagesVapor Pressure of A Liquid Solutionintania660% (1)
- BN-EG-UE109 Guide For Vessel SizingDocument28 pagesBN-EG-UE109 Guide For Vessel Sizingeulalio_méndezNo ratings yet
- Spo 1Document89 pagesSpo 1Fidal SibiaNo ratings yet
- Desalter Operation OptimizationDocument3 pagesDesalter Operation OptimizationRexx MexxNo ratings yet
- Heat Exchanger Input Correction FactorDocument11 pagesHeat Exchanger Input Correction FactorTrần Tuấn VũNo ratings yet
- Optimize Crude Oil Desalting with Membrane FiltrationDocument14 pagesOptimize Crude Oil Desalting with Membrane FiltrationDucViking100% (1)
- Colligative Properties ExplainedDocument26 pagesColligative Properties ExplainedYAWAR SAEED100% (1)
- DesalterDocument2 pagesDesalterHimanshu SharmaNo ratings yet
- Packed Column Design GuideDocument32 pagesPacked Column Design GuideAnaBelenMedranoBarrientosNo ratings yet
- Volumes 2Document1 pageVolumes 2Rafael ReyesNo ratings yet
- Desalter OperationDocument19 pagesDesalter OperationmohammedNo ratings yet
- VGS Vane Separators Are Recommended ForDocument1 pageVGS Vane Separators Are Recommended ForSanthu PeelaNo ratings yet
- Horizontal Three Phase Separator Sizing CalculationDocument3 pagesHorizontal Three Phase Separator Sizing CalculationDazzy 265No ratings yet
- CentrifugalDocument8 pagesCentrifugalKwang Je LeeNo ratings yet
- Cooling Tower ComparisonDocument4 pagesCooling Tower ComparisonKiran DasNo ratings yet
- Gas Processing ProblemDocument2 pagesGas Processing ProblemEngr Anees AhmadNo ratings yet
- Natural Gas Processing Separator TechniquesDocument37 pagesNatural Gas Processing Separator TechniquestahaNo ratings yet
- Process Description and ASPEN Computer Modelling oDocument32 pagesProcess Description and ASPEN Computer Modelling omehul10941No ratings yet
- Separator S-101: Vessel Diameter and HeightDocument29 pagesSeparator S-101: Vessel Diameter and HeightAjeng FadillahNo ratings yet
- Principles of Separation: Production and Test SeparatorsDocument28 pagesPrinciples of Separation: Production and Test SeparatorsهانيزايدNo ratings yet
- Conventional SeparatorsDocument27 pagesConventional SeparatorsMax Singh100% (1)
- Two Phase (Gas - Oil) Vertical Separator: As Per "Petroleum and Gas Field Processing - Hussein K. Abdel-Aal, Mohamed Aggour, M. A. Fahim"Document1 pageTwo Phase (Gas - Oil) Vertical Separator: As Per "Petroleum and Gas Field Processing - Hussein K. Abdel-Aal, Mohamed Aggour, M. A. Fahim"Vu TranNo ratings yet
- Sizing Sheet for 2-phase separator as per API 12J standardsDocument5 pagesSizing Sheet for 2-phase separator as per API 12J standardsWickyNo ratings yet
- Catalyst Enthalpy Hydrogen PeroxideDocument2 pagesCatalyst Enthalpy Hydrogen PeroxideAna GonzálezNo ratings yet
- Water Requirement in Chemical Process IndustriesDocument145 pagesWater Requirement in Chemical Process IndustriesKAUSTAV ROYNo ratings yet
- Losses in Pipe BendsDocument5 pagesLosses in Pipe BendsLove KumarNo ratings yet
- Calculos DemisterDocument2 pagesCalculos DemistermoviedohNo ratings yet
- Cvts - Tag No. Mpt-tv-4161Document2 pagesCvts - Tag No. Mpt-tv-4161biswasdipankar05No ratings yet
- Crude Oil Desalting ProcessDocument24 pagesCrude Oil Desalting ProcessamirejazNo ratings yet
- Optimize Desalter Operation for Salt RemovalDocument4 pagesOptimize Desalter Operation for Salt RemovalVangapanduSrinivasaraoNo ratings yet
- American Society of Mechanical Engineers Standard Boilers Pressure VesselsDocument8 pagesAmerican Society of Mechanical Engineers Standard Boilers Pressure VesselsPedro Oporto IIINo ratings yet
- Calculation Sheet For Make - Up Water Tank: #Value!Document4 pagesCalculation Sheet For Make - Up Water Tank: #Value!thanh_79No ratings yet
- Process Design: Vessel Sizing (Liquid & Vapour Separators)Document36 pagesProcess Design: Vessel Sizing (Liquid & Vapour Separators)Krishanu SahaNo ratings yet
- Preliminary Design of Sodium Carbonate Plant: Darft Assignment 2Document69 pagesPreliminary Design of Sodium Carbonate Plant: Darft Assignment 2Ardina Ayu WulandariNo ratings yet
- OIL-SU-02 Rev 0Document34 pagesOIL-SU-02 Rev 0HosseinNo ratings yet
- Crude Oil DesaltingDocument5 pagesCrude Oil DesaltingAhmed Mohamed Khalil0% (1)
- Oshun BrochureDocument13 pagesOshun Brochurehassan zakwanNo ratings yet
- Information Required For ESIA - Gie - 2018!01!18Document11 pagesInformation Required For ESIA - Gie - 2018!01!18hassan zakwanNo ratings yet
- Petroleum RefiningDocument74 pagesPetroleum Refiningmaidaimarine100% (1)
- Full TPost-Combustion CO2 Capture Using Chemical Absorptionext 01Document108 pagesFull TPost-Combustion CO2 Capture Using Chemical Absorptionext 01hassan zakwanNo ratings yet
- Performance and Reliability Improvements For The Ms5001 Gas TurbinesDocument39 pagesPerformance and Reliability Improvements For The Ms5001 Gas TurbinesMuhammad Irfan AnwarNo ratings yet
- Line Practice Concrete: Effect of Eccentric Loading of ColumnsDocument3 pagesLine Practice Concrete: Effect of Eccentric Loading of Columnsgnanasekar007No ratings yet
- 12 18 86Document13 pages12 18 86adrian2009-2020No ratings yet
- AcResin - The Acrylic HotmeltDocument12 pagesAcResin - The Acrylic HotmeltRajNo ratings yet
- Supercritical Fluid Chromatography and ExtractionDocument51 pagesSupercritical Fluid Chromatography and ExtractionJaya Krishna Choudary VelagapudiNo ratings yet
- Is 204-2 1992Document10 pagesIs 204-2 1992vpmohammedNo ratings yet
- Vince Delmonte - Non Nonsense Muscle Building HRT-1 ProtocolDocument11 pagesVince Delmonte - Non Nonsense Muscle Building HRT-1 ProtocolAnonymous QDX3ksFCeB50% (4)
- Corporate Induction Training Programme On Reactor Engineering (NE-04)Document34 pagesCorporate Induction Training Programme On Reactor Engineering (NE-04)Sourav BasakNo ratings yet
- Osmotic Dehydration of Fruits and Vegetables - Review PDFDocument20 pagesOsmotic Dehydration of Fruits and Vegetables - Review PDFRoque VirgilioNo ratings yet
- Soil Fertility and Nutrient ManagementDocument59 pagesSoil Fertility and Nutrient ManagementChandran RnNo ratings yet
- Solution Manual ThermodynamicsDocument0 pagesSolution Manual ThermodynamicsVigna Ruban Ram100% (1)
- Deinking of Waste Paper - FlotationDocument8 pagesDeinking of Waste Paper - FlotationMiko Still SpeedinNo ratings yet
- Some Thoughts On Chute Design PhilosophyDocument3 pagesSome Thoughts On Chute Design PhilosophyneilradcliffeNo ratings yet
- 5.well PerforationDocument13 pages5.well Perforationakshitppe11No ratings yet
- Mohr CircleDocument19 pagesMohr Circlemortaza7094No ratings yet
- Avk Y-Strainer, PN 16 910/21: N50-300, A2 Plug and Fasteners 001Document3 pagesAvk Y-Strainer, PN 16 910/21: N50-300, A2 Plug and Fasteners 001AgieYogaswaraNo ratings yet
- Experiment 4 - Potentiometric TitrationDocument11 pagesExperiment 4 - Potentiometric TitrationJoemer Absalon Adorna100% (2)
- All Unit Cells Consist of The: Ideal Arrangement of Atoms or Molecules Specific OrderDocument75 pagesAll Unit Cells Consist of The: Ideal Arrangement of Atoms or Molecules Specific Orderkhare_girishNo ratings yet
- Syllabus G9 Term 1 Dec 23Document10 pagesSyllabus G9 Term 1 Dec 23zaynab.z.sidNo ratings yet
- Aurora StoryDocument4 pagesAurora Storyapi-255055950No ratings yet
- In-Situ Ophthalmic GelDocument16 pagesIn-Situ Ophthalmic Gelkscri100% (5)
- Zinc OxideDocument1 pageZinc OxideAlexi Del Castillo MustaineNo ratings yet
- Hawk Sensor Error Codes Rev - DDocument8 pagesHawk Sensor Error Codes Rev - Ddewsq5062No ratings yet
- Clinical Chemistry ImmunoassaysDocument23 pagesClinical Chemistry ImmunoassaysVincent ReyesNo ratings yet
- Adhesion Testing MethodDocument3 pagesAdhesion Testing MethodMohd Effiezool YaserNo ratings yet
- Advanced Mock Test Liquid Solutions With AnswersDocument8 pagesAdvanced Mock Test Liquid Solutions With AnswersSai KrishnaNo ratings yet
- Tech Bull 210-1-Liquid ARDocument2 pagesTech Bull 210-1-Liquid ARMichelle KeatingNo ratings yet
- Xi Neet Iseet Chemistry PDFDocument21 pagesXi Neet Iseet Chemistry PDFFATHIMA80% (5)
- Design GuideDocument68 pagesDesign GuiderdsrajNo ratings yet
- Msds Colonial Monolaurin (16 Section)Document3 pagesMsds Colonial Monolaurin (16 Section)mndmattNo ratings yet
- SGC - Commodities & HS Codes - AJCEPT VJEPA 2015 From April 2015Document2 pagesSGC - Commodities & HS Codes - AJCEPT VJEPA 2015 From April 2015Nguyen Thanh PhuongNo ratings yet