Professional Documents
Culture Documents
12 115 PDF
Uploaded by
JacobMsangOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
12 115 PDF
Uploaded by
JacobMsangCopyright:
Available Formats
The Cleft Palate-Craniofacial Journal 50(6) pp.
730733 November 2013
Copyright 2013 American Cleft Palate-Craniofacial Association
ORIGINAL ARTICLE
Role of Facial Artery Musculomucosal Flap in Large and Recurrent
Palatal Fistulae
Rahul Shetty, M.B.B.S., M.S., M.Ch., Shashank Lamba, M.B.B.S., M.S., M.Ch., Ashish Kumar Gupta, M.B.B.S., M.S., M.Ch.
Objective: Palatal fistulas are not uncommon after palatoplasty. Although there are currently
many techniques that can be used to close large palatal fistulae, most of these procedures are
usually cumbersome and mostly unreliable with high recurrence rates. The facial artery
musculomucosal (FAMM) flap was described to circumvent these problems. The purpose of this
study was to review our experience with the FAMM flap to reconstruct palatal fistulas, most of
them being recurrent.
Materials and Methods: A retrospective analysis was done of 11 FAMM flaps performed
between January 2007 and March 2012.
Results: There were no major complications. Venous congestion was seen in two cases. Two
flaps developed terminal marginal necrosis. One patient had suture line dehiscence. There were
no recurrences of the fistula after repair. All patients had a satisfactory closure of the fistula.
Conclusion: FAMM flap is a reliable and versatile flap that provides like with the like tissue
and is a good option for closure of recurrent wide palatal fistulae.
KEY WORDS:
palatal fistulas
cleft palate surgery complications, facial artery musculomucosal flap (FAMM flap),
turnover ap is usually used. In cases of mid palatal
stulas with adequate surrounding soft tissue, a redo
palatoplasty is a good option. For large stulas, various
aps have been described including cheek/buccal
mucosa ap (Mukherji, 1969), tongue ap (GuerreroSantos and Altamirano, 1968), facial artery musculomucosal (FAMM) ap (Pribaz et al., 1992), and free
tissue transfer (Chen et al., 1992).
The FAMM ap was rst described by Pribaz et al. in
1992, and because of its versatility and reliability, it was
seen as a possible solution to this difcult problem.
Some studies have also assessed its use in oral cavity
reconstructions, with most of them being after oral
malignancy excisions (Joshi et al., 2005; Bianchi et al.,
2009). The vascular basis of the facial artery ap has
already been well described in the literature (Musgrave
and Bremner, 1960; Pribaz et al., 1992; Dupoirieux et
al., 1999; Lahiri and Richard, 2007). The FAMM ap
contains buccal mucosa, submucosa, part of the
buccinators, and facial artery with its venous plexus.
It is reliable both as a superiorly based or inferiorly
based ap. When stulas are in the anterior half of the
hard palate, superiorly based aps are used. For stulas
in the rest of the hard palate, inferiorly based aps are
used (Lahiri and Richard, 2007).
We present our experience with FAMM ap for closure
of palatal stulas. The aim was to assess the reliability and
associated complications of palatal stula closure with the
FAMM ap.
Palatal stulas are common and complicated sequelae of
cleft palate surgery. About 0% to 45% of all palatal cleft
surgeries result in a palatal stula (Musgrave and Bremner,
1960; Cohen et al., 1991; Losken et al., 2011). The problem
is compounded when 25% to 34% of palatal stula
surgeries result in recurrence (Cohen et al., 1991; Emory et
al., 1997; Muzaffar et al., 2001). Closure of palatal stula is
difcult because of lack of surrounding soft tissue in the
palate and excessive scarring due to previous surgeries.
Provision of fresh, unscarred, and well-vascularized tissue
in the form of a regional ap can be invaluable in treating
these stulas. The main goals of reconstruction of these
stulas are to restore internal oral lining, preserve or
improve the function of residual structures, replace mucosa
with tissues having similar features, and have the best
esthetic result possible.
A variety of surgical techniques have been suggested
for the closure of the stulas depending on their size and
location. Based on their size, stulas were classied as
small (,2 mm), medium (2 to 5 mm) and large (.5 mm;
Cohen et al, 1991). For small palatal stulas, a local
Dr. Rahul Shetty is Senior Postgraduate Registrar, Dr.
Shashank Lamba, is Assistant Professor, and Dr. Ashish Kumar
Gupta is Professor and Head, Department of Plastic and
Reconstructive Surgery, Christian Medical College, Vellore, India.
Submitted May 2012; Accepted November 2012.
Address correspondence to: Dr. Rahul Shetty, Department of
Plastic and Reconstructive Surgery, Christian Medical College,
Vellore-632004, TN, India. E-mail rahulplastic@hotmail.com.
DOI: 10.1597/12-115
730
Shetty et al., ROLE OF FAMM FLAP IN PALATAL FISTULAE
MATERIALS
AND
731
METHODS
We performed a retrospective analysis of FAMM aps
done between January 2007 and March 2012. Medical
records of these patients were checked and data reviewed
for demographic prole, complaints, etiology of stula, size
of stula, treatment, and complications. All aps were done
by the same surgeon. Fully informed consents were
obtained prior to surgery and principles outlined in the
Declaration of Helsinki were followed.
Operative Technique
The procedure was performed under general anesthesia. The nasal lining was repaired with turnover
aps (Fig. 1). These aps were designed to be larger
than the defect. The course of the facial artery was
marked with a handheld Doppler. The parotid duct
was identied and marked. This prevents accidental
injury to the duct. The ap was marked medial to the
duct, which limits the posterior extent of the ap. The
anterior ap marking starts 1 cm posterior to the oral
commissure. The size of the ap was tailored proportionally to the size of the defect. The width of the ap
was kept to about 2 to 2.5 cm to avoid tension in the
closure of the donor site. An initial incision was made 1
cm posterior to the oral commissure to identify the
superior labial artery, which could be traced back to
the facial artery. The incision was deepened through
the buccal mucosa, submucosa, and underlying muscles (buccinators and a small portion of the orbicularis
oris near the commissure) into the layer of buccal fat.
The ap was dissected in a retrograde or antegrade
manner depending on the site of stula, maintaining
the vessels in a central position in the ap (Fig. 2). The
ap was then swung over a pivot at the base of the
pedicle and used to cover the defect. Care was taken to
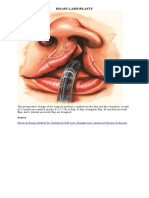
FIGURE 1 a: Palatal stula with nasal turnover ap marked on
adjacent normal tissue. Raised FAMM ap seen near oral commissure.
FIGURE 2 Facial artery identied in the ap and maintained in central
position throughout length of ap.
avoid kinking or twisting the pedicle. In the case of
superior-based aps, the pedicle was guided into the
oral cavity either by bridging over the dentition or
through a gap in the dentition if present. In the case of
inferiorly based aps, the pedicle was positioned to
cover the defect through the retromolar fossa.
Once completely raised, the ap was inset and the
donor site was closed primarily with 4-0 polyglactin
(Vicryl) interrupted sutures.
Bite block was used postoperatively in all cases.
Patients were put on a soft diet for 1 week and
discharged after 48 to 72 hours. The results were
assessed for anatomical continuity during follow-up
ofce visits (Figs. 3a and 3b).
RESULTS
A total of 11 patients were treated with the FAMM
ap. The average age of the patients was 12 years. There
were seven boys (63.63%) and four girls (36.36%). Nine
(81.1%) of the stulas were due to cleft palate surgery,
one was due to dental cyst excision, and one was due to
tumor excision (i.e., juvenile angiobroma). Nine
(81.8%) of the patients had recurrent stulas. The
stulae were between 1 and 2 cm in size. Nine of the
patients complained of discomfort due to nasal
regurgitation. Three (27.27%) aps were inferiorly
based and eight (72.72%) superiorly. Two aps
developed venous congestion (,4 to 6 hours), which
resolved on its own without any active intervention
(,48 hours). Two aps (18.8%) developed terminal
marginal necrosis. One ap (9.9%) developed suture
line dehiscence. All aps were divided and inset at 3
weeks. All our patients were followed up for a period
ranging from 6 months to 2 years, with a mean followup of 8 months. Satisfactory closure of the stula was
obtained in all patients (Table 1).
732
Cleft Palate-Craniofacial Journal, November 2013, Vol. 50 No. 6
FIGURE 3 a: Preoperative picture of patient with a wide anterior stula. b: After Reconstruction with a FAMM ap.
DISCUSSION
Closure of a hard palatal stula is a challenging problem.
In the literature, recurrence after palatal stula closure has
been reported to be 25% to 33% (Reid, 1962; Schultz, 1986;
Cohen et al., 1991). Risk of stula increases with every
failure. Therefore, all attempts should be made for
successful palate repair during the rst surgery. It is
desirable to repair palatal stulas in two well-vascularized
layers (separate nasal and oral) without tension (Mathes et
al., 2005). Attempts at repair with local palatal tissue can be
difcult because of the scarcity of tissue and residual
scarring from previous attempts at closure. Different
surgical techniques are described for closure of large palatal
stulas such as buccal ap, buccal pad ap, tongue ap, and
FAMM ap.
FAMM ap is considered a better option over other
musculomucosal aps by its axial pattern blood supply
and minimal donor site morbidity (Pribaz et al., 1992).
However, ap elevation can be technically challenging.
The tongue ap described by Guerrero-Santos and
Altamirano (1966) has been considered for recurrent
defects. It provides a large source of tissue for closure of
TABLE 1
problematic palatal stulas. A second stage surgery is
required for ap division usually after 3 weeks. In
addition, the texture, color, and consistency of the
tongue ap makes it less than ideal for palatal repair
(Mathes et al., 2005). The buccal mucosal ap is a
random-pattern ap and hence unreliable. It also
cannot be used for anterior stulas (Freedlander,
1989), and the tissue provided by this ap is limited
for large stulas. The buccal fat pad is another useful
option in managing mid and posterior palatal stulae.
Ashtiani et al. (2011) reported managing 28 of the 29
patients with palatal stula with the buccal fat pad ap.
As this ap is useful mainly for junctional stulae, it is
less versatile as compared with the FAMM ap.
Microvascular free-tissue transfer such as radial forearm ap (Chen et al., 1992) has been used to achieve
closure of particularly large palatal defects resistant to
other methods. However, use of free aps requires
competence in microsurgery, long operative time, and
prolonged hospitalization. It may also lead to donor site
morbidity and esthetically unsatisfactory results (Bianchi et al., 2009).
Patient Details
Sl No.
Age/Sex
Indication*
Location/size
Type of Flap
Postoperative Complications
1
2
3
4
5
6
7
8
9
10
11
4 years/F
16 years/M
39 years/M
16 years/M
23 years/F
12 years/F
14 years/M
7 years/F
11 years/M
16 years/M
15 years/M
UCLP
BCLP
Dental cyst excision
BCLP
CP
BCLP
CP
CP
BCLP
UCLP
Angiofibroma excision
Anterior fistula, 1.5 cm2
Anterior fistula, 1 cm2
Anterior fistula, 1.2 cm2
Midpalatal fistula, 2 cm2
Anterior fistula, 1.8 cm2
Posterior fistula, 1.2 cm2
(Group 3) anterior fistula, 1 cm2
Posterior fistula, 1.2 cm2
Posterior fistula, 2 cm2
Anterior fistula, 1 cm2
Posterior fistula, 1.9 cm2
Inferiorly based
Superiorly based
Superiorly based
Inferiorly based
Inferiorly based
Superiorly based
Superiorly based
Superiorly based
Superiorly based
Superiorly based
Superiorly based
Nil
Venous congestion
Distal third necrosis
Suture line dehiscence
Nil
Nil
Nil
Nil
Distal third necrosis
Venous congestion
Nil
* UCLP unilateral cleft and palate; BCLP bilateral cleft and palate; CP cleft of palate.
Final Outcome
Completely
Completely
Completely
Completely
Completely
Completely
Completely
Completely
Completely
Completely
Completely
healed
healed
healed
healed
healed
healed
healed
healed
healed
healed
healed
Shetty et al., ROLE OF FAMM FLAP IN PALATAL FISTULAE
None of the 11 patients in our study had any major
complications. Two of the aps developed venous
congestion in the immediate postoperative period,
which resolved without any active intervention. This
venous congestion is a common occurrence in FAMM
aps, as has been mentioned in the literature. Dupoirieux et al. (1999) mentioned that the vein follows the
artery at a variable distance of about 15 mm in the upper
part of the ap at the level the of ala nasi and at a lesser
distance (approximately 4 mm) near the mandible.
Some studies (Janfaza et al., 2001; Bianchi et al., 2009)
have indicated that venous drainage is by a plexus of
veins and not a single vein. Keeping these factors in
mind, we keep a broad base for all our aps, which
ensures adequate venous drainage. Two patients had a
marginal ap necrosis, which we attributed to failure to
centralize the artery in the ap over the entire course.
These aps healed by mucosalization and did not need
any further intervention. One patient had a suture line
dehiscence, probably due to the thinned mucosa to
which the ap was sutured. The ap was subsequently
advanced and resutured, following which the stula
healed uneventfully.
The base of the ap in our study was kept 2 to 2.5 cm
wide to ensure adequate venous drainage and ap
success. We use the FAMM ap for the oral lining after
the nasal lining is closed with a local turnover ap. This
step is of paramount importance in preventing recurrent
stulae. These turnover aps are designed to be larger
than the defect because the scarred palatal mucosa is
inelastic and covers less area than it appears when the
ap is designed. Preoperative identication of the
parotid duct is important to prevent its inadvertent
injury during ap elevation. Initial dissection of the
superior labial artery rst makes it easy to identify the
facial artery as it is the most important constant
collateral of the facial artery (Dupoirieux et al., 1999).
Overall results in our study were satisfactory.
CONCLUSION
The FAMM ap is a reliable and versatile ap that
provides like with the like tissue and is a good option for
closure of recurrent stulae of the hard palate. Adaptations
in technique including a two-layer closure of the stula and
identication of the facial artery with its inclusion along the
entire length of a sufciently wide pedicle will ensure success
of the ap.
733
REFERENCES
Anastassov GE, Schwartz S, Rodriguez E. Buccinator myomucosal
island ap for postablative maxillofacial reconstructions: a report
of 4 cases. J Oral Maxillofac Surg. 2002;60:816821.
Ashtiani AK, Bohluli B, Kalantar Motamedi MH, Fatemi MJ,
Moharamnejad N. Effectiveness of buccal fat in closing residual
midpalatal and posterior palatal stulas in patients previously
treated for clefts. J Oral Maxillofac Surg. 2011;69:e416e419.
Bianchi B, Ferri A, Ferrari S, Copelli C, Sesenna E. Myomucosal
cheek aps: applications in intraoral reconstruction using three
different techniques. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 2029;108:353359.
Chen HC, Ganos DL. Coessens BC, Kyutoku S, Noordhoff MS. Free
radial forearm ap for closure of difcult oronasal stulas in cleft
palate patients. Plast Reconstr Surg. 1992;90:757762.
Cohen SR, Kalinowski J, La Rossa D, Randall P. Cleft palate stulas:
a multivariate statistical analysis of prevalence, aetiology and
surgical management. Plast Reconstr Surg. 1991;87:10411047.
Dupoirieux L, Plane L, Gard C, Penneau M. Anatomical basis and
results of the facial artery musculomucosal ap for oral reconstruction. Br J Oral Maxillofac Surg. 1999;37:2528.
Emory RE, Clay RP, Bite U, Jackson IT. Fistula formation and repair
after palatal closure: an institutional perspective. Plast Reconstr
Surg. 1997;99:15351538.
Freedlander E. The fate of buccal mucosal aps in primary palatal
repair. Cleft Palate J. 1989;26:110112.
Guerrero-Santos J, Altamirano JT. The use of lingual aps in repair of
stulae of the hard palate. Plast Reconstr Surg. 1966;38:123128.
Janfaza P, Nadol JB, Galla RJ, Fabian RL, Montgomery WW.
Surgical Anatomy of the Head and Neck. Philadelphia: Lippincott
Williams and Wilkins; 2001.
Joshi A. Rajendrapasad JS, Shetty K. Reconstruction of intraoral
defects using facial artery musculomucosal ap. Br J Plast Surg.
2005;58:10611066.
Lahiri A, Richard B. Superiorly based facial artery musculomucosal
ap for large anterior palatal stulae in clefts. Cleft Palate
Craniofac J. 2007;44:523527.
Losken W, Aalst JV, Teotia SS. Achieving low stula rates: surgical
results and techniques. Craniofac J. 2011;48:312320.
Mathes SJ, Weinberger SE, Hentz VR. Plastic Surgery. 2nd ed.
Philadelphia: WB Saunders; 2005.
Mukherji MM. Cheek ap for short palates. Cleft Palate Craniofac J.
1969;6:415420.
Musgrave RH, Bremner JC. Complications of cleft palate surgery.
Plast Reconstr Surg. 1960;26:180189.
Muzaffar AR, Byrd HS, Rohrich RJ, Johns DF, LeBlanc D, Beran SJ,
Anderson C, Papaioannou AA. Incidence of cleft palate stula: an
institutional experience with twostage palatal repair. Plast
Reconstr Surg. 2001;108:15151518.
Pribaz J, Stephens W, Crespo L, Gifford G. A new intraoral ap:
facial artery musculomucosal FAMM) ap. Plast Reconstr Surg.
1992;90:421429.
Reid DAC. Fistulae in the hard palate following cleft palate surgery.
Br J Plast Surg. 1962;15:377384.
Schultz RC. Management and timing of cleft palate stula repair.
Plast Reconstr Surg. 1986;78:739747.
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Operating Room Surgical ProceduresDocument197 pagesOperating Room Surgical ProceduresChin Chan100% (6)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Pile Foundation As Per IRC 112Document59 pagesPile Foundation As Per IRC 112ARVIND SINGH RAWAT0% (1)
- Three-Phase SCR Battery Charger Installation GuideDocument20 pagesThree-Phase SCR Battery Charger Installation GuideJohnTPNo ratings yet
- Case Study - BronchopneumoniaDocument45 pagesCase Study - Bronchopneumoniazeverino castillo91% (33)
- General Indications: AnticoagulantsDocument15 pagesGeneral Indications: AnticoagulantswahidNo ratings yet
- Fabrication and Installation of Vertical Steel Pressure ShaftDocument23 pagesFabrication and Installation of Vertical Steel Pressure ShaftPasan RajasingheNo ratings yet
- The 4 Main Phases of Wound HealingDocument2 pagesThe 4 Main Phases of Wound HealingJacobMsangNo ratings yet
- Catatan Harian Seorang Mahasiswa KedokteranDocument14 pagesCatatan Harian Seorang Mahasiswa KedokteranJacobMsangNo ratings yet
- 017 UrologyDocument544 pages017 UrologyNashaat H. Alshawabkeh100% (1)
- Sepsis Update 2018Document32 pagesSepsis Update 2018putri endah100% (1)
- Teknik Skening USG AbdomenDocument5 pagesTeknik Skening USG AbdomenJacobMsangNo ratings yet
- PDFDocument25 pagesPDFJacobMsangNo ratings yet
- Clinical Anatomy of The Vulva, Vagina, Lower Pelvis, and PerineumDocument20 pagesClinical Anatomy of The Vulva, Vagina, Lower Pelvis, and PerineumJacobMsangNo ratings yet
- Contoh Pidato Bahasa Inggris Tentang Perpisahan Sekolah Dan ArtinyaDocument3 pagesContoh Pidato Bahasa Inggris Tentang Perpisahan Sekolah Dan ArtinyaJacobMsangNo ratings yet
- 80 118 2 PB PDFDocument7 pages80 118 2 PB PDFJacobMsangNo ratings yet
- 036 PDFDocument24 pages036 PDFJacobMsangNo ratings yet
- PDFDocument25 pagesPDFJacobMsangNo ratings yet
- 125 205 1 SM PDFDocument5 pages125 205 1 SM PDFJacobMsangNo ratings yet
- 10 5923 J Surgery 20140301 03 PDFDocument4 pages10 5923 J Surgery 20140301 03 PDFJacobMsangNo ratings yet
- 12 115 PDFDocument4 pages12 115 PDFJacobMsangNo ratings yet
- 10 1 1 432 9764 PDFDocument4 pages10 1 1 432 9764 PDFJacobMsangNo ratings yet
- Achieving Adequate Bowel Length For Anastomosis After A Left Colonic ResectionDocument3 pagesAchieving Adequate Bowel Length For Anastomosis After A Left Colonic ResectionJacobMsangNo ratings yet
- 0365 0596 Abd 90 02 0258 PDFDocument3 pages0365 0596 Abd 90 02 0258 PDFJacobMsangNo ratings yet
- PDFDocument17 pagesPDFJacobMsangNo ratings yet
- Anesthesia, Regional, Digital BlockDocument15 pagesAnesthesia, Regional, Digital BlockJacobMsangNo ratings yet
- Rumus Menghitung Balance CairanDocument1 pageRumus Menghitung Balance CairanHadi KusumahNo ratings yet
- Anatomical Steps of ThyroidectomyDocument7 pagesAnatomical Steps of ThyroidectomyJacobMsangNo ratings yet
- Download Video Pemeriksaan Fisik Internal MedicineDocument8 pagesDownload Video Pemeriksaan Fisik Internal MedicineJacobMsangNo ratings yet
- Anesthesia, Regional, Digital BlockDocument15 pagesAnesthesia, Regional, Digital BlockJacobMsangNo ratings yet
- Facial Scar Removal and Dog Bite TreatmentDocument3 pagesFacial Scar Removal and Dog Bite TreatmentJacobMsangNo ratings yet
- Nasal Defect After Mohs SurgeryDocument2 pagesNasal Defect After Mohs SurgeryJacobMsangNo ratings yet
- When Organs Fail One by OneDocument6 pagesWhen Organs Fail One by OneJacobMsangNo ratings yet
- Craniotomy VS CraniectoyDocument1 pageCraniotomy VS CraniectoyJacobMsangNo ratings yet
- Nonunion of The Fractured ClavicleDocument18 pagesNonunion of The Fractured ClavicleJacobMsangNo ratings yet
- Cyclopropane, Ethynyl - (Cas 6746-94-7) MSDS: CyclopropylacetyleneDocument5 pagesCyclopropane, Ethynyl - (Cas 6746-94-7) MSDS: CyclopropylacetyleneMiMi JoyNo ratings yet
- Carbon Steel Alloys Steel, Pipe Dimension With Weight Test Pressures According To ANSI B36, 10 For ASTM A53/A 106/API 5L/A335/ SpecificationDocument6 pagesCarbon Steel Alloys Steel, Pipe Dimension With Weight Test Pressures According To ANSI B36, 10 For ASTM A53/A 106/API 5L/A335/ SpecificationsanjibkrjanaNo ratings yet
- wk4 Stdconferencereflection ElmoreajaDocument4 pageswk4 Stdconferencereflection Elmoreajaapi-316378224No ratings yet
- Unit 12: CERT Basic Training Unit 7 ReviewDocument11 pagesUnit 12: CERT Basic Training Unit 7 Reviewdonald1976No ratings yet
- Isoflurane, Sevoflurane and AnestesiDocument124 pagesIsoflurane, Sevoflurane and Anestesizidni fatayanNo ratings yet
- (L-2) - Cell - Mar 03, 2020Document52 pages(L-2) - Cell - Mar 03, 2020puneetlokwani04No ratings yet
- LESSON 2 - TRANSMUTATION - Louise Peralta - 11 - FairnessDocument2 pagesLESSON 2 - TRANSMUTATION - Louise Peralta - 11 - FairnessLouise Joseph PeraltaNo ratings yet
- Allen: Chemical KineticsDocument4 pagesAllen: Chemical KineticsBidhan Chandra SarkarNo ratings yet
- Ascha_ASJ19_Nonsurgical Management of Facial Masculinization and FeminizationDocument15 pagesAscha_ASJ19_Nonsurgical Management of Facial Masculinization and Feminizationallen.515No ratings yet
- CEU - Catalytic ReactorsDocument3 pagesCEU - Catalytic ReactorsPong VongNo ratings yet
- AMB4519R9v06-3238 12ports (LB 4T, PCS-2.6G 4T Dual Beam) 2.6m Twin BeamDocument3 pagesAMB4519R9v06-3238 12ports (LB 4T, PCS-2.6G 4T Dual Beam) 2.6m Twin BeamMIGUEL PEREDA QUIJANONo ratings yet
- D 2144 - 01 - RdixndqDocument4 pagesD 2144 - 01 - RdixndqjayakumarNo ratings yet
- Strain Gauge Load Cells LPB0005IDocument2 pagesStrain Gauge Load Cells LPB0005ILordbyron23No ratings yet
- The Concepts Good Side and Bad Side of HumansDocument9 pagesThe Concepts Good Side and Bad Side of HumansFAITHINGODMISSIONARIESNo ratings yet
- Solution Manual For Safety Health and Environmental Concepts For The Process Industry 2nd EditionDocument8 pagesSolution Manual For Safety Health and Environmental Concepts For The Process Industry 2nd EditionRobert Hornback100% (34)
- Respiratory Epithelium: Human Histology Lecture Chapter 17 Ï The Respiratory SystemDocument16 pagesRespiratory Epithelium: Human Histology Lecture Chapter 17 Ï The Respiratory SystemEvandie OngNo ratings yet
- Sugar Crisis in Pakistan Research PaperDocument10 pagesSugar Crisis in Pakistan Research Paperrehan9891No ratings yet
- The Design of The 2016-17 Young Lives School Survey in EthiopiaDocument10 pagesThe Design of The 2016-17 Young Lives School Survey in EthiopiaFuadNo ratings yet
- An Interview - ExercisesDocument3 pagesAn Interview - ExercisesCarmen GloriaNo ratings yet
- SBR2018 - AbstractsDocument115 pagesSBR2018 - AbstractsGustavo ResendeNo ratings yet
- The International Research Congress On Integrative Medicine and Health 2014Document159 pagesThe International Research Congress On Integrative Medicine and Health 2014Sergio Jesús Huapaya GálvezNo ratings yet
- Advantest R3131 Spectrum Analyzer Operator ManualDocument277 pagesAdvantest R3131 Spectrum Analyzer Operator ManualMartin Argay100% (1)
- Series NRX With PXR - Type NF Low Voltage Power (Air) Circuit Breaker Instruction ManualDocument70 pagesSeries NRX With PXR - Type NF Low Voltage Power (Air) Circuit Breaker Instruction ManualHamilton GutierrezNo ratings yet
- XFY1548移动式筛分站术规格书Document16 pagesXFY1548移动式筛分站术规格书abangNo ratings yet
- Chloe Kho - Assignment 5 - MTT PracticeDocument5 pagesChloe Kho - Assignment 5 - MTT PracticeanthonyNo ratings yet