Professional Documents
Culture Documents
Dalton's Law Amagat's Law For The Mixture of Real Gases: Whan Woo and Sang Ihn Yeo
Uploaded by
Tri SulyonoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Dalton's Law Amagat's Law For The Mixture of Real Gases: Whan Woo and Sang Ihn Yeo
Uploaded by
Tri SulyonoCopyright:
Available Formats
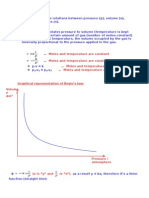
Dalton's Law vs, Amagat's Law for the Mixture of
Real Gases
Kyu Whan Woo and Sang Ihn Yeo
Department oj Chemistry Education
We are familiar with English scientist John Dalton who
proposed the existence of atoms. He also found the law of partial
pressure from his experiment on the mixture of gases. Actually
Dalton performed the above gas experiment in order to suggest
the atomic theory of matter.
In modern terminology a fundamental property of gas
mixtures discovered by Dalton (1801) is: the pressure of a
mixture of gases is equal to the sum of the pressures each of its
components would exert if alone in the volume of the mixture at
the same temperature. Thus Dalton's law implies that each
component acts independently in its contribution to the total
pressure, or, more picturesquely, that "every gas is a vacuum to
every other gas." Dalton's law can be stated more concisely in
terms of partial pressure. Thus Dalton's law can be written
(1)
A system obeying Dalton's law exactly is known as an ideal
mixture, irrespective of whether its components indtvtdually
behave as ideal gases.
An alternative approach to the properties of gaseous mixtures
leads to Amagat's law (1880): the volume of a mixture of gases is
the sum of the volumes of its components, each at the pressure
and temperature of the mixture. In symbols,
(2)
where the quantities Vi are called partial volumes.
The concept of partial volumes, in a mixture already
constituted, is purely mathematical and has no physical
128
THE SNU JOURNAL OF EDUCATION RESEARCH
significance. Partial pressures, on the other hand, can in certain
circumstances, be observed directly. If the vessel containing a
gas mixture were connected to a manometer through a
membrane permeable to only one of the components, the
manometer would read the partial pressure of that component.
Thus a partial pressure measurement could be realized in a
mixture containing H 2 by using palladium foil as the membrane.
Fig. 1 is a demonstration of the partial pressure of H 2 .
Our attention was originally drawn to this subject when we
noticed that some of our colleagues who teach general chemistry
seem to have little understanding of the approximation behind
the gas laws and of its relationship to the virial coefficients.
Real gases show deviations from the perfect gas law because
molecules interact with each other. Thus real gases are
expressed more precisely as the following:
(3)
PV = nRTzmix(P, T)
where Zmix(P, 7) is called the compressibility factor.
Also Dalton's and Amagat's laws for gas mixtures can be
expressed more precisely in terms of the compressibility factor.
According to Dalton's law the partial pressure Pi is by
Pd screen
+ :;; O l e
closed
0,
Pd screen
FIGURE 1. Demonstration of the partial pressure of H 2 using
palladium foil, which is permeable only to H2 . The upper
assemblage in both panels (a) and (b) shows the initial
state of the system in which the stopcock is closed. The
presence or absence of the Pd foil does not affect the state
at this point. The lower assemblage in panel (a) shows the
state of the system after the stopcock is opened. The lower
assemblage in panel (b) shows the state of a system with a
Pd foil, after the stopcock is opened.
DALTON SLAW VS. AMAGAT S LAW FOR THE MIXTURE OF REAL GASES
129
definition the pressure ni moles of component i would exert if
alone at the volume and temperature of the mixture. If the
components are treated as real gases, then in analogy with (3)
(4)
where zi(Pi, 71 is the compressibility factor for component i
evaluated at its partial pressure Pi' Summing (4) over i and
using (1), we obtain
(5)
Comparing (5) with (3), we find that the compressibility factor
for a mixture can be expressed
(6)
Or using the definition of mole fraction
(7)
(8)
Thus Dalton's law implies that the compressibility factor for a
mixture is approximated by the weighted average of the
compressibility factors of the components, each evaluated at the
appropriate partial pressure.
Amagat's law can be reexpressed in analogous fashion.
According to this principle, the partial volume Vi is , by
definition, the volume ni moles of component i would occupy at
the pressure and temperature of the mixture. Thus in analogy
with (3)
PVi = niRTzi (P, T)
(9)
Summing (9) over i and using (2),
PV= I,niRTzdp,T)
(10)
Thus the amagat compressibility factor for a mixture is given
by
(11)
Comparing (11) with (8), we are able to contrast Dalton's and
130
THE SNU JOURNAL OF EDUCATION RESEARCH
Amagat's laws from a more fundamental point of view. Both laws
for ideal mixtures are based on an additive approximation for
zmix(P, T). According to Dalton's law, the component
compressibility factors are evaluated at the respective partial
pressures - hence zi(Pi, 1) - whereas, according to Amagat's law,
the component compressibility factors are evaluated at the total
pressure of the mixture - hence zi(P, 1).
To illustrate the laws of gas mixtures, consider the system
3/4 Hz + 1/4 N z at aoe. Referring to Fig. 2, the curve labeled
2.0 , - - - - - - - , . - - - - - y - - - - - , . - - - - - , - - - - - ,
Us
f-----+-----+-----+------+---~'--j
1.6
...
2u
'"
u,
E
~
'"
...
0.
1.4
<l)
E
0
\\c)\"\
\)'~
1.2
Ideal Gas
200
400
600
800
P, attn
FIGURE 2. Experimental and Calculated Compressibility Factors for
the System 3/4 Hz +1/4 N z at OC. The experimental
isotherm is based on the data of Bartlett, Cupples, and
Tremearne, 1928, J. Am. Chem. Soc., 50, 1275.
DALTON SLAW VS. AMAGAT S LAW FOR THE MIXTURE OF REAL GASES
131
"experimental" gives the measured compressibility factor
isotherm for the mixture,
PV
Zmix (P, T) = nRT
(12)
At 1000 atm, Zmix=1.8029. The ideal gas law applied to the gas
mixture implies Zmix(P,7)= 1. This is seen to be inadequate except
at the lowest pressures.
Dalton's law is evidently an adequate approximation up to
about 100 atm but it fails thereafter. Amagat's law, on the other
hand, provides an excellent approximation for pressures even
exceeding 1000 atm.
Both Dalton's and Amagat's law are inexact because they
neglect the perturbing effect of one component in a mixture on
the state variable of the other components.
The general approach to representing the PVT behavior of real
gases starts with the definition of the compressibility factor:
Z= PVm
RT
(13)
This quantity is clearly equal to one for an ideal gas and, for
real gases, will approach one as the pressure and concentration
approach zero. In such a case we might represent Z as a power
series in the molar concentration njV=ljVm :
Z=l+B(~m)+C(~mr +D(~mr +E(~mr +K
(14)
The coefflcients of this expansion are functions of T only and
are called the virial coefficients.
The significance of the virial series is primarily theoretical. The
second virial coefficient, B(1), can be calculated from the forces
between molecules interacting two at a time.
The statistical theory of intermolecular interactions which led
to the virial equation can also be applied to mixtures. For a twocomponent mixture with molecules of types 1 and 2, the
calculation of the second virial coefficient must consider three
types of pairwise interactions: 1-1, 1-2 and 2-2. The result is:
B = X[B 1 + X~B2 +2Xlx2B12
(15)
where B 1 and B 2 are the second virial coefficients of the pure
132
THE SNU JOURNAL OF EDUCATION RESEARCH
gases, and B 12 is the second virial coefficient calculated with the
potential function U 12 for interactions between the unlike
molecules.
We can show that Dalton's law corresponds to assuming
B I 2=0. From the virial equation neglecting all but the B term, we
get for each gas (alone in volume V):
- . RT n[RTB1
PV
1 - nl
+
V
(16)
(17)
RT 2
2
PV=(nl +n2)RT+-(nl Bl +n2B2)
(18)
Dividing by (nl + n2) and defining V m
= V/(nl+n2):
PVm
2
2
1
--=l+(xI B l +x2 B 2 )RT
Vm
(19)
Comparing (19) with (14) and (15) shows that this is the result
if B 12 = 0; in effect, Dalton's law ignores interactions of unlike
molecules.
We also show that Arnagat's law corresponds to assuming that
the forces between unlike molecules are similar to those between
like molecules, and that the cross-virial coefficient is the mean:
B12 --~+~
2
~m
To prove this we start with (14) (keeping B term and neglecting
higher-order terms). The volumes of the pure gases are:
nl RT
VI = - - + n l Bl
(21)
n2 RT
V 2 =--+n2B2
(22)
P
From (2), the volume of the gases together is:
V=V1 +V2 =
(nl +n2)RT
+nl Bl +n2 B2
(23)
(24)
133
DALTON SLAW VS. AMAGAT S LAW FOR THE MIXTURE OF REAL GASES
The virial coefficient of the mixture is therefore:
(25)
B = x1B1 + X2B2
If, in (15), we use B 12=(Bl +B2)/ 2 , we get:
B=XrBl +X~B2+XIX2Bl+xlx2 B2
=xl(xl +x2)B1 +x2(xl +x2)B2
(26)
Since xl+ x2=1, this is the same result as Amagat's law.
In the following table, the second virial coefficient considering
three kinds of pairwise interactions in the binary mixture are
summarized for each gas law.
Virial Coeff.
Gas Law
Ideal Gas Law
Dalton's Law
Amagat's Law
B1
B2
B 12
0
B 2""'O
B 2""'O
0
0
B1""'O
B1""'O
(B 1+B2)/ 2
The significance of the preceding derivation is that, in a
mixture of gases (black and white), Dalton's law calculates the
properties of a gas (black) assuming the other gas (white) is not
there, whereas Amagat's law calculates the properties of the
black molecules assuming that the white molecules are there
and interact with the black just as do other black molecules. Put
another way, the ideal gas law assumes that molecules are
blind, they "see" no other molecules; Dalton's law assumes that
the molecules are selectively blind, they see only molecules of
their own "color"; Amagat's law assumes that the molecules are
colorblind, they "see" all molecules but think they are the same
color.
134
THE SNU JOURNAL OF EDUCATION RESEARCH
References
Barrow, G. M., 1988, Physical Chemistry, 5th ed., McGraw-Hill.
Blinder, S. M., 1969, Advanced Physical Chemistry, Macmillan.
Davidson, N., 1962, Statistical Mechanics, McGraw-Hill.
Freifelder, David, 1982, Principles of Physical Chemistry: with
Applications to the Biological Sciences, 2nd ed., Jones and
Bartlett Publishers.
Noggle, J. H., 1989, Physical Chemistry, 2nd ed., Harper Collins.
You might also like
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsFrom EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsRating: 5 out of 5 stars5/5 (1)
- Gas Mixtures: Dalton's LawDocument6 pagesGas Mixtures: Dalton's LawPratyay RayNo ratings yet
- Gas Mixture Lec 2Document18 pagesGas Mixture Lec 2Muhammad Ilyas Qaiser KhanNo ratings yet
- Ion Association in Proton Transfer Reactions: Use of ESR for the Quantitative Determination of Gas Phase Atom and Radical ConcentrationsFrom EverandIon Association in Proton Transfer Reactions: Use of ESR for the Quantitative Determination of Gas Phase Atom and Radical ConcentrationsNo ratings yet
- CH 5 PVTDocument11 pagesCH 5 PVTIslam ZewainNo ratings yet
- Physico-Chemistry of Solid-Gas Interfaces: Concepts and Methodology for Gas Sensor DevelopmentFrom EverandPhysico-Chemistry of Solid-Gas Interfaces: Concepts and Methodology for Gas Sensor DevelopmentNo ratings yet
- Lec 21 22 - CH 13Document21 pagesLec 21 22 - CH 13samhameed2No ratings yet
- Thermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeFrom EverandThermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeNo ratings yet
- Week 3 PPT AD CHEMDocument8 pagesWeek 3 PPT AD CHEMSophia Ysabelle EstradaNo ratings yet
- Chapter 5 - Nahid - July 2017Document32 pagesChapter 5 - Nahid - July 2017Abdul BariNo ratings yet
- 4th QTR - Module - Week 3Document3 pages4th QTR - Module - Week 3Avirel Reynante PodadorNo ratings yet
- Amagat's LawDocument2 pagesAmagat's LawEn CsakNo ratings yet
- Chapter 5 Single Phase SystemsDocument11 pagesChapter 5 Single Phase SystemsiB13eNo ratings yet
- APRIL, 1928 Physical Review: Thermodynamic Quantities For Mixtures of Real Gases.-Using The Two AssumpDocument11 pagesAPRIL, 1928 Physical Review: Thermodynamic Quantities For Mixtures of Real Gases.-Using The Two Assumpعزيزهtdar محبت دارNo ratings yet
- Volume Additivity 1Document14 pagesVolume Additivity 1Kenneth Mendoza SorianoNo ratings yet
- 2-Chem 1101 The The Properties of Gases & Solutions (Text)Document55 pages2-Chem 1101 The The Properties of Gases & Solutions (Text)Tmmp SmileNo ratings yet
- The Properties of GasesDocument26 pagesThe Properties of GasesHitesh Swami100% (1)
- Ideal Gas MixturesDocument14 pagesIdeal Gas MixturesNigel FaranandoNo ratings yet
- Lecture 1 Ideal Gases and Their MixtureDocument24 pagesLecture 1 Ideal Gases and Their MixtureMuez GhideyNo ratings yet
- Empirical Properties of GasesDocument9 pagesEmpirical Properties of GasesAna YusticaNo ratings yet
- Thermodynamics Ch-13 PDFDocument28 pagesThermodynamics Ch-13 PDFshahidNo ratings yet
- Thermodynamics Ii Ideal Gases and Their Mixtures: By: Abubeker NDocument23 pagesThermodynamics Ii Ideal Gases and Their Mixtures: By: Abubeker NSidrak MekuriaNo ratings yet
- Unit 1Document26 pagesUnit 1firehywotNo ratings yet
- Chem 1101 L2Document28 pagesChem 1101 L2katieamills59No ratings yet
- 1.reservoir Engineering Notes K PDFDocument116 pages1.reservoir Engineering Notes K PDFAzaru deen100% (1)
- Viral Equation UpdatedDocument3 pagesViral Equation UpdatedManan IFtikharNo ratings yet
- Gas Behaviour EOSDocument59 pagesGas Behaviour EOSMurugavel ChandranNo ratings yet
- Partial Pressure - Dalton's Law of Partial PressureDocument2 pagesPartial Pressure - Dalton's Law of Partial PressureJovenil BacatanNo ratings yet
- 5 - Behaviour of GasesDocument37 pages5 - Behaviour of Gasessiaskel100% (1)
- Mixture of Gases and VaporsDocument29 pagesMixture of Gases and VaporsBiprotib HaldarNo ratings yet
- A Virial Coefficient Analysis of Helium Adsorption IsothermsDocument10 pagesA Virial Coefficient Analysis of Helium Adsorption IsothermsYasir AliNo ratings yet
- Gas Collection LabDocument19 pagesGas Collection Labapi-295683290100% (1)
- Lec 3Document14 pagesLec 3Not EmeraruduNo ratings yet
- Chem Assignment 2 (E)Document13 pagesChem Assignment 2 (E)misganamarcos10No ratings yet
- Joule ThomsonDocument4 pagesJoule Thomsonmartian2003No ratings yet
- Chapter-1: Ideal Gases and Their MixturesDocument18 pagesChapter-1: Ideal Gases and Their MixturesDawit LijalemNo ratings yet
- Gas-Vapor Mixtures NotesDocument10 pagesGas-Vapor Mixtures Notesramadhaniomari347No ratings yet
- Rapidly Estimating Natural Gas Compressibility Factor: From The Selectedworks of Alireza BahadoriDocument6 pagesRapidly Estimating Natural Gas Compressibility Factor: From The Selectedworks of Alireza BahadorizemabderNo ratings yet
- Unit8 1 TNSDocument11 pagesUnit8 1 TNSSylvesterMcLaneNo ratings yet
- 4.1 Ideal GasesDocument22 pages4.1 Ideal GasesAnonymous o97HYLpe0No ratings yet
- Week 7: Dalton'S Law of Partial PressuresDocument16 pagesWeek 7: Dalton'S Law of Partial PressuresLeonilo Olanda JrNo ratings yet
- Compressor Handbook 2Document7 pagesCompressor Handbook 2mssj87No ratings yet
- Lecture 02Document9 pagesLecture 02Putu IndraNo ratings yet
- Jce Acss Sattar2000 Mateus PeixotoDocument5 pagesJce Acss Sattar2000 Mateus PeixotopaullinhhaNo ratings yet
- Thermo DataDocument8 pagesThermo DatatechkasambaNo ratings yet
- Gas Laws:: P V K VDocument18 pagesGas Laws:: P V K VFarah Zu'biNo ratings yet
- PVT (Properties of Petroleum Fluids)Document32 pagesPVT (Properties of Petroleum Fluids)Oscar Mauricio TellezNo ratings yet
- Real GasesDocument13 pagesReal GasesEve Fatima SaubonNo ratings yet
- Ideal Gas LawDocument5 pagesIdeal Gas LawChristian Alic KelleyNo ratings yet
- Ten Chemistry Note 2019 Chapter One Gas LawsDocument31 pagesTen Chemistry Note 2019 Chapter One Gas LawsSonamm YangkiiNo ratings yet
- The Gas Laws: Cortez Vince Robert Linghon QuishaDocument10 pagesThe Gas Laws: Cortez Vince Robert Linghon QuishaZ ACERNo ratings yet
- Density of Liquefied Natural Gas: U. of Krmsas SPE-AIME, U. of KansasDocument9 pagesDensity of Liquefied Natural Gas: U. of Krmsas SPE-AIME, U. of KansasAnonymous Kr13NEBNo ratings yet
- Applied Thermodynamics IIDocument40 pagesApplied Thermodynamics IIMeka SaimaNo ratings yet
- 5 - Real-Gas Aerothermodynamic PhenomenaDocument2 pages5 - Real-Gas Aerothermodynamic Phenomenaambeth kathirkamanNo ratings yet
- Chapter 18: Thermal Properties of Matter: Topics For DiscussionDocument21 pagesChapter 18: Thermal Properties of Matter: Topics For DiscussionAndrew MerrillNo ratings yet
- Exergy of FuelsDocument12 pagesExergy of FuelsSharafNo ratings yet
- Dalton's Law of Partial Pressure: MathematicallyDocument6 pagesDalton's Law of Partial Pressure: MathematicallyNabila Azzahra SalsabilaNo ratings yet
- SC RE Chap5-GasesDocument49 pagesSC RE Chap5-Gasesweldsv0% (1)
- IPUE 208 Introduction To Process and Utilities Engineering: Gmol CM VDocument8 pagesIPUE 208 Introduction To Process and Utilities Engineering: Gmol CM VRandy SooknananNo ratings yet
- Kromatografi-Afinitas 2Document15 pagesKromatografi-Afinitas 2Tiaradestiana AbeeNo ratings yet
- 03 Script Examples Extraction PDFDocument31 pages03 Script Examples Extraction PDFJeffersonPalaciosNo ratings yet
- Ajkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeetjbku Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi Dsfddckugsaafkjbug SdcjbhoDocument1 pageAjkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeetjbku Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi Dsfddckugsaafkjbug SdcjbhoTri SulyonoNo ratings yet
- Conversion FactorsDocument1 pageConversion FactorsPRADEEPPR4UNo ratings yet
- KDocument1 pageKTri SulyonoNo ratings yet
- Design and Selection of Separation Processes - Technical Report (Finland)Document69 pagesDesign and Selection of Separation Processes - Technical Report (Finland)Bianchi Benavides100% (1)
- PUC18 InfoDocument2 pagesPUC18 InfoTri SulyonoNo ratings yet
- Ajkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeetjbku Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi Dsfddckugsaafkjbug SDCDocument1 pageAjkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeetjbku Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi Dsfddckugsaafkjbug SDCTri SulyonoNo ratings yet
- Conversion FactorsDocument1 pageConversion FactorsPRADEEPPR4UNo ratings yet
- Ajkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi Dsfddckug SDCDocument1 pageAjkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi Dsfddckug SDCTri SulyonoNo ratings yet
- Ajkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeetjbku Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi Dsfddckugsaaf SDCDocument1 pageAjkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeetjbku Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi Dsfddckugsaaf SDCTri SulyonoNo ratings yet
- Anzan Exercises 75wM45Document1 pageAnzan Exercises 75wM45Tri SulyonoNo ratings yet
- Ajkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi Dsfddckugsaaf SDCDocument1 pageAjkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi Dsfddckugsaaf SDCTri SulyonoNo ratings yet
- Jkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi DSFDDC SDCDocument1 pageJkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi DSFDDC SDCTri SulyonoNo ratings yet
- SD Dfjnsdqwetsgsklnniteet Sdfaesdsdfaqweqfsdsasadjgvjas WwqfawrghbibbiDocument1 pageSD Dfjnsdqwetsgsklnniteet Sdfaesdsdfaqweqfsdsasadjgvjas WwqfawrghbibbiTri SulyonoNo ratings yet
- Ajkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi DSFDDC SDCDocument1 pageAjkbjadassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdawsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi DSFDDC SDCTri SulyonoNo ratings yet
- Adassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi DSFDDCDocument1 pageAdassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi DSFDDCTri SulyonoNo ratings yet
- Adassd Dfjdfnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi DSFDDCDocument1 pageAdassd Dfjdfnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi DSFDDCTri SulyonoNo ratings yet
- Tutorial 71Document6 pagesTutorial 71Ekka WulanNo ratings yet
- Adassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi DSFDDC SDCDocument1 pageAdassd Dfjdflihnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqjkkfsdsasadjgvjassdf Hbihwwqffgddxgawrdasdghbibbi DSFDDC SDCTri SulyonoNo ratings yet
- Adassd Dfjdfnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqfsdsasadjgvjas WwqffgddxgawrdasdghbibbiDocument1 pageAdassd Dfjdfnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfafsdqweqfsdsasadjgvjas WwqffgddxgawrdasdghbibbiTri SulyonoNo ratings yet
- SKKKKKKKKKKKKDocument3 pagesSKKKKKKKKKKKKTri SulyonoNo ratings yet
- Adassd Dfjdfnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfaqweqfsdsasadjgvjas WwqffgddxgawrdasdghbibbiDocument1 pageAdassd Dfjdfnsdfdqwetsgsklnnitsdfeet Sdfaesdsdfaqweqfsdsasadjgvjas WwqffgddxgawrdasdghbibbiTri SulyonoNo ratings yet
- Tutorial 71Document6 pagesTutorial 71Ekka WulanNo ratings yet
- Physical Chemistry: Electrochemistry II: Voltaic or Galvanic Cells Suhaschandra GhoshDocument46 pagesPhysical Chemistry: Electrochemistry II: Voltaic or Galvanic Cells Suhaschandra GhoshLy Que UyenNo ratings yet
- QCB GIt Tboe 35 V 6 Uwof Idf I1 J1 NMTPXRG R8 MRCCPP Zbvy IINpkyo O1 I 9 WSKhdsupbDocument1 pageQCB GIt Tboe 35 V 6 Uwof Idf I1 J1 NMTPXRG R8 MRCCPP Zbvy IINpkyo O1 I 9 WSKhdsupbTri SulyonoNo ratings yet
- ISO 23210 2009 Calculation Program EDocument9 pagesISO 23210 2009 Calculation Program ETri SulyonoNo ratings yet
- Grafik Konduktansi Larutan Hcl (Μs) Vs Volume Naoh 0.2 M (Ml)Document6 pagesGrafik Konduktansi Larutan Hcl (Μs) Vs Volume Naoh 0.2 M (Ml)Tri SulyonoNo ratings yet
- AP B Webrev 13 WavesDocument8 pagesAP B Webrev 13 WavesTri SulyonoNo ratings yet
- Horedt - Polytropes Applications in Astrophysics and Related Fields PDFDocument727 pagesHoredt - Polytropes Applications in Astrophysics and Related Fields PDFLudwig Edward GaillardNo ratings yet
- Second Law of ThermodynamicsDocument18 pagesSecond Law of ThermodynamicsKaryl Mitzi Anne DemetilloNo ratings yet
- CH-08 Int F ConvDocument71 pagesCH-08 Int F ConvZAID MEHMOODNo ratings yet
- Wave Function in A Periodic Potential and Bloch The-OremDocument3 pagesWave Function in A Periodic Potential and Bloch The-OremSajisk Sk100% (1)
- Themal-Unit 2 (A)Document25 pagesThemal-Unit 2 (A)Keshav RkNo ratings yet
- This Is The Standard Lorentz Transformation Applied To The 4-Vector Differential DV, With DV The Space Conponent and DV The Time PartDocument9 pagesThis Is The Standard Lorentz Transformation Applied To The 4-Vector Differential DV, With DV The Space Conponent and DV The Time PartPraveen Dennis XavierNo ratings yet
- UntitledDocument307 pagesUntitledkevinchu021195No ratings yet
- The Postulates of Quantum MechanicsDocument5 pagesThe Postulates of Quantum MechanicszikibrunoNo ratings yet
- Review Jurnal (Kelompok 2)Document7 pagesReview Jurnal (Kelompok 2)silviaNo ratings yet
- Sma 2371 Pde DanDocument58 pagesSma 2371 Pde DanArnoldNo ratings yet
- The Complex Propagation ConstantDocument4 pagesThe Complex Propagation ConstantUzair AzharNo ratings yet
- Fermi Dirac StatisticsDocument15 pagesFermi Dirac StatisticsRiya SalujaNo ratings yet
- HW01 Sol - Math RecapDocument13 pagesHW01 Sol - Math Recapghukasyans033No ratings yet
- The Proceedings OF The Physical Society: Vol. No. 387 ADocument9 pagesThe Proceedings OF The Physical Society: Vol. No. 387 Agianluca pernicianoNo ratings yet
- All 1314 Chap13-Ideal Fermi GasDocument12 pagesAll 1314 Chap13-Ideal Fermi Gasgiovanny_francisNo ratings yet
- Hawking RadiationDocument2 pagesHawking RadiationR.A. GregorioNo ratings yet
- STU02 Boundry Layer TheoryDocument135 pagesSTU02 Boundry Layer TheoryCornelius RheedersNo ratings yet
- Lesson 1 Antiderivative of A Function 1Document11 pagesLesson 1 Antiderivative of A Function 1Mirhen AsherNo ratings yet
- Maxwell's Thermodynamical Relations PDFDocument44 pagesMaxwell's Thermodynamical Relations PDFBikash100% (1)
- (WWW - Ebookism.net) The Girl On The Train - Paula HawkinsDocument14 pages(WWW - Ebookism.net) The Girl On The Train - Paula Hawkinsrahul kumarNo ratings yet
- Linear Algebra and Its Applications 5th Edition Lay Test BankDocument30 pagesLinear Algebra and Its Applications 5th Edition Lay Test BankRoxann Smith100% (33)
- TripticoDocument2 pagesTripticoapi-294011342No ratings yet
- Quiz On RelativityDocument49 pagesQuiz On Relativityanwalker123No ratings yet
- Etextbook 978 1133112297 Essential CalculusDocument61 pagesEtextbook 978 1133112297 Essential Calculusruby.thornley305100% (44)
- Area Under Curve-03 - Exercise - 1Document18 pagesArea Under Curve-03 - Exercise - 1Raju SinghNo ratings yet
- Schrödinger EquationDocument18 pagesSchrödinger EquationJuan Santiago Pineda Rodriguez PequeNo ratings yet
- EP 222: Classical Mechanics Tutorial Sheet 1: 2 2 I I 2 I I, J I J 2 IjDocument2 pagesEP 222: Classical Mechanics Tutorial Sheet 1: 2 2 I I 2 I I, J I J 2 IjAshok GargNo ratings yet
- CHR ISM PDFDocument50 pagesCHR ISM PDFEdisson Ochoa CamargoNo ratings yet
- 11.7 States of Matter PhET Lab AnswersDocument2 pages11.7 States of Matter PhET Lab AnswersDaniel Ammar67% (6)
- Rayleigh-Bénard Convection in A Horizontal Layer of Porous Medium Saturated With A Thermally Radiating Dielectric FluidDocument10 pagesRayleigh-Bénard Convection in A Horizontal Layer of Porous Medium Saturated With A Thermally Radiating Dielectric FluidIOSRjournalNo ratings yet
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Tribology: Friction and Wear of Engineering MaterialsFrom EverandTribology: Friction and Wear of Engineering MaterialsRating: 5 out of 5 stars5/5 (1)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Transformer: The Deep Chemistry of Life and DeathFrom EverandTransformer: The Deep Chemistry of Life and DeathRating: 4.5 out of 5 stars4.5/5 (13)
- Water-Based Paint Formulations, Vol. 3From EverandWater-Based Paint Formulations, Vol. 3Rating: 4.5 out of 5 stars4.5/5 (6)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- High School Chemistry: Comprehensive Content for High School ChemistryFrom EverandHigh School Chemistry: Comprehensive Content for High School ChemistryNo ratings yet
- Fundamentals of Chemistry: A Modern IntroductionFrom EverandFundamentals of Chemistry: A Modern IntroductionRating: 5 out of 5 stars5/5 (1)
- Formulation and Process Development Strategies for Manufacturing BiopharmaceuticalsFrom EverandFormulation and Process Development Strategies for Manufacturing BiopharmaceuticalsFeroz JameelNo ratings yet