Professional Documents
Culture Documents
SDS-Polyacrylamide Gel Electrophoresis of Proteins
Uploaded by
Young LoveCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SDS-Polyacrylamide Gel Electrophoresis of Proteins
Uploaded by
Young LoveCopyright:
Available Formats
SDS-Polyacrylamide Gel Electrophoresis of Proteins -- Sambrook and ...
1 of 9
file:///C:/Users/MILI%20JOON/Documents/protocol/Electrophoresis,...
Please cite as: CSH Protocols; 2006; doi:10.1101/pdb.prot4540
Protocol
SDS-Polyacrylamide Gel Electrophoresis of Proteins
Joseph Sambrook and David W. Russell
This protocol was adapted from "Commonly Used Techniques in Molecular Cloning," Appendix 8, in Molecular Cloning,
Volume 3, 3rd edition (eds. Sambrook and Russell). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA,
2001.
INTRODUCTION
This protocol describes the separation of proteins by SDS-polyacrylamide gel electrophoresis. SDS is used
with a reducing agent and heat to dissociate the proteins. SDS-polypeptide complexes form and migrate
through the gels according to the size of the polypeptide. By using markers of known molecular weight,
the molecular weight of the polypeptide chain(s) can be estimated.
MATERIALS
Reagents
Acrylamide solutions (see Table 1 and Table 2 for recipes)
Premixed stock solutions are commercially available (e.g., Invitrogen)
Ammonium persulfate stock solution (10% w/v)
Isobutanol (overlay for gels containing ~10% acrylamide)
SDS (0.1%) (overlay for gels containing ~8% acrylamide)
Protein standard molecular-weight markers (e.g., Invitrogen)
Protein samples to be resolved (e.g., purified protein or cell lysates)
SDS stock solution (10% w/v, electrophoresis grade) for resolving and stacking gels
Exclusive use of one brand of SDS is recommended to ensure reproducible results.
1X SDS gel-loading buffer
TEMED (electrophoresis grade)
Tris-Cl (1.5 M, pH 8.8) and (1.0 M, pH 6.8)
1X Tris-glycine buffer
8/21/2008 11:24 AM
SDS-Polyacrylamide Gel Electrophoresis of Proteins -- Sambrook and ...
2 of 9
file:///C:/Users/MILI%20JOON/Documents/protocol/Electrophoresis,...
Equipment
Erlenmeyer flask or disposable plastic tube
Hamilton microliter syringe or micropipettor equipped with gel-loading tips
Hypodermic needle (blunt) attached to a syringe
Pasteur pipette
Power supply (capable of supplying up to 500 V and 200 mA)
Squirt bottle for H2O
Vertical electrophoresis apparatus (e.g., Invitrogen)
Use only one type of apparatus in a given lab if possible. This makes it easier to compare the results
obtained by different investigators and allows parts to be reused.
Water bath or heating block, preset to 100C or, for extremely hydrophobic proteins, 45-55C
METHOD
Pouring SDS-Polyacrylamide Gels
1. Assemble the glass plates according to the manufacturers instructions.
2. Determine the volume of the gel mold (this information is usually provided by the manufacturer).
In an Erlenmeyer flask or disposable plastic tube, prepare the resolving gel using the appropriate
volume of solution containing the desired concentration of acrylamide using the values given in
Table 1. Polymerization will begin as soon as the TEMED has been added. Without delay, swirl the
mixture rapidly and proceed to the next step.
The concentration of ammonium persulfate recommended here and in Step 5 is higher than that
used by some investigators. This eliminates the need to rid the acrylamide solution of dissolved
oxygen by degassing.
3. Pour the acrylamide solution into the gap between the glass plates. Leave sufficient space for the
stacking gel (the length of the teeth of the comb plus 1 cm). Use a Pasteur pipette to overlay the
acrylamide solution carefully with 0.1% SDS (for gels containing ~8% acrylamide) or isobutanol (for
gels containing ~10% acrylamide). Place the gel in a vertical position at room temperature.
The overlay prevents oxygen from diffusing into the gel and inhibiting polymerization. Isobutanol
dissolves the plastic of some minigel apparatuses.
4. After polymerization is complete (30 minutes), pour off the overlay and wash the top of the gel
several times with deionized H2O to remove any unpolymerized acrylamide. Drain as much fluid as
possible from the top of the gel, and then remove any remaining H2O with the edge of a paper
towel.
8/21/2008 11:24 AM
SDS-Polyacrylamide Gel Electrophoresis of Proteins -- Sambrook and ...
3 of 9
file:///C:/Users/MILI%20JOON/Documents/protocol/Electrophoresis,...
5. In a disposable plastic tube, prepare the stacking gel using the appropriate volume of solution
containing the desired concentration of acrylamide using the values given in Table 2.
Polymerization will begin as soon as the TEMED has been added. Without delay, swirl the mixture
rapidly and proceed to the next step.
6. Pour the stacking gel solution directly onto the surface of the polymerized resolving gel.
Immediately insert a clean Teflon comb into the stacking gel solution. Avoid trapping air bubbles.
Add more stacking gel solution to fill the spaces of the comb completely. Place the gel in a vertical
position at room temperature.
Teflon combs should be cleaned with H2O and dried with ethanol just before use.
Preparation of Samples and Running the Gel
7. While the stacking gel is polymerizing, prepare the samples in the appropriate volume of 1X SDS
gel-loading buffer and heat them to 100C for 3 minutes to denature the proteins.
Be sure to denature a sample containing marker proteins of known molecular weights. Mixtures of
appropriately sized polypeptides are available from commercial sources.
Extremely hydrophobic proteins, such as those containing multiple transmembrane domains, may
precipitate or multimerize when boiled for 3 minutes at 100C. To avoid these pitfalls, heat the
samples for 1 hour at a lower temperature (45-55C) to denature.
8. After polymerization is complete (30 minutes), remove the Teflon comb carefully. Use a squirt
bottle to wash the wells immediately with H2O to remove any unpolymerized acrylamide. If
necessary, straighten the teeth of the stacking gel with a blunt hypodermic needle attached to a
syringe. Mount the gel in the electrophoresis apparatus. Add Tris-glycine electrophoresis buffer to
the top and bottom reservoirs. Remove any bubbles that become trapped at the bottom of the gel
between the glass plates with a bent hypodermic needle attached to a syringe.
Do not pre-run the gel before loading the samples, since this will destroy the discontinuity of the
buffer systems.
9. Load up to 15 l of each of the samples in a predetermined order into the bottom of the wells.
Use a Hamilton microliter syringe or a micropipettor, equipped with gel-loading tips, that is washed
with buffer from the bottom reservoir after each sample is loaded. Load an equal volume of 1X SDS
gel-loading buffer into any wells that are unused.
10. Attach the electrophoresis apparatus to an electric power supply (the positive electrode should
be connected to the bottom buffer reservoir). Apply a voltage of 8 V/cm to the gel. After the dye
front has moved into the resolving gel, increase the voltage to 15 V/cm and run the gel until the
bromophenol blue reaches the bottom of the resolving gel (~4 hr). Then, turn off the power supply.
11. Remove the glass plates from the electrophoresis apparatus and place them on a paper towel.
Use an extra gel spacer to carefully pry the plates apart. Mark the orientation of the gel by cutting a
corner from the bottom of the gel that is closest to the left-most well (slot 1).
Do not cut the corner from gels that are to be used for immunoblotting.
8/21/2008 11:24 AM
SDS-Polyacrylamide Gel Electrophoresis of Proteins -- Sambrook and ...
4 of 9
file:///C:/Users/MILI%20JOON/Documents/protocol/Electrophoresis,...
12. At this stage, the gel can be fixed, stained with Coomassie Brilliant Blue or silver salts,
fluorographed or autoradiographed, or used to establish an immunoblot.
REFERENCES
Harlow E. and Lane D. 1988. Antibodies: A laboratory manual. Cold Spring Harbor Laboratory, Cold
Spring Harbor, New York.
Caution
Acrylamide:bisacrylamide
Acrylamide:bisacrylamide, see Acrylamide; Bisacrylamide
Caution
Ammonium persulfate
Ammonium persulfate (NH4)2S2O8 is extremely destructive to tissue of the mucous membranes and upper
respiratory tract, eyes, and skin. Inhalation may be fatal. Wear appropriate gloves, safety glasses, and
protective clothing. Always use in a chemical fume hood. Wash thoroughly after handling.
Caution
General warning
This material contains hazardous components. Please see recipe for full details.
Caution
Isobutanol
Isobutanol (Isobutyl alcohol) is extremely flammable and may be harmful by inhalation or ingestion. Wear
appropriate gloves and safety glasses. Keep away from heat, sparks, and open flame.
8/21/2008 11:24 AM
SDS-Polyacrylamide Gel Electrophoresis of Proteins -- Sambrook and ...
5 of 9
file:///C:/Users/MILI%20JOON/Documents/protocol/Electrophoresis,...
Caution
SDS (Sodium dodecyl sulfate)
SDS (sodium dodecyl sulfate) is toxic, an irritant, and poses a risk of severe damage to the eyes. It may be
harmful by inhalation, ingestion, or skin absorption. Wear appropriate gloves and safety goggles. Do not
breathe the dust.
Caution
TEMED
TEMED N,N,N',N'-Tetramethylethylenediamine is extremely destructive to tissues of the mucous
membranes and upper respiratory tract, eyes, and skin. Inhalation may be fatal. Prolonged contact can
cause severe irritation or burns. Wear appropriate gloves, safety glasses, and other protective clothing. Use
only in a chemical fume hood. Wash thoroughly after handling. Flammable: Vapor may travel a
considerable distance to source of ignition and flash back. Keep away from heat, sparks, and open flame.
Recipe
2X SDS gel-loading buffer
100 mM Tris-Cl (pH 6.8)
4% (w/v) SDS (electrophoresis grade)
0.2% (w/v) bromophenol blue
20% (v/v) glycerol
200 mM dithiothreitol (DTT)
200 mM -mercaptoethanol can be used instead of DTT.
Store the SDS gel-loading buffer without thiol reagents at room temperature. Add the thiol reagents just
before the buffer is used.
Recipe
Ammonium persulfate
8/21/2008 11:24 AM
SDS-Polyacrylamide Gel Electrophoresis of Proteins -- Sambrook and ...
6 of 9
file:///C:/Users/MILI%20JOON/Documents/protocol/Electrophoresis,...
To prepare a 10% (w/v) solution: Dissolve 1 g ammonium persulfate in 10 ml of H2O and store at 4C.
Ammonium persulfate decays slowly in solution, so replace the stock solution every 2-3 weeks.
Ammonium persulfate is used as a catalyst for the copolymerization of acrylamide and bisacrylamide gels.
The polymerization reaction is driven by free radicals generated by an oxido-reduction reaction in which a
diamine (e.g., TEMED) is used as the adjunct catalyst.
Recipe
SDS (10%) stock solution
Dissolve 10 g of SDS in 80 mL of H2O, and then add H2O to 100 mL. This stock solution is stable for 6
mo at room temperature.
Recipe
Tris-Cl
Tris base
HCl
To prepare a 1 M solution, dissolve 121.1 g of Tris base in 800 mL of H2O. Adjust the pH to the desired
value by adding concentrated HCl.
pH
HCl
7.4
70 mL
7.6
60 mL
8.0
42 mL
Allow the solution to cool to room temperature before making final adjustments to the pH. Adjust the
volume of the solution to 1 L with H2O. Dispense into aliquots and sterilize by autoclaving.
If the 1 M solution has a yellow color, discard it and obtain Tris of better quality. The pH of Tris solutions
is temperature-dependent and decreases ~ 0.03 pH units for each 1C increase in temperature. For
example, a 0.05 M solution has pH values of 9.5, 8.9, and 8.6 at 5C, 25C, and 37C, respectively.
Recipe
8/21/2008 11:24 AM
SDS-Polyacrylamide Gel Electrophoresis of Proteins -- Sambrook and ...
7 of 9
file:///C:/Users/MILI%20JOON/Documents/protocol/Electrophoresis,...
Tris-glycine buffer
Prepare a 5x stock solution in 1 liter of H2O.
15.1 g Tris base
94 g glycine (electrophoresis grade)
50 ml of 10% SDS (electrophoresis grade)
The 1x working solution is 25 mM Tris-Cl/250 mM glycine/0.1% SDS. Use Tris-glycine buffers for
SDS-polyacrylamide gels.
Table
Solutions for preparing 5% stacking gels for Tris-glycine
SDS-polyacrylamide gel electrophoresis
Volume (ml) of Components Required to Cast Gels of Indicated
Volumes
Components
Gel Volume
1 ml
2 ml
3 ml
4 ml
5 ml
6 ml
8 ml
10 ml
0.68
1.4
2.1
2.7
3.4
4.1
5.5
6.8
30% acrylamide mix
0.17
0.33
0.5
0.67
0.83
1.0
1.3
1.7
Tris-Cl (1.0 M, pH 6.8)
0.13
0.25
0.38
0.5
0.63
0.75
1.0
1.25
SDS (10%)
0.01
0.02
0.03
0.04
0.05
0.06
0.08
0.1
ammonium persulfate
0.01
0.02
0.03
0.04
0.05
0.06
0.08
0.1
0.001
0.002
0.003
0.004
0.005
0.006
0.008
0.01
H2O
(10%)
TEMED
Modified from Harlow and Lane (1988).
Table
Solutions for preparing resolving gels for Tris-glycine
SDS-polyacrylamide gel electrophoresis
8/21/2008 11:24 AM
SDS-Polyacrylamide Gel Electrophoresis of Proteins -- Sambrook and ...
8 of 9
file:///C:/Users/MILI%20JOON/Documents/protocol/Electrophoresis,...
Volume (ml) of Components Required to Cast Gels of Indicated
Volumes and Concentrations
Components Gel
5 ml
Volume =>
10 ml
15 ml
20 ml
25 ml
30 ml
40 ml
50 ml
2.6
5.3
7.9
10.6
13.2
15.9
21.2
26.5
30% acrylamide mix 1.0
2.0
3.0
4.0
5.0
6.0
8.0
10.0
Tris-Cl (1.5 M, pH
1.3
2.5
3.8
5.0
6.3
7.5
10.0
12.5
SDS (10%)
0.05
0.1
0.15
0.2
0.25
0.3
0.4
0.5
10% ammonium
persulfate
0.05
0.1
0.15
0.2
0.25
0.3
0.4
0.5
0.004
0.008
0.012
0.016
0.02
0.024
0.032
0.04
2.3
4.6
6.9
9.3
11.5
13.9
18.5
23.2
30% acrylamide mix 1.3
2.7
4.0
5.3
6.7
8.0
10.7
13.3
Tris-Cl (1.5 M, pH
1.3
2.5
3.8
5.0
6.3
7.5
10.0
12.5
SDS (10%)
0.05
0.1
0.15
0.2
0.25
0.3
0.4
0.5
10% ammonium
persulfate
0.05
0.1
0.15
0.2
0.25
0.3
0.4
0.5
0.003
0.006
0.009
0.012
0.015
0.018
0.024
0.03
1.9
4.0
5.9
7.9
9.9
11.9
15.9
19.8
30% acrylamide mix 1.7
3.3
5.0
6.7
8.3
10.0
13.3
16.7
Tris-Cl (1.5 M, pH
1.3
2.5
3.8
5.0
6.3
7.5
10.0
12.5
SDS (10%)
0.05
0.1
0.15
0.2
0.25
0.3
0.4
0.5
10% ammonium
persulfate
0.05
0.1
0.15
0.2
0.25
0.3
0.4
0.5
0.002
0.004
0.006
0.008
0.01
0.012
0.016
0.02
1.6
3.3
4.9
6.6
8.2
9.9
13.2
16.5
30% acrylamide mix 2.0
4.0
6.0
8.0
10.0
12.0
16.0
20.0
6% gel
H2O
8.8)
TEMED
8% gel
H2O
8.8)
TEMED
10% gel
H2O
8.8)
TEMED
12% gel
H2O
8/21/2008 11:24 AM
SDS-Polyacrylamide Gel Electrophoresis of Proteins -- Sambrook and ...
9 of 9
file:///C:/Users/MILI%20JOON/Documents/protocol/Electrophoresis,...
Tris-Cl (1.5 M, pH
1.3
2.5
3.8
5.0
6.3
7.5
10.0
12.5
SDS (10%)
0.05
0.1
0.15
0.2
0.25
0.3
0.4
0.5
10% ammonium
persulfate
0.05
0.1
0.15
0.2
0.25
0.3
0.4
0.5
0.002
0.004
0.006
0.008
0.01
0.012
0.016
0.02
1.1
2.3
3.4
4.6
5.7
6.9
9.2
11.5
30% acrylamide mix 2.5
5.0
7.5
10.0
12.5
15.0
20.0
25.0
Tris-Cl (1.5 M, pH
1.3
2.5
3.8
5.0
6.3
7.5
10.0
12.5
SDS (10%)
0.05
0.1
0.15
0.2
0.25
0.3
0.4
0.5
10% ammonium
persulfate
0.05
0.1
0.15
0.2
0.25
0.3
0.4
0.5
0.002
0.004
0.006
0.008
0.01
0.012
0.016
0.02
8.8)
TEMED
15% gel
H2O
8.8)
TEMED
Modified from Harlow and Lane (1988).
Copyright 2006 by Cold Spring Harbor Laboratory Press. Online ISSN: 1559-6095 Terms of Service All
rights reserved. Anyone using the procedures outlined in these protocols does so at their own risk. Cold
Spring Harbor Laboratory makes no representations or warranties with respect to the material set forth in
these protocols and has no liability in connection with their use. All materials used in these protocols, but
not limited to those highlighted with the Warning icon, may be considered hazardous and should be used
with caution. For a full listing of cautions, click here.
All rights reserved. No part of these pages, either text or images, may be used for any reason other than
personal use. Reproduction, modification, storage in a retrieval system or retransmission, in any form or
by any means-electronic, mechanical, or otherwise-for reasons other than personal use is strictly
prohibited without prior written permission.
CiteULike
Connotea
Del.icio.us
Digg
Technorati
What's this?
8/21/2008 11:24 AM
You might also like
- Isolation of Plasmids From EDocument4 pagesIsolation of Plasmids From ElinubinoyNo ratings yet
- Gel Electrophoresis Lab AutosavedDocument8 pagesGel Electrophoresis Lab Autosavedapi-313889454No ratings yet
- Protein Electrophoresis LabDocument8 pagesProtein Electrophoresis LabMarie St. Louis100% (1)
- Antigen-Antibody Reactions In Vivo: Methods in Immunology and Immunochemistry, Vol. 5From EverandAntigen-Antibody Reactions In Vivo: Methods in Immunology and Immunochemistry, Vol. 5No ratings yet
- Methylation Diagram PDFDocument1 pageMethylation Diagram PDFmetNo ratings yet
- ElectrophoresisDocument17 pagesElectrophoresisShilpaKamathamNo ratings yet
- Sds-Polyacrylamide Gel Electrophoresis IntroductionDocument5 pagesSds-Polyacrylamide Gel Electrophoresis IntroductionmejohNo ratings yet
- Monoclonal Antibody Technology: The Production and Characterization of Rodent and Human HybridomasFrom EverandMonoclonal Antibody Technology: The Production and Characterization of Rodent and Human HybridomasRating: 4 out of 5 stars4/5 (1)
- Factors Affecting PCR Amplifications and The TroubleshootingDocument27 pagesFactors Affecting PCR Amplifications and The TroubleshootingXavier LooNo ratings yet
- Plasmid LabDocument10 pagesPlasmid LabAhmed J AlhindaweNo ratings yet
- Mini PrepDocument6 pagesMini PrepWilson GomargaNo ratings yet
- Polymerase Chain Reaction (PCR)Document21 pagesPolymerase Chain Reaction (PCR)greateinsteinNo ratings yet
- Lab2 BCA Assay HandoutDocument6 pagesLab2 BCA Assay HandoutsandalailaNo ratings yet
- Lab 8 Cell Culture Lab & TransfectionDocument13 pagesLab 8 Cell Culture Lab & Transfectiondead_knightNo ratings yet
- MTT AssayDocument2 pagesMTT AssayHameedhaNo ratings yet
- Bacteria TransformationDocument28 pagesBacteria TransformationAtif Khan100% (1)
- PCR PDFDocument43 pagesPCR PDFAmirNo ratings yet
- Importance of Tris EDTADocument15 pagesImportance of Tris EDTADarshana JuvekarNo ratings yet
- Ethanol PrecipitationDocument4 pagesEthanol PrecipitationPriyanka_1No ratings yet
- ElectrophoresisDocument7 pagesElectrophoresisnavedNo ratings yet
- 3.1.1 HBsAg Testing (ELISA Method)Document6 pages3.1.1 HBsAg Testing (ELISA Method)Jeevan VkiNo ratings yet
- Biotechnology Lab Manual Offered To Iii Year B.Tech Chemical EngineeringDocument34 pagesBiotechnology Lab Manual Offered To Iii Year B.Tech Chemical Engineeringanon_348923763No ratings yet
- PH Meter Use and CalibrationDocument10 pagesPH Meter Use and CalibrationVlarick JongNo ratings yet
- Steps in Tissue Processing For Paraffin Sections: Presented By: Analee Nicole RilleraDocument15 pagesSteps in Tissue Processing For Paraffin Sections: Presented By: Analee Nicole RilleraNico LokoNo ratings yet
- Introduction To SDS PAGEDocument8 pagesIntroduction To SDS PAGESukeshNo ratings yet
- Sample Lab ReportDocument5 pagesSample Lab Reportapi-232072092No ratings yet
- PCR An OverviewDocument41 pagesPCR An OverviewnassradabourNo ratings yet
- ConjugationDocument2 pagesConjugationIwan MuzakiNo ratings yet
- Introduction To Bioinformatics 1Document109 pagesIntroduction To Bioinformatics 1Murugan AnnamalaiNo ratings yet
- Lippinocott's Q& ADocument195 pagesLippinocott's Q& Ag_komolafe100% (1)
- DNA Quantification ProtocolDocument4 pagesDNA Quantification Protocolme_dayakar100% (1)
- PCR Types and Its ApplicationsDocument68 pagesPCR Types and Its ApplicationsShefali Pawar100% (1)
- Agarose Gel ElectrophoresisDocument11 pagesAgarose Gel ElectrophoresisAbrar 111No ratings yet
- Practical Hand Outs-Molecular Biology (BIO302)Document33 pagesPractical Hand Outs-Molecular Biology (BIO302)Anaya MalikNo ratings yet
- DNA Extraction and Agarose Gel ElectrophoresisDocument5 pagesDNA Extraction and Agarose Gel ElectrophoresisMelan Yap0% (1)
- How A Micropipette Works 2Document3 pagesHow A Micropipette Works 2api-437132233No ratings yet
- Pglo Lab ReportDocument4 pagesPglo Lab Reportapi-345256671No ratings yet
- Identification of Unknown PlasmidDocument9 pagesIdentification of Unknown Plasmidapi-233148262No ratings yet
- Dna Purification and Extraction Practical ReportDocument8 pagesDna Purification and Extraction Practical ReportAnselmo ManishaNo ratings yet
- PCR Lab Write UpDocument4 pagesPCR Lab Write Upmadypaddie50% (2)
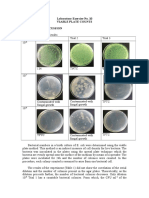
- Laboratory Exercise No. 10 Viable Plate Counts Results and DiscussionDocument3 pagesLaboratory Exercise No. 10 Viable Plate Counts Results and Discussionvanessa olga100% (1)
- Phenomics Workshop ReportDocument42 pagesPhenomics Workshop ReportEdin HamzicNo ratings yet
- Elisa GuideDocument30 pagesElisa GuideNatalia LarionovaNo ratings yet
- MTT Assay Sample ReportDocument5 pagesMTT Assay Sample ReportAlexander VincentNo ratings yet
- BT 0312 - Animal Cell and Tissue Culture LaboratoryDocument47 pagesBT 0312 - Animal Cell and Tissue Culture LaboratoryammaraakhtarNo ratings yet
- Bioinstrumentation Lab ManualDocument19 pagesBioinstrumentation Lab ManualRitayan DeyNo ratings yet
- DNA Quality-Spectrophotometry and ElectrophoresisDocument5 pagesDNA Quality-Spectrophotometry and Electrophoresislovina candra kirana100% (1)
- Restriction Enzyme Digestion of DNADocument2 pagesRestriction Enzyme Digestion of DNAMustansir BhoriNo ratings yet
- RNAi and Epigenetics Source BookDocument172 pagesRNAi and Epigenetics Source BookWenliang Zhang100% (1)
- Bacterial Transformation Lab (6a)Document7 pagesBacterial Transformation Lab (6a)Chris PriceNo ratings yet
- Western BlottingDocument29 pagesWestern BlottingThoppe Rajendran SriramNo ratings yet
- Lab ReportDocument7 pagesLab ReportAlliedschool DefencecampusNo ratings yet
- Molecular BiotechnologyDocument40 pagesMolecular BiotechnologyBhaskar GangulyNo ratings yet
- 6.introduction To TFF - Karen PDFDocument40 pages6.introduction To TFF - Karen PDFpenelopezeus39No ratings yet
- Types of Cell CultureDocument5 pagesTypes of Cell CultureSarah PavuNo ratings yet
- Southern Blotting KLP 7Document21 pagesSouthern Blotting KLP 7nindimediartika100% (1)
- Mutiplexpcr Primer DesignDocument11 pagesMutiplexpcr Primer DesignAnn Irene DomnicNo ratings yet
- Manual On Antimicrobial Susceptibility TestingDocument63 pagesManual On Antimicrobial Susceptibility TestingSai Sridhar100% (1)
- DNA Extraction Reagents - FunctionsDocument1 pageDNA Extraction Reagents - Functionsharpreet157100% (5)
- Size Exclusion Chromatography HandbookDocument139 pagesSize Exclusion Chromatography HandbookJoshua Del CarmenNo ratings yet
- Biology Lecture, Chapter 5Document84 pagesBiology Lecture, Chapter 5Nick GoldingNo ratings yet
- General Biology 2Document398 pagesGeneral Biology 2Joyae ChavezNo ratings yet
- Coatings Paints BrochureDocument2 pagesCoatings Paints BrochureroladinNo ratings yet
- CHAPTER 2 BiologyDocument10 pagesCHAPTER 2 BiologyMohamad ShukriNo ratings yet
- Rethrosyntehsis of Natural ProductsDocument306 pagesRethrosyntehsis of Natural ProductsMed AjNo ratings yet
- Method For Preparation of Vitamin C and Method For Determination of Vitamin C in TabletsDocument18 pagesMethod For Preparation of Vitamin C and Method For Determination of Vitamin C in TabletsTi MaNo ratings yet
- Amino AcidsDocument10 pagesAmino AcidsWingki Ari Angga100% (1)
- Beneficiation of AvocadoDocument10 pagesBeneficiation of AvocadoJUANNo ratings yet
- Chapter 4 and 5 - Qualitative Research - Practical Research 1Document25 pagesChapter 4 and 5 - Qualitative Research - Practical Research 1Jesha JuanNo ratings yet
- CatecholaminesDocument30 pagesCatecholaminesashutoshkafle100% (1)
- TLC TraduccionDocument16 pagesTLC TraduccionKarlos Lds NvNo ratings yet
- OrganicChemistryChapter5 PDFDocument19 pagesOrganicChemistryChapter5 PDFJuliet Tatiana CumbeNo ratings yet
- The Big TEGO. Products Services Data Sheets-75-150-16!76!31-61Document31 pagesThe Big TEGO. Products Services Data Sheets-75-150-16!76!31-61DWI RAHMASARI FATMAWATINo ratings yet
- Liquid FuelsDocument77 pagesLiquid FuelsMNButtNo ratings yet
- Yacon SyrupDocument40 pagesYacon SyrupTrilceNo ratings yet
- Narayana GT 1 Sol PDFDocument9 pagesNarayana GT 1 Sol PDFgyandattNo ratings yet
- Sara Naji TabasiDocument13 pagesSara Naji TabasiVasincuAlexandruNo ratings yet
- (Solvent) Diol Aldehyde-Alcohol Ketone-Alcohol Compound 1 Compound 2 Compound 3Document10 pages(Solvent) Diol Aldehyde-Alcohol Ketone-Alcohol Compound 1 Compound 2 Compound 3Ilias YacNo ratings yet
- Gas Emission From Landfills: An Overview of Issues and Research NeedsDocument57 pagesGas Emission From Landfills: An Overview of Issues and Research NeedsPedro AlarcónNo ratings yet
- Iit Jam Organic - Theory Book: IndexDocument28 pagesIit Jam Organic - Theory Book: IndexYbynybybyhNo ratings yet
- Food Canning Waste in Industrial Processes: April 2016Document6 pagesFood Canning Waste in Industrial Processes: April 2016Loredana Veronica ZalischiNo ratings yet
- 1 1Document2 pages1 1nur azizahNo ratings yet
- Daelim Polybutene CatalogueDocument9 pagesDaelim Polybutene CatalogueFernando García Pacheco100% (2)
- 3 Cosmetics PDFDocument3 pages3 Cosmetics PDFAkshit MalikNo ratings yet
- 12 Chemistry23 24 sp01Document14 pages12 Chemistry23 24 sp01bhattkrrish339No ratings yet
- Colling Klein, Bonomi, Maciel Filho - 2018 - Integration of Microalgae Production With Industrial Biofuel Facilities A Critical ReviewDocument17 pagesColling Klein, Bonomi, Maciel Filho - 2018 - Integration of Microalgae Production With Industrial Biofuel Facilities A Critical ReviewfvassisNo ratings yet
- A Comparative Study For The Organic Byproducts FroDocument16 pagesA Comparative Study For The Organic Byproducts FroArmiee InfiniteNo ratings yet
- 9701 w03 Ms 1+2+3+4+5+6Document29 pages9701 w03 Ms 1+2+3+4+5+6Bismaht0% (1)
- Chem 31 PROCEDURES (Practicals)Document9 pagesChem 31 PROCEDURES (Practicals)FMDCNo ratings yet
- 1602 01684 PDFDocument61 pages1602 01684 PDFSandra MedeirosNo ratings yet