Professional Documents
Culture Documents
Catàlisis 4rt Curs Curs 2015 16 Grau Química Problemes Unitat 1
Uploaded by
ssxOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Catàlisis 4rt Curs Curs 2015 16 Grau Química Problemes Unitat 1
Uploaded by
ssxCopyright:
Available Formats
Catlisis
Curs201516
PROBLEMESUNITAT1
4rtcurs
GrauQumica
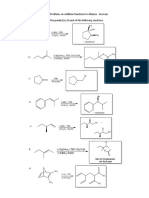
1. Themostimportant(andalsothemostexpensive)grapefruitaromacompoundisthebicyclicterpenenootkatone.Itis
manufacturedbyoxidationofvalencene,whichisextractedfromValenciaoranges.Nextfigureshowstworoutesfor
thisoxidation,astoichiometricreactionusingchromiumtrioxide,andacatalyticalternativeusingsodiumhypochlorite
(bleach)inthepresenceof1mol%osmiumtetraoxidecatalyst:
(a) Comparetheatomeconomybetweenthetworeactionsaccordingto:
(b)CalculatetheEfactorsvaluesforbothoptions.
(c)GiventhatbothCrOandOsO4areequallytoxic,estimatetheQvaluesinbothcases,andexplainwhichoption
youfavorandwhy.
2.
The classic synthesis of hydroquinone starts with aniline, and uses stoichiometric MnO2, sulfuric acid, and iron,
accordingtothefollowingreactions(equationsarenotbalanced);
O
NH2
+ MnO2 + H2SO4
O
+ (NH4)2SO4 + MnSO4 + H2O
O
+ Fe + H2O
OH
+ FeO
O
OH
(a) Calculatetheatomeconomyforthisprocess
(b)Anilineitselfismadebynitrationofbenzenetonitrobenzene,followedbyhydrogenation.Usingtheprinciples
ofgreenchemistry,drawaschemeforanewprocessformakinghydroquinone,startingdirectlyfrombenzene
(feelfreetoinventanycatalystsyouneed).
(c) Search on the Internet for information on the Upjohn hydroquinone process, and compare that with your
synthesisroute.WhataretheadvantagesoftheUpjohnprocessandofyoursynthesiscomparedtotheclassic
route?Arethereanydisadvantages?
3.
Fluorescentlightbulbscontainmercury,whichisreleasedintotheenvironmentwhenthebulbsaredisposedofin
landfills.Incandescent(regular)lightbulbscontainnomercury,andsodisposalisnotaproblem.However,regular
bulbsusemoreelectricitythanfluorescentones,whichmeansburningmorecoalatthepowerstation,andburning
coalalsoreleasesmercuryintotheenvironment.Atypicalfluorescentbulbconsumes11Wandburnsfor5000h,
whileatypicalincandescentoneconsumes75Wandburnsfor1000h.
(a)Constructtwolifecyclecharts,oneforfluorescentlightbulbsandoneforincandescentlightbulbs.
(b)Assumingthatcoalcontainstypically20ppmmercuryimpurities,whichtypeoflightbulbisbetter,fluorescentor
incandescent,fortheenvironment?
Hint:Ifyoudonotfindabettervalue,considerthattheefficiencyofpowerplantthatgenerateselectricityburning
coalis30%
4.
The asymmetric hydrogenation of aryl ketones is an important step in the synthesis of many pharmaceutical
intermediates.BlaserandcoworkersshowedthatRucomplexeswithFecyclopentadienyl.sandwichcomplexesare
good catalysts for this reaction. The next figure shows the different substrates tested, along with the time,
conversion, and substrate/catalyst ratio. Using these data, calculate for each reaction the catalyst TON and the
averageTOFalongthewholereaction.
5.
Naproxenisanonsteroidalanalgesicandantiinflammatorydrugwidelyused.Thedrugissuppliedasthepure(S)
enantiomer, since the (R) enantiomer is a liver toxin. Although enantiomerically pure Naproxen is industrially
produced by resolution of the racemate, it can be prepared by asymmetric hydroformylation, followed by the
oxidation of the enantomerically enriched aldehyde to the corresponding carboxylic acid, by using in the
hydroformylationstepthehomochiralcatalystRhH(L)(CO)2(L=(R,S)BINAPHOS)developedbyNozakietal.
Assumethatyouarerunningapilotplantinvestigatingthisreaction.Startingwith153.0molsof2methoxy6
vinylnaphthaleneyougetthefollowingreactionmixture:
Calculate:
a) Thereactionconversion
b) The chemoselectivity, the regioselectivity in the branched aldehyde, and the enantioselectovity in the (S)
enantiomer.
c) Theyieldin(S)2(6methoxynaphthalen2yl)propanal
6.
The following graphics represent the % of conversion versus time (in minutes) in experiments corresponding the
hydrogenationof(S)limonenewithRhCl(PPh3)3catalystsindifferentreactionconditions:
Exp1:0.100mmolofcatalyst,12.0mmolof(S)limonene,P(H2)=20bar;T=80oCwith10mloftolueneassolvent.
Exp2:0.400mmolofcatalyst,200mmolof(S)limonene,P(H2)=30bar;T=50oCwith60mloftolueneassolvent.
Fromthedataabove,calculate:
a) TheinitialTOFofeachreaction
b) TheaverageTOFforeachreactionduringthefirst20minutes
c) ThemaximumTONforthehydrogenationof(S)limonenecatalyzedbyRhCl(PPh3)3
You might also like
- Organic C CCCC CCCCDocument88 pagesOrganic C CCCC CCCCKugan KishurNo ratings yet
- Terpenoids and SteroidsDocument370 pagesTerpenoids and SteroidsJessica PizzoNo ratings yet
- CHEM 210-1 Organic Chemistry NotesDocument26 pagesCHEM 210-1 Organic Chemistry NotesRobert GardnerNo ratings yet
- Alkenes TutorialDocument8 pagesAlkenes TutorialVarshLokNo ratings yet
- Common Foundation Organic Q in A LevelDocument21 pagesCommon Foundation Organic Q in A Level黄维燕No ratings yet
- Organic-Synthesis & ChiralityDocument34 pagesOrganic-Synthesis & ChiralityAlaa Al HamedNo ratings yet
- 2007 ADocument4 pages2007 AAmiro MayraNo ratings yet
- Austrian 25Document13 pagesAustrian 25Vo Tung LamNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Ukraine Chemistry Olympiad - 2009Document29 pagesUkraine Chemistry Olympiad - 2009AryanNo ratings yet
- (Oxford Chemistry Primers, 01) Susan E. Thomas - Organic Synthesis - The Roles of Boron and Silicon-Oxford University Press (1992) PDFDocument96 pages(Oxford Chemistry Primers, 01) Susan E. Thomas - Organic Synthesis - The Roles of Boron and Silicon-Oxford University Press (1992) PDFUjjal Das100% (4)
- Advanced Enzymes White PaperDocument16 pagesAdvanced Enzymes White PaperAnubhav TripathiNo ratings yet
- Practice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersFrom EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersRating: 3 out of 5 stars3/5 (2)
- Practice E12Document10 pagesPractice E12Stephen BoandohNo ratings yet
- 1st Final Examination of OcDocument2 pages1st Final Examination of OcRahmanida SusianaNo ratings yet
- Alkyne Revision SlideDocument43 pagesAlkyne Revision SlideAditya RamNo ratings yet
- Complex Chemistry: Problem 1 4 PointsDocument13 pagesComplex Chemistry: Problem 1 4 PointsATHAYYA FORTUNANo ratings yet
- Final Worksheet For Pre-Engineering StudentsDocument5 pagesFinal Worksheet For Pre-Engineering Studentshermela697No ratings yet
- Chemistry Past Paper Ch4.1Document13 pagesChemistry Past Paper Ch4.1Raymond ChanNo ratings yet
- Aldehyde KetoneDocument5 pagesAldehyde Ketonehareharanbt22No ratings yet
- Cuaderno de Trabajo - 2019-2Document35 pagesCuaderno de Trabajo - 2019-2Monica BravoNo ratings yet
- Quaternary Ammonium HalidesDocument5 pagesQuaternary Ammonium HalidesromeroeqNo ratings yet
- Chemistry 2018 FinalDocument24 pagesChemistry 2018 FinalmilapdhruvcomputerworkNo ratings yet
- Topic 15 Organic Chemistry: Carbonyls, Carboxylic Acids and ChiralityDocument9 pagesTopic 15 Organic Chemistry: Carbonyls, Carboxylic Acids and ChiralitysalmaNo ratings yet
- Mark Scheme: University of Malta Matriculation Certificate Examination Intermediate Level MAY 2010Document17 pagesMark Scheme: University of Malta Matriculation Certificate Examination Intermediate Level MAY 2010Bernice JohnsonNo ratings yet
- Effects of Complexants On (Ni Co MN) Co Morphology and Electrochemical Performance of Lini Co MN ODocument10 pagesEffects of Complexants On (Ni Co MN) Co Morphology and Electrochemical Performance of Lini Co MN OHEIDY JOVANA HUANCA RAMOSNo ratings yet
- Attachment 1Document5 pagesAttachment 1Eva AberaNo ratings yet
- Acjc 08 Paper 3Document8 pagesAcjc 08 Paper 3Zenaida AtinorNo ratings yet
- Free Radicals 12 QuesDocument62 pagesFree Radicals 12 Quesdinesh111180No ratings yet
- Practice Problems On Addition Reactions To Alkenes With AnswersDocument4 pagesPractice Problems On Addition Reactions To Alkenes With AnswersSangetha ChelladoraiNo ratings yet
- Atoms, Molecules and Stoichiometry STPMDocument5 pagesAtoms, Molecules and Stoichiometry STPMIna DinNo ratings yet
- 2018 PU2 H2 05 Halogen Derivatives Tutorial AnswerDocument12 pages2018 PU2 H2 05 Halogen Derivatives Tutorial AnswerNeil SharmaNo ratings yet
- Cuaderno de Trabajo - 2019-2Document35 pagesCuaderno de Trabajo - 2019-2Monica BravoNo ratings yet
- 2008 Promo 1Document15 pages2008 Promo 1shinkir0No ratings yet
- 180 Question Bank 2nd PucDocument10 pages180 Question Bank 2nd PucudaysrinivasNo ratings yet
- Aldehydes Ketones Carboxylic AcidsDocument22 pagesAldehydes Ketones Carboxylic AcidsvenkithebossNo ratings yet
- CHEM1007Session FinalExamPracticeDocument2 pagesCHEM1007Session FinalExamPracticeRoop SinghNo ratings yet
- INCHO10Document39 pagesINCHO10Amit SharmaNo ratings yet
- Acfrogbyyb W54zpzfswkn8k3vq0clq6et8mk Ne Px62hvrlk5chrlql9xx83xtq2sr0dqcuhrswcoglr Ueky068cras4ph7jxkmy 143kq0wnhekbynbh 4 Eq1p0kvslajoriecir6ikqqswDocument8 pagesAcfrogbyyb W54zpzfswkn8k3vq0clq6et8mk Ne Px62hvrlk5chrlql9xx83xtq2sr0dqcuhrswcoglr Ueky068cras4ph7jxkmy 143kq0wnhekbynbh 4 Eq1p0kvslajoriecir6ikqqswThanh Hằng NgôNo ratings yet
- CBSE Sample Paper Class 12 Chemistry Set 4Document5 pagesCBSE Sample Paper Class 12 Chemistry Set 4Sidharth SabharwalNo ratings yet
- HL ChemistryDocument12 pagesHL ChemistryVithursan ThangarasaNo ratings yet
- 2010 SAJC H2 Chem Prelim P1Document19 pages2010 SAJC H2 Chem Prelim P1Giovanni AndersonNo ratings yet
- Class 12 Important QuestionsDocument4 pagesClass 12 Important Questionsmisraadyasha6No ratings yet
- Industrial - Engineering Chemistry Process Design and Development Volume 11 Issue 3 1972 (Doi 10.1021 - I260043a002) Kurtz, B. E. - Homogeneous Kinetics of Methyl Chloride ChlorinationDocument7 pagesIndustrial - Engineering Chemistry Process Design and Development Volume 11 Issue 3 1972 (Doi 10.1021 - I260043a002) Kurtz, B. E. - Homogeneous Kinetics of Methyl Chloride ChlorinationbagasAjNo ratings yet
- Sure-Shot Questions-Chemistry Class XII: 1markDocument5 pagesSure-Shot Questions-Chemistry Class XII: 1markudit pandyaNo ratings yet
- (WWW - Entrance Exam - Net) Chemistry GsebDocument8 pages(WWW - Entrance Exam - Net) Chemistry GsebmjdNo ratings yet
- Effects of Synthesis Conditions On The Structural and Electrochemical PropertiesDocument5 pagesEffects of Synthesis Conditions On The Structural and Electrochemical PropertiesjoseNo ratings yet
- Chemistry Mid 2 Beyour - DoctorDocument20 pagesChemistry Mid 2 Beyour - Doctorsyeda ruqaiyah ashfaqNo ratings yet
- Which of The Following Statements About The Equivalence Point of An AcidDocument10 pagesWhich of The Following Statements About The Equivalence Point of An AcidCorrine PerezNo ratings yet
- CHM 1311 Final Exam PDFDocument12 pagesCHM 1311 Final Exam PDFMutahir KhattakNo ratings yet
- Code:SP/LV-2 Sample Paper: General InstructionsDocument3 pagesCode:SP/LV-2 Sample Paper: General InstructionsKhogen MairembamNo ratings yet
- Examen Campinas InglesDocument7 pagesExamen Campinas InglesSharon Laurente RamónNo ratings yet
- AIEEE Grand Test - 1Document13 pagesAIEEE Grand Test - 1Sayan Kumar KhanNo ratings yet
- Chemistry s5 Theory and Pract.Document29 pagesChemistry s5 Theory and Pract.ngabonzizayusuf9No ratings yet
- Anic Chemistry AK 2018-19Document22 pagesAnic Chemistry AK 2018-19XXXNo ratings yet
- Chemistry Notes For Town BoysDocument5 pagesChemistry Notes For Town BoysArnabNo ratings yet
- Organic TestDocument4 pagesOrganic Testpritam neogiNo ratings yet
- 2014 H2 Alkanes Tut (Teachers)Document14 pages2014 H2 Alkanes Tut (Teachers)Chen ZhihaoNo ratings yet
- Chemistry For Students of Mechanical Engineering Studiengang BachelorDocument9 pagesChemistry For Students of Mechanical Engineering Studiengang BachelorAsif KhanNo ratings yet
- Aieee 2011 ChemistryDocument8 pagesAieee 2011 ChemistryEdward StewartNo ratings yet
- Chemistry STPM Sem 3 MSAB Pre-Trial QuestionDocument6 pagesChemistry STPM Sem 3 MSAB Pre-Trial QuestionKenneth Chan43% (7)
- Selected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionFrom EverandSelected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionNo ratings yet
- Novel Nanoscale Hybrid MaterialsFrom EverandNovel Nanoscale Hybrid MaterialsBhanu P. S. ChauhanNo ratings yet
- Application of IC-MS and IC-ICP-MS in Environmental ResearchFrom EverandApplication of IC-MS and IC-ICP-MS in Environmental ResearchRajmund MichalskiNo ratings yet
- G43337a2016 17iENGDocument4 pagesG43337a2016 17iENGssxNo ratings yet
- 2016/2017 Work Placement II: Use of Languages ContactDocument3 pages2016/2017 Work Placement II: Use of Languages ContactssxNo ratings yet
- 2016/2017 Design of Integrated Systems For Digital ProcessingDocument4 pages2016/2017 Design of Integrated Systems For Digital ProcessingssxNo ratings yet
- G43337a2016 17iENGDocument4 pagesG43337a2016 17iENGssxNo ratings yet
- G43335a2016 17iENGDocument5 pagesG43335a2016 17iENGssxNo ratings yet
- 2016/2017 Business Management and Administration: Use of Languages ContactDocument3 pages2016/2017 Business Management and Administration: Use of Languages ContactssxNo ratings yet
- 2016/2017 Biopsychological Aspects of People: Use of Languages ContactDocument5 pages2016/2017 Biopsychological Aspects of People: Use of Languages ContactssxNo ratings yet
- 2016/2017 Pattern Analysis and Recognition: Use of Languages ContactDocument6 pages2016/2017 Pattern Analysis and Recognition: Use of Languages ContactssxNo ratings yet
- G103682a2016 17iENG PDFDocument5 pagesG103682a2016 17iENG PDFssxNo ratings yet
- G103682a2016 17iENG PDFDocument5 pagesG103682a2016 17iENG PDFssxNo ratings yet
- G103682a2016 17iENG PDFDocument5 pagesG103682a2016 17iENG PDFssxNo ratings yet
- 2016/2017 Biopsychological Aspects of People: Use of Languages ContactDocument5 pages2016/2017 Biopsychological Aspects of People: Use of Languages ContactssxNo ratings yet
- Fragrances: Chiral Chemistry in FlavoursDocument6 pagesFragrances: Chiral Chemistry in Flavoursmohamed tharwatNo ratings yet
- CML 100 Questions PDFDocument7 pagesCML 100 Questions PDFDivyansh GuptaNo ratings yet
- Application of Biocatalysis and Biotransformations To The Synthesis of PharmaceuticalsDocument19 pagesApplication of Biocatalysis and Biotransformations To The Synthesis of PharmaceuticalsAndrés David PolancoNo ratings yet
- Drugs of The Future 2002, 27 (2) 143-158Document16 pagesDrugs of The Future 2002, 27 (2) 143-158Rajesh TammanaNo ratings yet
- Bullitine CSIRDocument46 pagesBullitine CSIRSrikanth Naik ANo ratings yet
- Steriochemistry SteriochemistryDocument98 pagesSteriochemistry SteriochemistryPankaj SenNo ratings yet
- CML 100 QuestionsDocument7 pagesCML 100 QuestionsamanNo ratings yet
- Bossing Nicole PDFDocument47 pagesBossing Nicole PDFMark CastilloNo ratings yet
- Adv Synth Catal - 2011 - Busacca - The Growing Impact of Catalysis in The Pharmaceutical IndustryDocument40 pagesAdv Synth Catal - 2011 - Busacca - The Growing Impact of Catalysis in The Pharmaceutical IndustrymariNo ratings yet
- (13653075 - Pure and Applied Chemistry) Reflections On The Total Synthesis of Natural Products - Art, Craft, Logic, and The Chiron ApproachDocument16 pages(13653075 - Pure and Applied Chemistry) Reflections On The Total Synthesis of Natural Products - Art, Craft, Logic, and The Chiron ApproachyaswanthNo ratings yet
- Chem - Organic SynthesisDocument8 pagesChem - Organic SynthesiswhoyaNo ratings yet
- 02a PDFDocument19 pages02a PDFSyed Ali Akbar BokhariNo ratings yet
- Chemistry PG 2015 Admn On05sept2015Document96 pagesChemistry PG 2015 Admn On05sept2015ZiyadNo ratings yet
- Schiff Base Catalysts For The Asymmetric Strecker Reaction Identified and Optimized From Parallel Synthetic LibrariesDocument2 pagesSchiff Base Catalysts For The Asymmetric Strecker Reaction Identified and Optimized From Parallel Synthetic LibrariesТNo ratings yet
- Improved Methodology For The Preparation of Chiral AminesDocument170 pagesImproved Methodology For The Preparation of Chiral AminesEric David López DomínguezNo ratings yet
- Improved Synthesis of Enantiopure 4-Hydroxy (2.2) ParacyclophaneDocument3 pagesImproved Synthesis of Enantiopure 4-Hydroxy (2.2) ParacyclophaneDiogo DiasNo ratings yet
- 2006 J. Org. Chem. 2006, 71, 7083-7086Document4 pages2006 J. Org. Chem. 2006, 71, 7083-7086sudheeshNo ratings yet
- Stereochemistry Practce PDFDocument6 pagesStereochemistry Practce PDFFerminNo ratings yet
- Bs Alkal 2018 11 001Document117 pagesBs Alkal 2018 11 001Shar KhanNo ratings yet
- 10 1002@ (Sici) 1521-3765 (19990401) 5:4 1320::aid-Chem1320 3 0 Co 2-#Document11 pages10 1002@ (Sici) 1521-3765 (19990401) 5:4 1320::aid-Chem1320 3 0 Co 2-#Yassine SabekNo ratings yet
- 18-Noyori Asymmetric Hydrogenation ReactionDocument11 pages18-Noyori Asymmetric Hydrogenation ReactionImdadur RahamanNo ratings yet
- Preamble and Syllabus MSC ChemistryDocument34 pagesPreamble and Syllabus MSC ChemistrycomgmailNo ratings yet
- ZXC ZCDocument26 pagesZXC ZCboiroyNo ratings yet
- Jurnal Aldehid Dan KetonDocument170 pagesJurnal Aldehid Dan KetonMsv Desma AdriansyahNo ratings yet
- (Catalysis by Metal Complexes 31) Evgenii Klabunovskii, Gerard v. Smith (Auth.), Evgenii Klabunovskii, Gerard v. Smith, Ágnes Zsigmond (Eds.)-Heterogeneous Enantioselective Hydrogenation_ Theory and pDocument313 pages(Catalysis by Metal Complexes 31) Evgenii Klabunovskii, Gerard v. Smith (Auth.), Evgenii Klabunovskii, Gerard v. Smith, Ágnes Zsigmond (Eds.)-Heterogeneous Enantioselective Hydrogenation_ Theory and pCarlos EsescritorNo ratings yet