Professional Documents
Culture Documents
Mycorrhizal Effectiveness and Manganese Toxicity in Soybean As Affected by Soil Type and Endophyte
Uploaded by
biodaviOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mycorrhizal Effectiveness and Manganese Toxicity in Soybean As Affected by Soil Type and Endophyte
Uploaded by
biodaviCopyright:
Available Formats
Mycorrhizal effectiveness and manganese toxicity in soybean
329
MYCORRHIZAL EFFECTIVENESS AND MANGANESE
TOXICITY IN SOYBEAN AS AFFECTED BY
SOIL TYPE AND ENDOPHYTE
Marco Antonio Nogueira1; Elke Jurandy Bran Nogueira Cardoso2,3*
1
UEL - Depto. de Microbiologia, C.P. 6001 - 86051-990 - Londrina, PR - Brasil.
USP/ESALQ - Depto. de Solos e Nutrio de Plantas, C.P. 9 - 13418-900 - Piracicaba, SP - Brasil.
3
CNPq scholar.
*Corresponding author <ejbncard@esalq.usp.br>
2
ABSTRACT: Mycorrhizal plants may present Mn toxicity alleviation and this depends on the plant-endophyteenvironment interaction. The effectiveness of three arbuscular mycorrhizal fungi (AMF) (Glomus
macrocarpum, G. etunicatum, G. intraradices) and a control without AMF in two soils: Typic Rhodudalf
with high Mn availability and a Typic Quartzipsamment, with low Mn availability, was evaluated in a timecourse experiment at 3, 6, 9 and 12 weeks after soybean (Glycine max L.) seedling emergence. The objective
was to select the most effective AMF species to enhance plant growth and to assess its effects upon Mn
uptake by plants and Mn availability in the soil. For the sandy soil, AMF inoculation resulted in increased
plant biomass, especially with G. intraradices and G. etunicatum. Lower Mn concentrations were observed
in shoot and root of mycorrhizal plants. For the clayey soil, there was also an increase in plant biomass, but
only for plants inoculated with G. intraradices and G. etunicatum. Mycorrhizal plants presented higher Mn
concentrations in shoot and root and there was an increase of available Mn in the soil, in relation to the
control, especially in the treatment with G. macrocarpum. When inoculated with G. macrocarpum, plants
presented Mn toxicity symptoms and reduced biomass in comparison to control plants. The effects of
mycorrhizal inoculation, either positive or negative, were most intense at 9 and 12 weeks.
Key words: Mn, availability, metal, mycorrhiza, toxicity, uptake
EFICINCIA MICORRZICA E TOXIDEZ DE MANGANS EM SOJA
EM FUNO DO TIPO DE SOLO E DA ESPCIE DO ENDFITO
RESUMO: Plantas micorrizadas podem apresentar atenuao da toxidez causada pelo excesso de Mn, mas
isso depende da interao planta-endfito-ambiente. Avaliou-se a eficincia de trs espcies de fungos
micorrzicos arbusculares (FMA) (Glomus macrocarpum, G. etunicatum, G. intraradices, e um controle sem
FMA, em quatro pocas de coleta (3, 6, 9 e 12 semanas aps a emergncia das plntulas de soja (Glycine max
L.)) num Nitossolo Vermelho eutrofrrico tpico, muito argiloso, com alta disponibilidade de Mn e num
Neossolo Quartzarnico tpico, com baixa disponibilidade de Mn. O objetivo foi avaliar a eficincia micorrzica
desses FMA nesses substratos, em diversas pocas, bem como os efeitos sobre a disponibilidade de Mn no
substrato e sua absoro pelas plantas. No substrato arenoso, a micorrizao aumentou a biomassa das plantas,
com destaque para G. intraradices e G. etunicatum. Nesse caso, houve menor concentrao de Mn na parte
area e razes das plantas. No substrato argiloso, a micorrizao com G. intraradices e G. etunicatum aumentou
a biomassa das plantas, mas tambm aumentou a disponibilidade de Mn no substrato e a concentrao na
parte area e razes em relao ao controle, com mais intensidade nas plantas com G. macrocarpum. Nesse
caso, houve sintomas de toxidez de Mn e diminuio da biomassa das plantas. Os efeitos da micorrizao,
positivos ou negativos, foram mais expressivos com 9 e 12 semanas.
Palavras-chave: Mn, absoro, disponibilidade, metal, micorriza, toxicidade
INTRODUCTION
Mycorrhizal associations are related to several
benefits to the host plant. Besides the improvement of the
nutritional state, other benefits like increasing of plant resistance to pathogens, to ionic stresses such as Mn excess (Cardoso, 1985; Bethlenfalvay & Franson, 1989) and
to drought stress are also relevant. Nevertheless, depending on the interactions between the endophyte, the host
Scientia Agricola, v.60, n.2, p.329-335, Abr./Jun. 2003
and the environment, the effects may as well be negative
or deleterious to the host plant (Medeiros et al., 1995;
Nogueira & Cardoso, 2000).
Although there is no known host specificity for
arbuscular mycorrhizal fungi (AMF), root colonization
and growth response depend on the interaction of both
symbionts and they are affected by several conditions,
such as soil pH (Lambais & Cardoso, 1988), fungal species or isolate (Lambais & Cardoso, 1990) and the stage
Nogueira & Cardoso
330
of development of the host plant (Bethlenfalvay et al.,
1982b). Therefore, very often one can not find a simple
correlation between the percent root colonization and host
growth responses (Allen, 2001).
The lack of correlation between infectivity and
mycorrhizal effectiveness may be related to the time that
it takes for the establishment of the symbiosis, mainly for
annual crops (Abbott & Robson, 1981). Nogueira &
Cardoso (2000) observed that Gigaspora margarita, besides presenting a slow phase of root colonization, also
produced less total external mycelium compared to G.
intraradices. This behavior probably limited plant response to mycorrhization and induced a transient plant
growth depression in relation to the non-mycorrhizal control. As the plant response to mycorrhization may vary
along the plant cycle, wrong interpretations may arise
from short time experiments, mainly those in which the
AMF presents a slow phase of root colonization, without enough time to eventually have a host growth increase
(Bethlenfalvay et al., 1982b). The proportion between the
external and internal mycorrhizal mycelium may also influence the symbiosis effectiveness (Abbott & Robson,
1981). A negative response for host growth (parasitism)
may be the result of great internal fungal colonization and
lack of enough external growth for an effective mining
of the nearby soil (Bethlenfalvay et al., 1982a).
Infectivity as well as symbiotic effectiveness for
the different endophytes vary according to the plant-fungus-environment interactions, and this poses the need for
selecting the most effective interaction for each condition.
The aim of this work was to evaluate the effectiveness
of three AMF species in promoting soybean development,
and their effects on the availability and uptake of Mn by
plants grown in two soils: one sandy, with low Mn availability and one clayey, with high Mn availability.
MATERIAL AND METHODS
The experiment was installed in a greenhouse
with pots containing four kg of soil, previously autoclaved at 121oC for 2h. Treatments consisted of two soil
types: sandy (0-0.2 m layer of a Typic Quartzipsamment,
containing 830, 40 and 130 g kg-1 of sand, silt and clay,
respectively) and clayey (0-0.2 m layer of a Typic
Rhodudalf, containing 250, 150 and 600 g kg-1 of sand,
silt and clay, respectively), in combination with three species of AMF (Glomus macrocarpum, G. etunicatum, G.
intraradices) and a non-inoculated control, with six replicates. Plants were harvested at 3, 6, 9 and 12 weeks after emergence. The choice of these three AMF was based
on previous studies in which their effectiveness in increasing soybean growth, as well as in alleviating the toxicity caused by Mn excess was verified (Cardoso, 1985).
The fertilization of the clayey soil was based on
its chemical analysis (Table 1). There was no need for
liming or K and S supplement. Biological fixation was
responsable for N supply by inoculating the plantlets at
emergence with 4 mL of a cell suspension (c.a. 1 x 107
mL-1) of the strains SEMIA 587 and SEMIA 5019 of
Bradyrhizobium elkanii. Phosphorus was added according to the results obtained by Nogueira & Cardoso (2000),
aiming to favour the mycotrophic condition, by giving 51
mg of P per pot as ground (<0.75 mm) triple superphosphate. Boron (0.48 mg as H3BO3 per pot) and Mo (0.15
mg as Na2MoO4.2H2O per pot) were furnished in a nutrient solution.
Liming and fertilization of the sandy soil were
performed with the objective of having conditions similar to those of the clayey soil in relation to nutrient availability. The soil was limed to reach a cation saturation
level (V%) of 87%, the same observed in the clayey soil,
Table 1 - Chemical characteristics of the soils utilized in the experiment before (B) and after (A) autoclaving. Sandy = Typic
Quartzipsamment; Clayey = Typic Rhodudalf.
pH
C a C l2
S a mp le
M. O .
g d m- 3
S a nd y
C la ye y
3.9
K+
S- SO 4
- - - - mg d m- 3 - - - 2
8
14
10
C a 2+
M g2+
Al3+
H+ Al
SB
- - - - - - - - - - - - - - - - - - - - - mmo lc d m- 3 - - - - - - - - - - - - - - - - - - - - - 0.5
47
4.5
52
--- % --9
67
3.8
14
1.1
38
9.1
47
19
47
5.5
28
14
11
5.8
48
19
25
73.8
98
74
6.2
29
16
14
8.0
82
27
18
11 7
135
87
mic ro nutrie nts
B
Cu
Fe
Mn
Zn
-3
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - mg d m - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - S a nd y
0.40
0.5
75.6
A
C la ye y
B
A
0.44
0.21
0.3
124.0
9.8
0.9
0.70
7.9
10.8
35.8
3.5
8.8
8.4
365.0
5.1
Scientia Agricola, v.60, n.2, p.329-335, Abr./Jun. 2003
3.6
1.1
Mycorrhizal effectiveness and manganese toxicity in soybean
by adding 3.7 g of dolomitic lime per pot (CaO 42%,
MgO 25%). Phosphorus was also added as ground triple
superphosphate at a rate of 83.25 mg P per pot. Potassium and S were given at rates of 1.67 g of KCl and 65
mg of CaSO4.2H2O per pot, respectively. Boron (1.17 mg
as H3BO3), Cu (1.5 mg as CuSO4.5H2O), Zn (0.9 mg as
ZnSO4.7H2O) and Mo (0.15 mg as Na2MoO4.2H2O) were
also added as nutrient solution. Iron and Mn were not
added to both soils.
The inoculation of each AMF species was made
with a spore suspension, extracted by wet sieving
(Gerdemann & Nicolson, 1963) from a soil cultivated
with Brachiaria decumbens. About 240 spores were
added per pot and incorporated into the 5 cm uppermost
layer. All pots received 5 mL of a filtrate obtained by
shaking 100 g of soil with 1.5 L of distilled water. The
suspension was filtered through several sieves, the last
one with 45 mm mesh. The objective of this procedure
was to reestablish the original microbial community of
the soils in all treatments, except for AMF propagules.
Five seeds of the soybean cultivar IAC-8 were
planted per pot after disinfection with a 25% NaClO commercial solution for 5minutes, and rinsing with distilled
water. One week after emergence, plantlets were thinned
to one per pot. Plants were watered daily with distilled
water.
At each harvesting period, 48 pots were harvested. Shoots were cut, washed in a sequence of distilled
water, 0.01 mol L-1 HCl solution, and deionized water and
dried at 60oC until reaching constant weight, to obtain the
shoot dry weight (SDW). Soil was removed from roots,
then washed in tap water and the same sequence described
above was followed to obtain the root dry weight (RDW).
Root samples were clarified and stained (Phillips &
Hayman, 1970) for the evaluation of percent root colonization by AMF by means of a squared counting plate
(Giovanneti & Mosse, 1980).
Shoots and roots were ground in a 60 mesh sieve
mill and digested with nitric-perchloric acid solution to
determine the percentage of P and Mn in plant tissues.
Phosphorus was determined by metavanadate colorimetry
and Mn by atomic absorption spectrophotometry. There
was not enough material for nutrient analysis in the roots.
A soil sample was stored at 5oC to estimate the
total external mycelium length (TEM), according to
Sylvia (1992), modified by Melloni & Cardoso (1999).
These determinations were performed in duplicate, and
the mean was used for calculations. To assure the maximum recovery of external mycelium from both soils,
50 mL of a dispersing solution of sodium pyrophosphate
(20 g L-1) were added to the samples, incubating them for
40 minutes before extraction. Another soil sample was air
dried, passed through 2 mm sieves and analyzed for available Mn, extracted by Mehlich-I solution (HCl 0.05 mol
L-1 + H2SO4 0.05 mol L-1, 25:2,5, shaken for 15 minutes
Scientia Agricola, v.60, n.2, p.329-335, Abr./Jun. 2003
331
and filtered through filter paper Whatman 42). The extract was evaluated in a Perkin-Elmer atomic absorption
spetrophotometer model 560.
Results were statistically analyzed for each harvesting time as a 4 x 2 factorial by the statistical software The Statistical Analysis System (SAS) (SAS, 1991),
employing the t test at P < 0.05. Data for percent root colonization were transformed to (x + 0.5)1/2 before analysis.
The mycorrhizal effectiveness was calculated according
to Lambais & Cardoso (1990), by using the total plant
dry weight (SDW + RDW).
RESULTS
Manganese availability in the soil increased after autoclaving to eliminate native AMF. This increase
was about ten times in the clayey soil and almost three
times in the sandy soil (Table 1).
SDW and RDW (Table 2) were influenced more
expressively by mycorrhizal treatments at the 12 weeks
harvest. In the sandy soil there was no difference with
regard to SDW between the treatments with G.
etunicatum and G. intraradices. The AMF effect on RDW
was seen only at 12 weeks, especially with G. etunicatum
and G. intraradices, when compared to control plants and
those with G. macrocarpum. In the clayey soil, however,
G. etunicatum promoted a greater plant growth, while
plants with G. macrocarpum had a SDW equivalent to
the non-mycorrhizal control, and they presented Mn toxicity symptoms as described by Foy et al. (1978).
Generally, for the sandy soil, concentrations of P
were greater in mycorrhizal plants in relation to the control, both in shoots and roots (Figure 1). In plants inoculated with G. macrocarpum, grown in the clayey soil,
concentrations of P in the tissues were, in general, the same
as those of the control plants. On the other hand, plants with
G. etunicatum and G. intraradices presented greater P concentrations in both shoots and roots. The Mn concentrations
in plants grown in the sandy soil, either in shoots, or in roots
(Figure 2), were greater than in the control plants. In the
sandy soil, plants did not present Mn toxicity symptoms at
any harvesting period. Conversely, mycorrhizal plants grown
in the clayey soil, in general, presented greater Mn concentrations, mainly when inoculated with G. macrocarpum. This
caused Mn toxicity symptoms, which probably contributed
to reduce plant growth (Table 2). At 12 weeks of age, Mn
concentrations in shoots and roots of plants with G.
macrocarpum were 1300 and 3400 mg kg-1, respectively,
while in the other treatments, Mn concentration did not surpass 700 mg kg-1 in the shoots and 600 mg kg-1 in the roots.
Interestingly, 3- and 6-week old plants with G. etunicatum
presented the highest Mn concentration in the shoots (about
700 mg kg-1), which coincided with the lowest values of
SDW and RDW at 6 weeks (Table 2) and the appearance
of Mn toxicity symptoms in leaves.
Nogueira & Cardoso
332
Table 2 - Shoot (SDW) and root (RDW) dry weights of soybean plants, every three weeks, cultivated in a clayey and a sandy
soil, with or without (control) arbuscular mycorrhizal fungi. Same letters at the same harvesting period and soil do
not differ (t test at P < 0.05).
W EEK S
AM F
3
S DW
C o ntr o l
G . in t r a r a d ic e s
G . e t u n ic a t u m
G . m a c ro c a r p u m
RD W
PH O SPH O RU S,g kg-1
SH O O TS
RO O TS
a
ab
bc
ns
a ns
ab
ab
b
1200
a
b
a
b
b
c
c
a
ba
b
a
a
b
a
aaa
b b
RD W
S DW
12 3
Control
G .etunicatum
Clayey
a
a
a a
a
ba
S DW
G .intraradices
G .m acrocarpum
Sandy
RD W
12
RD W
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - s a nd y - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 0.40 a
0.12 a
2.08 a
0.53 a
6.98 b
1.38 a
13.16 c
2.68 b
0.50 a
0.15 a
2.71 a
0.68 a
9.63 a
1.66 a
26.50 a
4.81 a
0.52 a
0.15 a
2.60 a
0.54 a
10.43 a
1.62 a
25.32 a
4.36 a
0.35 a
0.09 a
2.52 a
0.57 a
8.75 ab
1.62 a
18.20 b
3.29 b
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - c la ye y - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 0.65 a
0.27 a
2.42 b
0.88 a
6.80 b
1.84 b
13.32 bc
3.75 b
0.70 a
0.31 a
3.44 a
0.92 a
9.30 a
2.00 ab
16.83 b
3.42 b
0.64 a
0.23 a
2.20 b
0.67 a
11 . 1 8 a
2.29 a
21.5 a
4.69 a
0.70 a
0.29 a
2.40 b
0.73 a
4.78 b
1.08 c
9.98 c
2.18 c
Control
G .etunicatum
3
S DW
a
a
aa
bb
b b
12
W EEK S
M A N G A N ESE,m g kg-1
SH O O TS
RO O TS
C o ntr o l
G . in t r a r a d ic e s
G . e t u n ic a t u m
G . m a c ro c a r p u m
G .intraradices
G .m acrocarpum
Clayey
Sandy
a
a
800
400 ns
a
b
b b
b
b b
b b b
a
a
b
b b
aa
b
bb
b
a
3300
900
600
300
a
a
bbb
bb
bbb b
12 3
ab
bc
c
c
a
bbb
b
b b
12
W EEK S
Figure 1 - P concentration, every three weeks, in shoot and root of
soybean plants, cultivated in a clayey and a sandy soil,
with or without (control) arbuscular mycorrhizal fungi.
Same letters at the same harvesting period and soil do
not differ (t test at P < 0.05); n.s. = non-significant.
Figure 2 - Mn concentration, every three weeks, in the shoot and
root of soybean plants, cultivated in a clayey and a sandy
soil, with or without (control) arbuscular mycorrhizal
fungi. Same letters at the same harvesting period and
soil do not differ (t test at P < 0.05); n.s. = non-significant.
Total external mycelium (TEM) in the soil from
mycorrhizal treatments was, in general, greater than in the
soil with control plants, except for G. macrocarpum in the
clayey soil (Figure 3). The average values of TEM found
in the clayey soil were higher than in the sandy soil. Root
colonization reached 70% at 9 weeks in the sandy soil (Figure 3). In the clayey soil, root colonization also reached
this value in treatments with G. intraradices and G.
etunicatum, but G. macrocarpum presented the lowest levels of root colonization, about 12% at 9 weeks. In all mycorrhizal treatments, the greatest level of root colonization
occurred at 9 weeks, followed by a decrease at 12 weeks.
The concentration of available manganese was
higher in the clayey soil (Figure 4). In the sandy soil, Mn
concentration varied from 5 to 8 mg dm-3 and there was
no effect of the mycorrhizal treatments. In the clayey soil,
however, Mn availability varied from 84 to 414 mg dm-3
and increased for the mycorrhizal treatments, especially
with G. macrocarpum.
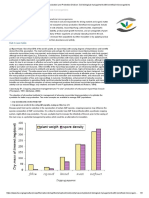
All AMF species in the sandy soil resulted in an
increased host growth as compared to control plants, except at three weeks, when G. macrocarpum decreased plant
growth (Figure 5). In the clayey soil, plants with G.
etunicatum presented a slight negative effect at 3 and 6
weeks, but this was the most effective treatment at the other
harvesting periods. G. macrocarpum was the less effective
AMF, resulting, at 9 and 12 weeks, in about 30% less biomass of the host plant in relation to the control plants. In
both soils, the effect of the endophytes on plant development increased with plant age.
Scientia Agricola, v.60, n.2, p.329-335, Abr./Jun. 2003
G .intraradices
G .etunicatum
G .m acrocarpum
80 Sandy
Clayey
a
a
60
40
ab
ab
bb
bb
c
d
ab
30
n.s.
a
a
45
n.s.
30
a
a
n.s.
12
Clayey
3
w eeks
60
b
b
a
ab
75
15
a
a
90 Sandy
60
333
b ab
RO O T
CO LO N IZA TIO N ,%
Control
12
W EEK S
Figure 3 - Total external mycelium length and percent root
colonization of soybean plants colonized by arbuscular
mycorrhizal fungi, every three weeks, in the shoot and
root of soybean plants, cultivated in a clayey and a
sandy soil, with or without (control) mycorrhizal fungi.
Same letters at the same harvesting period and soil do
not differ (t test at P < 0.05); n.s. = non-significant.
M Y CO RRH IZA L EFFECTIV EN ESS,%
TO TA L EX TERN A L
-1
M Y CELIU M ,m g
Mycorrhizal effectiveness and manganese toxicity in soybean
-30
90
60
6
w eeks
30
0
-30
90
60
9
w eeks
30
0
-30
90
60
12
w eeks
30
-3
A V A ILA BLE M n,m g dm
G .intraradices
G .m acrocarpum
Control
G .etunicatum
8 Sandy
Clayey
400
7
n.s.
aa
n.s.
300
n.s.
n.s.
12
b
bc
G .intraradices
G .etunicatum
G .m acrocarpum
200
-30
b
c
100
12
Figure 5 - Arbuscular mycorrhizal fungi effectiveness on soybean
plant growth in relation to the non-mycorrhizal control,
every three weeks, in a clayey and a sandy soil.
W EEK S
Figure 4 - Available manganese, every three weeks, in a clayey and
a sandy soil, cultivated with soybean, with or without
(control) arbuscular mycorrhizal fungi. Same letters at
the same harvesting period and soil do not differ (t test at
P < 0.05); n.s. = non-significant.
DISCUSSION
The finding that plants associated with G.
macrocarpum in the clayey soil presented more Mn toxicity symptoms and lower growth was surprising, since
this behavior was expected to occur in the control plants.
In previous experiments, plants inoculated with G.
macrocarpum, but not the same isolate, presented lower
Mn concentration and suppression of Mn toxicity symptoms (Cardoso, 1985). The enhancement or alleviation of
Mn toxicity in mycorrhizal plants is not exclusively attributed to the AMF species, but may be the result of several interactions attributed to changes in host physiology,
with reflections on the microbial community in the
Scientia Agricola, v.60, n.2, p.329-335, Abr./Jun. 2003
mycorrhizosphere (Filion et al., 1999) and on the biological processes of Mn oxidation (Nealson et al., 1988) and
reduction (Kothari et al., 1991). This hypothesis is reinforced by the increase of Mn availability in the clayey
soil with AMF inoculated plants. In this case, the increase
of Mn availability resulted in the increase of Mn concentration in the plant. Nevertheless, mycorrhizal plants associated with G. etunicatum and G. intraradices also presented higher P concentrations in the tissues, in spite of
their greater biomass. The higher P concentration in these
plants may have enabled better conditions to support the
higher Mn concentration in the tissues, suppressing the
Mn toxicity symptoms in these cases. Bethlenfalvay &
Franson (1989) observed that, although mycorrhizal
plants presented greater Mn concentrations, there were no
toxicity symptoms. This might have occurred because of
an increase of internal tolerance to Mn (Foy et al., 1978)
by plants better fed with P. One of the tolerance mechanisms may be related to a precipitation reaction between
Mn2+ and phosphate anions inside the plants, reducing
334
Nogueira & Cardoso
Mn 2+ activity and, consequently, its adverse effects
(Murphy et al., 1981). Habte & Soedarjo (1996) verified
the formation of low solubility products between Mn and
P in the soil, resulting in Mn3(PO4)2. There is no evidence
that these reactions did also occur inside the plant in this
experiment, but it is a plausible hypothesis. The value
for the concentration of Mn in the plant tissue does not
inform about the real activity of Mn2+ ions. One portion
of the total Mn could be perhaps precipitated by reactions with phosphate ions, but because of the use of the
nitric-perchloric digestion process, all Mn could have
been solubilized and quantified. Electron microscopy
with X-ray microanalysis might give a better idea about
the microdistribution of these possible precipitates inside plant cells (Memon et al., 1981). The lower Mn
concentration found in the mycorrhizal plants in
the sandy soil probably occurred because of a dilution
effect caused by a better growth, because the decrease
in Mn concentration was proportional to the plant
biomass increase.
The negative effect of G. macrocarpum in plants
grown in clayey soil may be related to certain characteristics of the endophyte. Lower TEM production and the
lowest root colonization rates may have been the factors
responsible for the lower plant growth, and that indicates
the lack of adaptation of this AMF species to that soil.
Nogueira and Cardoso (2000) also observed this behavior for other AMF species when associated to soybean,
with negative effects on mycorrhizal effectiveness. Microbial interactions may affect mycorrhization and also
plant growth when bacteria antagonistic to the AMF become dominant in the rhizosphere (Andrade et al., 1995).
Independently of the real cause, the AMF acted as a C
sink for the host plant (Bethlenfalvay et al., 1982a).
The increase of Mn availability in the soil after
autoclaving is attributed to the thermal breakage of organic chelators, one of the main factors that regulate Mn
availability. Miyazawa et al. (1993) observed that Mn
availability decreased with the incubation time, as a result of microbial synthesis of new chelators, but this is
also influenced by the activity of Mn oxidizers (Nealson
et al., 1988) and Mn reducers (Kothari et al., 1991). In
the sandy soil, there was a general decrease of Mn availability with time. In the treatments with AMF in the
clayey soil, there was an increase of Mn availability. It
is not possible to state definitely whether or not the increase of Mn availability, especially in the treatment
with G. macrocarpum, can be attributed to changes in
the balance between Mn oxidizers or reducers in the
rhizosphere as a result of quantitative or qualitative
changes in the root exudates resulting from AMF interactions (Ames et al., 1984) or if it was a direct effect of
root exudates, such as organic acids or reducing compounds, modulated by the presence of mycorrhiza
(Habte & Soedarjo, 1996).
Scientia Agricola, v.60, n.2, p.329-335, Abr./Jun. 2003
A decrease in root colonization was observed
from 9 to 12 weeks, and this may have resulted from an
extra C sink related to the pod formation. Possibly root
colonization was restricted by the limitation of energetic
sources for growth and maintenance of the AMF. In addition, there was active root growth up to 12 weeks, causing dilution of the root colonization rate observed at 9
weeks. This behavior probably would not occur if root
colonization results were expressed as absolute values of
colonized root length, instead of relative values as root
colonization percentage (Allen, 2001).
Variations observed in the mycorrhizal effectiveness at different harvesting periods show that results obtained at early plant stages are different from those obtained at late stages. For example, considering the evaluation at 6 weeks in the clayey soil, G. etunicatum was
ineffective and G. macrocarpum had no effect on the
plants. Nevertheless, at 12 weeks, the inverse was observed: plants with G. etunicatum presented better
growth, while plants with G. macrocarpum presented
growth reduction in relation to the control. These observations indicate the importance of evaluating plants after they have had enough time to complete mycorrhizal
symbiosis establishment and to express the beneficial or,
eventually, adverse effects on plant growth.
CONCLUSIONS
Mycorrhizal effectiveness varied from beneficial
to adverse or indifferent according to the AMF, soil type
and stage of plant development. Mycorrhiza alters Mn
availability in the soil, and this affects the Mn concentration in the plants. In the ineffective interaction, mycorrhiza may increase the expression of Mn toxicity symptoms in the host plant. In the effective interaction, in spite
of increased Mn availability in the soil and increased Mn
concentration in plants, no Mn toxicity symptoms were
detected in the plants.
ACKNOWLEDGMENTS
To Denise de Lourdes Colombo Mescolotti and
Lus Fernando Baldesin for their assistance during the installation, conduction and analysis of the experiments.
REFERENCES
ABBOTT, L.K.; ROBSON, A.D. Infectivity and effectiveness of vesicular
arbuscular mycorrhizal fungi: effect of inoculation type. Australian
Journal of Agricultural Research, v.32, p.631-639, 1981.
ALLEN, M.F. Modeling arbuscular mycorrhizal infection: Is % infection
an appropriate variable? Mycorrhiza, v.10, p.255-258, 2001.
AMES, R.N.; REID, C.P.P.; INGHAM, E.R. Rhizosphere bacterial
population responses to root colonization by a vesicular-arbuscular
mycorrhizal fungus. New Phytologist, v.96, p.555-563, 1984.
ANDRADE, G.; AZCN, R.; BETHLENFALVAY, G.J. A rhizobacterium
modifies plant and soil responses to the mycorrhizal fungus Glomus
mosseae. Applied Soil Ecology, v.2, p.195-202, 1995.
Mycorrhizal effectiveness and manganese toxicity in soybean
BETHLENFALVAY, G.J.; BROWN, M.S.; PACOVSKY, R.S. Relationships
between host and endophyte development in mycorrhizal soybeans. New
Phytologist, v.90, p.537-543, 1982a.
BETHLENFALVAY, G.J.; PACOVSKY, R.S.; BROWN, M.S. et al.,
Mycotrophic growth and mutualistic development of host plant and
fungal endophyte in an endomycorrhizal symbiosis. Plant and Soil,
v.68, p.43-54, 1982b.
BETHLENFALVAY, G.J.; FRANSON, R.L. Manganese toxicity alleviated
by mycorrhizae in soybean. Journal of Plant Nutrition, v.12, p.953970, 1989.
CARDOSO, E.J.B.N. Efeito de micorriza vesculo-arbuscular e fostatode-rocha na simbiose soja-Rhizobium. Revista Brasileira de Cincia
do Solo, v.9, p.125-130, 1985.
FILION, M.; ST-ARNAUD, M.; FORTIN, J.A. Direct interaction between
the arbuscular mycorrhizal fungus Glomus intraradices and different
rhizosphere microorganisms. New Phytologist, v.141, p.525-533, 1999.
FOY, C.D.; CHANEY, R.L.; WHITE, M.C. The physiology of metal toxicity
in plants. Annual Review of Plant Physiology, v.29, p.511-566, 1978.
GERDEMANN, J.W.; NICOLSON, T.H. Spores of mycorryzal Endogone
extracted from soil by wet sieving end decanting. Transactions of the
British Mycological Society, v.46, p.235-244, 1963.
GIOVANNETI, M.; MOSSE, B. An evaluation of techniques for measuring
vesicular arbuscular mycorrhizal infection in roots. New Phytologyst,
v.84, p.489-500, 1980.
HABTE, M.; SOEDARJO, M. Response of Acacia mangium to vesiculararbuscular mycorrhizal inoculation, soil pH, and soil P concentration in
an oxisol. Canadian Journal of Botany, v.74, p.155-161, 1996.
KOTHARI, S.K.; MARSCHNER, H.; RMHELD, V. Effect of a vesiculararbuscular mycorrhizal fungus and rhizosphere micro-organisms on
manganese reduction in the rhizosphere and manganese concentrations
in maize (Zea mays L.). New Phytologist, v.117, p.649-655, 1991.
LAMBAIS, M.R.; CARDOSO, E.J.B.N. Avaliao da germinao de
esporos de fungos micorrzicos vesculo-arbusculares e da colonizao
micorrzica de Stylosanthes guianansis em solo cido e distrfico.
Revista Brasileira de Cincia do Solo, v.12, p.249-255, 1988.
Scientia Agricola, v.60, n.2, p.329-335, Abr./Jun. 2003
335
LAMBAIS, M.R.; CARDOSO, E.J.B.N. Response of Stylosanthes
guianensis to endomycorrhizal fungi inoculation as affected by lime and
phosphorus applications. Plant and Soil, v.129, p.283-289, 1990.
MEDEIROS, C.A.B.; CLARK, R.B.; ELLIS, J.R. Effects of excess
manganese on mineral uptake in mycorrhizal sorghum. Journal of
Plant Nutrition, v.18, p.201-217, 1995.
MELLONI, R.; CARDOSO, E.J.B.N Quantificao de miclio
extrarradicular de fungos micorrzicos arbusculares em plantas ctricas
I. Mtodo empregado. Revista Brasileira de Cincia do Solo, v.23,
p.53-58, 1999.
MEMON, A.R.; CHINO, M.; HARA, K. et al., Microdistribution of
manganese in the leaf tissues of different plant species as revealed by
X-ray microanalyzer. Physiologia Plantarum, v.53, p.225-232, 1981.
MIYAZAWA, M.; PAVAN, M.A.; MARTIN-NETO, L. Provvel mecanismo
de liberao de mangans no solo. Pesquisa Agropecuria Brasileira,
v.28, p.725-731, 1993.
MURPHY, L.S.; ELLIS Jr., R.; ADRIANO, D.C. Phosphorus-micronutrient
interaction effects on crop production. Journal of Plant Nutrition,
v.3, p.593-613, 1981.
NEALSON, K.H.; TEBO, B.M.; ROSSON, R.A. Occurence and
mechanisms of microbial oxidation of manganese. Advances in Applied
Microbiology, v.33, p.279-318, 1988.
NOGUEIRA, M.A.; CARDOSO, E.J.B.N. Colonizao radicular e produo
de miclio externo por duas espcies de fungos micorrzicos arbusculares
em soja. Revista Brasileira de Cincia do Solo, v.24, p.329-338, 2000.
PHILLIPS, J.M.; HAYMAN, A.S. Improved procedures for clearing roots
and staining parasitic and vesicular-arbuscular mycorrhizal fungi for
assessment of infection. Transactions of the British Mycological
Society, v.55, p.158-161, 1970.
SAS INSTITUTE. Procedure guide for personal computers. Release
6.11. 5.ed. Cary: Statistical Analysis System Institute, 1991.
SYLVIA, D.M. Quantification of external hyphae of vesicular-arbuscular
mycorrhizal fungi. Methods in Microbiology, v.24, p.54-65, 1992.
Received December 07, 2001
You might also like
- Case Study Rheumatoid ArthritisDocument16 pagesCase Study Rheumatoid ArthritisJessy Mallo100% (2)
- Cancer Biology How Science Works by Carsten Carlberg, Eunike VelleuerDocument179 pagesCancer Biology How Science Works by Carsten Carlberg, Eunike VelleuerAndreeaPopescuNo ratings yet
- Agronomy: Effects of Manganese Nanoparticle Exposure On Nutrient Acquisition in Wheat (Triticum Aestivum L.)Document16 pagesAgronomy: Effects of Manganese Nanoparticle Exposure On Nutrient Acquisition in Wheat (Triticum Aestivum L.)Juan PabloNo ratings yet
- 8776 21642 1 SMDocument10 pages8776 21642 1 SM9315 Nabila Alya IslamiNo ratings yet
- Effects of Applying Arbuscular Mycorrhizal Fungi To Nitrogen Cycle Microorganisms in Soils With CoffeeDocument16 pagesEffects of Applying Arbuscular Mycorrhizal Fungi To Nitrogen Cycle Microorganisms in Soils With Coffeebrandonggualotuna2004No ratings yet
- M C Jaizme Vega Et Al - Effect of Arbuscular Mycorrhizal Fungi (Amf) and Other Rhizosphere Microorganisms On Development of The Banana Root SystemDocument15 pagesM C Jaizme Vega Et Al - Effect of Arbuscular Mycorrhizal Fungi (Amf) and Other Rhizosphere Microorganisms On Development of The Banana Root SystemandresuribeNo ratings yet
- Liu 2000Document6 pagesLiu 2000Debrup GhoshNo ratings yet
- Accumulation and Export of Micronutrients in Potato Fertilized - NdmokgqDocument10 pagesAccumulation and Export of Micronutrients in Potato Fertilized - NdmokgqSTAR BOX MUSICNo ratings yet
- A Study On The Effects of Different BiofertilizerDocument5 pagesA Study On The Effects of Different BiofertilizerOliver TalipNo ratings yet
- 40 Agri MehrabanDocument3 pages40 Agri Mehrabanshirazi60No ratings yet
- Effect - of - Pesticides - On - Plant - Growth - Pro PicsDocument5 pagesEffect - of - Pesticides - On - Plant - Growth - Pro Picsatifmd250No ratings yet
- Arbuscular Mycorrhizal Fungus Rhizophagus Irregularis Influences Key Physiological Parameters of Olive TreesDocument10 pagesArbuscular Mycorrhizal Fungus Rhizophagus Irregularis Influences Key Physiological Parameters of Olive TreesbeneNo ratings yet
- Riginal Rticle: M - Jam I M Oeini1 M - Arm In, M .R. Asgharipour and Sara Karimi YazdiDocument8 pagesRiginal Rticle: M - Jam I M Oeini1 M - Arm In, M .R. Asgharipour and Sara Karimi YazdiRivan SuryadevanggaNo ratings yet
- None Ab8fdfb6Document13 pagesNone Ab8fdfb6Misel 1No ratings yet
- Sugarcane MonocroppingDocument15 pagesSugarcane MonocroppingAlexa MariusNo ratings yet
- Denton 2007Document8 pagesDenton 2007Gregorio AroneNo ratings yet
- Foliar Application of Amino Acids and MicronutrientsDocument5 pagesFoliar Application of Amino Acids and MicronutrientsShaina OrnopiaNo ratings yet
- ART RuiOliveira 2019Document9 pagesART RuiOliveira 2019Trang MốcNo ratings yet
- TMP 47 D8Document8 pagesTMP 47 D8FrontiersNo ratings yet
- DiversityDocument25 pagesDiversityK. Jeremy Hidalgo ArnezNo ratings yet
- Ajol File Journals - 82 - Articles - 56104 - Submission - Proof - 56104 973 94877 1 10 20100702Document10 pagesAjol File Journals - 82 - Articles - 56104 - Submission - Proof - 56104 973 94877 1 10 20100702PabloYaupiEspinozaNo ratings yet
- 0100 2945 RBF 40 5 e 024Document11 pages0100 2945 RBF 40 5 e 024David CataguaNo ratings yet
- Chitosan Application in Maize (Zea Mays) To Counteract The Effects of Abiotic Stress at Seedling LevelDocument8 pagesChitosan Application in Maize (Zea Mays) To Counteract The Effects of Abiotic Stress at Seedling LevelRamadi WiditaNo ratings yet
- Effect of Glomus Fasciculatum On The Growth and Yield of Tomato (Solanum Lycopersicum L.) in Meloidogyne Incognita Infested SoilDocument3 pagesEffect of Glomus Fasciculatum On The Growth and Yield of Tomato (Solanum Lycopersicum L.) in Meloidogyne Incognita Infested SoilShailendra RajanNo ratings yet
- Improvement of ArbusculaireDocument5 pagesImprovement of Arbusculairehanane boutajNo ratings yet
- Article 219365Document15 pagesArticle 219365aungh8088No ratings yet
- Pertumbuhan Kedelai (Glycine Max Mikoriza Arbuskular (Fma) Dan Konsorsium MikrobaDocument14 pagesPertumbuhan Kedelai (Glycine Max Mikoriza Arbuskular (Fma) Dan Konsorsium MikrobaRiska RamadaniNo ratings yet
- Jurnal Kakao Di Tanah AndosolDocument9 pagesJurnal Kakao Di Tanah AndosolFaradila KookieNo ratings yet
- 33 2 129 PDFDocument9 pages33 2 129 PDFRoyce SumagaysayNo ratings yet
- Revista Brasileira de Engenharia Agrícola e AmbientalDocument6 pagesRevista Brasileira de Engenharia Agrícola e AmbientalJesu JesuNo ratings yet
- Cadmio 3Document11 pagesCadmio 3greco lavayenNo ratings yet
- Trichoderma AsperellumDocument8 pagesTrichoderma AsperellumEnsiklopedia BiologiNo ratings yet
- NSJV Almond Field Day - Fumigant HandoutsDocument4 pagesNSJV Almond Field Day - Fumigant HandoutsDavid DollNo ratings yet
- Soil Poster 2017Document1 pageSoil Poster 2017Noor Khairani Muhamad BasriNo ratings yet
- Microbes for Climate Resilient AgricultureFrom EverandMicrobes for Climate Resilient AgriculturePrem Lal KashyapNo ratings yet
- Malathion Degradation by Azospirillum Lipoferum BeijerinckDocument10 pagesMalathion Degradation by Azospirillum Lipoferum BeijerinckUmesh MogleNo ratings yet
- Artículo Indizado 2014 - Biofumigación-SolarizaciónDocument11 pagesArtículo Indizado 2014 - Biofumigación-Solarizacióncatay de la cruz fernando joseNo ratings yet
- Diversity of Arbuscular Mycorrhizal Fungi Spores in Maize (Zea Mays L.) Plantations in Côte D'ivoireDocument11 pagesDiversity of Arbuscular Mycorrhizal Fungi Spores in Maize (Zea Mays L.) Plantations in Côte D'ivoireGermain DrohNo ratings yet
- 1 s2.0 S0325754120301255 MainDocument10 pages1 s2.0 S0325754120301255 Mainrinca239No ratings yet
- 1809-6891-Cab-20 - Sds Dfsdfe-38586Document12 pages1809-6891-Cab-20 - Sds Dfsdfe-38586FADASDADSNo ratings yet
- Copper Resistence of Different Ectomycorrhizal Fungi Such As PisolithusDocument9 pagesCopper Resistence of Different Ectomycorrhizal Fungi Such As PisolithusIsabelle_BebelleNo ratings yet
- PDFDocument14 pagesPDFMayMaya10No ratings yet
- Tekaya2017 Article ArbuscularMycorrhizalFungusRhiDocument9 pagesTekaya2017 Article ArbuscularMycorrhizalFungusRhiImed CheraiefNo ratings yet
- Biomassa e Atividade Microbiana Do Solo em Área de Cultivo de SojaDocument7 pagesBiomassa e Atividade Microbiana Do Solo em Área de Cultivo de SojaPaulo DiasNo ratings yet
- Functional Diversity of Mycorrhiza and Sustainable Agriculture: Management to Overcome Biotic and Abiotic StressesFrom EverandFunctional Diversity of Mycorrhiza and Sustainable Agriculture: Management to Overcome Biotic and Abiotic StressesNo ratings yet
- 1276 1535725406 PDFDocument14 pages1276 1535725406 PDFramotNo ratings yet
- Toxics 09 00179 v2Document18 pagesToxics 09 00179 v2Yami LujanNo ratings yet
- Herdari, Brook, Jones Barley P 2023Document16 pagesHerdari, Brook, Jones Barley P 2023Robert BrookNo ratings yet
- 10 1016@j Jhazmat 2019 120813Document9 pages10 1016@j Jhazmat 2019 120813arthurgladysntabalaNo ratings yet
- Article 1 Biofertilizers and Sustainable AgricultureDocument12 pagesArticle 1 Biofertilizers and Sustainable AgriculturePaolita CortesNo ratings yet
- Beneficial Microorganisms For Sustainable Agriculture: January 2012Document6 pagesBeneficial Microorganisms For Sustainable Agriculture: January 2012king balochNo ratings yet
- DECOMPOSITION AND NUTRIENT RELEASE OF LEGUMINOUS PLANTS IN COFFEE AGROFORESTRY SYSTEMS - MATOS Et Al, 2010Document9 pagesDECOMPOSITION AND NUTRIENT RELEASE OF LEGUMINOUS PLANTS IN COFFEE AGROFORESTRY SYSTEMS - MATOS Et Al, 2010Heder SchuabNo ratings yet
- Docu 12Document12 pagesDocu 12Franco Cerna CuevaNo ratings yet
- Hill 2000Document12 pagesHill 2000Redd ZhuangNo ratings yet
- Plant Production and Protection Division - Soil Biological Management With Beneficial MicroorganismsDocument3 pagesPlant Production and Protection Division - Soil Biological Management With Beneficial MicroorganismsCyberSquare IndiaNo ratings yet
- 1 s2.0 S1161030121000654 MainDocument12 pages1 s2.0 S1161030121000654 MainAB LimaNo ratings yet
- Jurnal Internasional The Effect of ManureDocument11 pagesJurnal Internasional The Effect of ManureIkram AffandiNo ratings yet
- Articulo para BIOKADocument13 pagesArticulo para BIOKACamila MontesinosNo ratings yet
- Phytophthora MegakaryaDocument17 pagesPhytophthora MegakaryaMd Ashikur RahmanNo ratings yet
- Massot 2016Document7 pagesMassot 2016Victoria Andrea VitaliNo ratings yet
- AMF and Soil FertilityDocument15 pagesAMF and Soil FertilityCharu KaushikNo ratings yet
- Canola Traits and Some Soil Biological Parameters in Response To Fertilization and Tillage ManagementDocument6 pagesCanola Traits and Some Soil Biological Parameters in Response To Fertilization and Tillage ManagementTensae EwunetieNo ratings yet
- Recurso 29Document146 pagesRecurso 29biodaviNo ratings yet
- Recurso 29 1Document17 pagesRecurso 29 1biodaviNo ratings yet
- PSPP Users' Guide: GNU PSPP Statistical Analysis Software Release 0.10.2Document206 pagesPSPP Users' Guide: GNU PSPP Statistical Analysis Software Release 0.10.2biodaviNo ratings yet
- Recurso 20Document3 pagesRecurso 20biodaviNo ratings yet
- Classificacoes Publicadas Ciencias Agrarias I 2017 1496941693120Document71 pagesClassificacoes Publicadas Ciencias Agrarias I 2017 1496941693120biodaviNo ratings yet
- C B W - D M S: Apacity Uilding Orkshop ATA Anagement OftwareDocument20 pagesC B W - D M S: Apacity Uilding Orkshop ATA Anagement OftwarebiodaviNo ratings yet
- 2004 Papageorgiou GovindjeeDocument43 pages2004 Papageorgiou GovindjeebiodaviNo ratings yet
- Eddy Covariance LI-CORDocument345 pagesEddy Covariance LI-CORbiodaviNo ratings yet
- Manual Imaging PamDocument221 pagesManual Imaging PambiodaviNo ratings yet
- Plant Ecophysiology - BIOL 255: Course Outline 2011Document5 pagesPlant Ecophysiology - BIOL 255: Course Outline 2011biodaviNo ratings yet
- ChymosinDocument242 pagesChymosinvbortizlNo ratings yet
- Concepts of BiologyDocument1,007 pagesConcepts of BiologyEduardo Panadero CuarteroNo ratings yet
- Meselson and Stahl Experiment DeterminedDocument1 pageMeselson and Stahl Experiment DeterminedCarolina RelvaNo ratings yet
- Book ListDocument3 pagesBook ListYekitaSNo ratings yet
- Mutations of Voltage-Gated Sodium Channel Genes SCN1A and SCN2A in Epilepsy, Intellectual Disability, and AutismDocument19 pagesMutations of Voltage-Gated Sodium Channel Genes SCN1A and SCN2A in Epilepsy, Intellectual Disability, and Autismyasmin al ammariNo ratings yet
- Ayurveda and The Science of AgingDocument8 pagesAyurveda and The Science of AgingRGuti13No ratings yet
- Antiviral DrugsDocument12 pagesAntiviral DrugsAmelia RumiNo ratings yet
- ENS ReportDocument13 pagesENS ReportAirastormNo ratings yet
- IB DP Biology 1.4 Membrane Transport QuestionDocument19 pagesIB DP Biology 1.4 Membrane Transport QuestionikaNo ratings yet
- In Vitro Propagation of Cannabis Sativa L. and Evaluation of Regenerated Plants For Genetic Fidelity and Cannabinoids Content For Quality AssuranceDocument14 pagesIn Vitro Propagation of Cannabis Sativa L. and Evaluation of Regenerated Plants For Genetic Fidelity and Cannabinoids Content For Quality AssuranceOmar Arias AndradeNo ratings yet
- General Biology Module 3Document12 pagesGeneral Biology Module 3reyloyd tabontabonNo ratings yet
- The Importance of A Balanced DietDocument4 pagesThe Importance of A Balanced DietdimdamflyNo ratings yet
- 04 Tissue, Glands and MembranesDocument52 pages04 Tissue, Glands and MembranesBryan Mae H. Degorio100% (1)
- v4n2 9pdfDocument10 pagesv4n2 9pdfissaninNo ratings yet
- 2023 Projects Microbiology-Hons-BookletDocument56 pages2023 Projects Microbiology-Hons-Bookletchand198No ratings yet
- PlatyhelminthesDocument4 pagesPlatyhelminthesSyamsul Bahri HsNo ratings yet
- Nutritional Aspects of Exercise and Sport Performance PlannerDocument2 pagesNutritional Aspects of Exercise and Sport Performance PlannerUSman TArarNo ratings yet
- Grace Et Al 2003b SAJBDocument63 pagesGrace Et Al 2003b SAJBhilmaNo ratings yet
- NCERT Bio FactsDocument7 pagesNCERT Bio FactsGomathi MgmNo ratings yet
- Environmental Enrichment and Neuronal Plasticity - Oxford HandbooksDocument42 pagesEnvironmental Enrichment and Neuronal Plasticity - Oxford HandbooksAdela Fontana Di TreviNo ratings yet
- Nitrate Reduction in Sulfate Reducing BacteriaDocument10 pagesNitrate Reduction in Sulfate Reducing BacteriaCatalinaManjarresNo ratings yet
- What Does A Forensic DNA Analyst Do?Document1 pageWhat Does A Forensic DNA Analyst Do?kamal asgharNo ratings yet
- Microorganisms Friend or Foe Part-4Document32 pagesMicroorganisms Friend or Foe Part-4rajesh duaNo ratings yet
- GCSE Week 09 InheritanceDocument36 pagesGCSE Week 09 InheritanceFrankie BarnesNo ratings yet
- Soil Microbiology PTTDocument35 pagesSoil Microbiology PTTZainab RBNo ratings yet
- Contoh Review Artikel (Presenter)Document2 pagesContoh Review Artikel (Presenter)Tri SuwandiNo ratings yet
- Principles of Nerual ScienceDocument28 pagesPrinciples of Nerual ScienceRaunak MukherjeeNo ratings yet
- Classification Notes PDFDocument20 pagesClassification Notes PDFBobNo ratings yet