Professional Documents
Culture Documents
V3i8 Ijertv3is080595
Uploaded by
Arly Demenz'ionOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
V3i8 Ijertv3is080595
Uploaded by
Arly Demenz'ionCopyright:
Available Formats
International Journal of Engineering Research & Technology (IJERT)
ISSN: 2278-0181
Vol. 3 Issue 8, August - 2014
The Fluidity of a Model Recycle-Friendly Al-Si
Cast Alloy for Automotive Engine Cylinder Head
Application
Ghebrezghi T. Zeru* and B. R. Mose
S. M. Mutuli
Department of Mechanical Engineering
Jomo Kenyatta University of Agriculture and Technology
Nairobi, Kenya
Department of Mechanical and Manufacturing
Engineering, University of Nairobi,
Nairobi, Kenya
Keywords Aluminium Recycling, Al-Si, Fluidity, Al-Si
modification Fe intermetallics
I.
INTRODUCTION
Automotive components are currently manufactured from
different Al-Si alloys. The production of these components
from primary aluminium alloys has become a huge burden in
terms of economic benefits and environmental aspects. Hence,
recycling aluminium alloys is adopted as a strategy in foundry
industries for production of various components in the
transport and other structural applications [1, 2]. However, the
recycled alloys are prone to various deleterious effects on
castability and mechanical performance mainly because of the
high levels of impurity elements [3]. The situation is more
complicated for products such as automotive engine cylinder
heads which are routinely produced from a wide variety of
cast aluminium alloys. The mechanical properties and
castability of Al-Si alloys are known to be affected by many
factors. Castability, especially fluidity depends on a number of
variables such as channel thickness, melt head, mould
temperature, superheat, alloying composition and inclusion [4,
5]. Fluidity is highly affected by degree of superheat. The
increase in melt temperature by 1C, in the temperature
interval 700-730C increases the fluidity length by 1% [6].
The presence of oxide inclusions decreases fluidity,
particularly at low pouring temperature [7]. A Solid oxide film
might form a continuous wrapping around the stream and
restrict the melt flow depending on the strength and specific
gravity of the film [8]. Fluidity in foundry is expressed as a
distance a molten metal flows in a mould of constant crosssectional area before it stopped by solidification [5]. Spiral test
IJERTV3IS080595
experiments are usually conducted to determine the fluidity of
cast aluminium alloys. Bouska [9] conducted spiral tests and
showed that increase strontium significantly decreases the
flow length of the aluminium melt. The effects of titanium and
strontium on the fluidity of liquid A319 and A356 alloys were
investigated by Sanchez et al [10]. It was found that increase
in titanium enhances the fluidity of the A319 alloy due to its
high potential to refine the grain size. Further, the fluidity
index of the A356 alloy was found to increase with the
increase of Sr. Strontium addition at levels 0.015% and 0.02%
were found to increase fluidity while addition of 0.28% Al5Ti-1B and combined addition of 0.02% Sr and 0.28% Al5Ti-1B decreases fluidity of alloys LM25 and LM27 [11].
Addition of grain refiner on alloys of AlSi7Mg and AlSi11Mg
was reported to affect fluidity. For both alloys, an increase in
fluidity is observed as the content of grain refiner increases
above 0.12% Ti, while the fluidity is diminished with
increased grain refinement below 0.12% Ti. Moreover,
refinement of the alloys with 0.015%wt B shows the highest
fraction solid at dendrite coherency, the smallest grain size
and the best fluidity [12].
IJE
RT
Abstract This study investigated the fluidity of a model
recycle-friendly Al-Si cast alloy for automobile engine cylinder
head application. The fluidity measurements were carried out
using spiral sand moulds fabricated from silica sand bonded by
sodium silicate/CO2. The fluidity of the base alloy was found to
possess excellent flow length in a curved channel while the
addition of 0.02% Sr was observed to reduce the fluidity by
5.2%. The increase of iron content of the base alloy to 0.38% Fe
decreased the fluidity by 21.9%. This is attributed to the
formation of intermetallic phases that blocks the interdendritic
channels which are responsible to compensate for solidification
shrinkage. However, combined addition of 0.9% Fe and 0.45%
Mn was observed to reduce the fluidity by 12.1%.
A report by Sahoo and Sivaramakrishnan [13] shows that
modification of Al-8.3wt% Fe-0.8wt% V- 0.9wt% Si alloy by
Mg had much better fluidity than the unmodified. This is
possible due to the formation of Mg phases which have high
heat of fusion and hence delays the solidification of the alloy.
Addition of 0.1%wt Cr to LM6 alloy has been reported to
decrease fluidity due to formation of sludge [14]. A study
made by Gowri and Samuel [15] on 380 alloys, indicated that
with the addition of Fe content of 1.5 and 1.7wt% decreased
fluidity by 4% and 6%, respectively. The additions of 1.3wt%
Zn to the same alloy caused a decrease in fluidity of 5%.
Studies made by Mose et al [11] showed that increase of
Fe level from 0.4-0.48% increased the fluidity of LM 25 alloy
while increasing beyond this level decrease the fluidity of the
alloy. In a similar research addition of Fe between 0.41 to
0.6% to the alloy LM27 has been shown to increase the
fluidity.
The objective of this study is to investigate the fluidity of a
model alloy developed from scrap cylinder heads with the
addition of eutectic modifier (Sr) and with some level of
impurity (Fe).
www.ijert.org
(This work is licensed under a Creative Commons Attribution 4.0 International License.)
1504
International Journal of Engineering Research & Technology (IJERT)
ISSN: 2278-0181
Vol. 3 Issue 8, August - 2014
II.
EXPERIMENTAL METHODS
A. Alloy Preparation
A model secondary alloy investigated was obtained by
melting different aluminium engine cylinder head scraps in a
70 kg capacity oil fired graphite crucible furnace to 730C.
And then cast into conical 4 kg capacity ingot molds
fabricated from silica sand bonded by sodium silicate/CO2
binder, targeting to avoid the iron pick up that could be
generated when using ingot moulds of mild steel sheets. The
model secondary alloy obtained from scrap cylinder heads is
coded as KA1 and is found to be approximately equivalent to
the JIS-AC2B standard alloy. The chemical composition of
this base alloy is given in TABLE 1.
TABLE 1. Chemical composition (in wt. %) of the secondary Al-Si alloy.
Alloy
KA1
Si
6.01
Zn
0.12
Cu
2.62
Cr
0.02
Mg
0.24
Ni
0.02
Fe
0.28
Pb
0.01
Mn
0.21
Sn
0.01
Ti
0.02
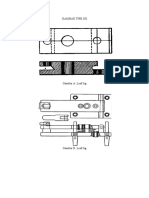
Fig. 2. (a) The spiral pattern and the mould (b) complete set-up of the spiral
fluidity mould (c) The sectional view of the fluidity set-up
IJE
RT
B. Preparation of Spiral Moulds
The mould used for testing the fluidity of the alloy was
fabricated from silica sand bonded by sodium silicate / CO2.
The mould consisted of a pouring basin incorporated with the
cope, a rectangular tapered sprue with small circular section
that extended to the drag, and spiral cavity completely in the
drag and the wide opening of the cavity was also pointing
upward. The dimensions of the mould were 290mm x 275mm
x 40 mm and with pressure head height 99 mm. The moulds
were fully vented during fabrication to remove the moisture.
An aluminium spiral pattern was cast in already existing
permanent spiral mould that had been designed and machined
from cast iron during a previous study [11]. The pattern was
then subsequently used in the preparation of the spiral sand
moulds. Moreover, the spiral pattern geometry was as show in
Fig. 1. The spiral pattern, the complete set-up and sectional
view of the fluidity mould used for successive casting are also
indicated in Fig. 2.
Fig.1 Dimensional details of the spiral fluidity pattern (in mm)
IJERTV3IS080595
C. Element Additions
In this study, the effect of Fe, Mn and eutectic Si
modification by Sr on the fluidity of model secondary alloy
was investigated. The Sr modifier was available in the form of
Al-10% Sr master alloy metallic rods while Fe and Mn were
available as Al - 75% Fe and Al - 75% Mn briquettes. The
effect of Sr was investigated by through addition of 0.02 wt.%
Sr while that of Fe was investigated by increasing the Fe
content in model alloy to 0.38 wt.% and 0.9 wt.%. However,
the 0.9 wt. % Fe alloy also included a Mn addition of 0.45 wt.
%.
D. Melting and Pouring
The ingots of the model alloy were heated to a temperature
of 7600C in an electrical resistance furnace located at
University of Nairobi. The alloying process has been done
when the temperature of the charged ingot reached between
7300C to 7500C. The pouring temperature of the molten metal
was maintained within 720 30C for all successive tests. Ktype thermocouple was employed to measure the temperature
of the molten metal while it was in the 4kg capacity SiC
crucible. Pouring time range that is the time between filling
the basin and removal of the stopper rod was kept the same for
each experiment. The pouring basin was filled completely as
fast as possible before and after the removal of the stopper rod
to give the same head pressure for all successive tests. Three
tests were taken for each of the alloy developed to incorporate
the possible errors that could be emerge due to personal,
mould and test variables.
www.ijert.org
(This work is licensed under a Creative Commons Attribution 4.0 International License.)
1505
International Journal of Engineering Research & Technology (IJERT)
ISSN: 2278-0181
Vol. 3 Issue 8, August - 2014
E. Alloy Preparation
Specimens for microstructural analysis were sectioned 5
mm from the tip of the fluidity spirals. The specimens were
polished with different grades of SiC paper and finally
polished with diamond paste of decreasing grain size of 6m,
1m and 1/4m. Optical micrographs were then taken using
Optika-Optical Microscope B -353MET.
III.
vents (holes for escaping the gases from the mould) are on the
cavity/channel, the flow of the molten metal will be obstructed
therefore reduces the flow length. These effects were observed
during the experiment which resulted in discarding the casting
that had such defects.
Fig. 4 and Fig. 5 shows the test result when the recycled
aluminium alloy was solidified in the spiral cavity fluidity
mould and micrograph of the cast spiral respectively.
RESULTS AND DISCUSSION
TABLE 3. shows the average results of three test
measured values and standard deviation of each of the alloy's
fluidity length. From TABLE 3 it can be seen that there was a
decrease in fluidity length of the alloy with the addition of
modifier and impurity elements. With the addition of eutectic
silicon modifier that is 0.02% Sr it has been observed to
decrease the fluidity by 5.2% and an increase of iron content
of the alloy to 0.38% Fe resulted a further decrease on the
average fluidity length by 21.9%. Iron addition in combination
with manganese in the ratio of 2:1 (Fe: Mn) was observed to
decrease the flow length by 12.1%.
TABLE 3. Experimental results of the alloys fluidity test
Alloys
Standard
deviation
(cm)
Base alloy (KA1)
KA1+0.02% Sr
Length in
(cm)
116.5
110.5
KA1 + 0.38% Fe
90.5
2.3
KA1 + 0.9% Fe +
0.45% Mn
102.4
1.6
1.8
Percentage
Difference
(%)
5.2%
Fig. 5 shows the microstructure of specimens taken 5mm
away from the tip of the casting spiral. It shows the primary
aluminum dendrites, eutectic, Fe-intermetallics, Cuintermetallics and oxides. Fig. 5(a) reveals that the alloy
contains some copper phases nucleated along the thin iron
phases, eutectic and oxides. Uniform distribution of phases of
the eutectic silicon and Fe- intermetallics were observed in
this alloy and no network of intermetallis were observed that
could choke up the flowing molten metal. However, in Fig.
5(b) it was observed that strontium modification resulted in a
network of intermetallic phases. These structures can block
the flowing molten metal and then decreases the fluidity of the
alloy if they solidify early before a coherent solid network is
formed.
IJE
RT
Average
Fig.4. The outcome of the alloys fluidity when it is solidified
21.9%
12.1%
Fig. 3 shows the effect of the alloying elements on the
fluidity of the base alloy when they are added in small
percentage. The bar chart shows the variation of average
recorded fluidity length changes when alloying elements
strontium, iron and manganese are added to the base alloy.
Fig. 3 Fluidity variation with Sr, Fe and combined Fe + Mn
The deviation of the measured test results are indicated by
standard deviation. These deviations are associated mostly
with mould parameters, temperature and personal errors. Such
effects can be reduced through the proper control of mood
parameters such as vent size and position and controlling of
pouring temperature of each successive test. For instance if the
IJERTV3IS080595
www.ijert.org
(This work is licensed under a Creative Commons Attribution 4.0 International License.)
1506
International Journal of Engineering Research & Technology (IJERT)
ISSN: 2278-0181
Vol. 3 Issue 8, August - 2014
CONCLUSION
This study shows that the base alloy possessed the best
castability (fluidity) while the addition of eutectic modifier
and the impurity level were observed to decrease the fluidity.
The addition of modifier reduces fluidity by forming
intermetallic network. The secondary alloy has high
propensity to reduce its fluidity with small addition of Fe
below its critical level. However, the neutralizing effect of
Mn is observed to result in fine Fe intermetallics in the
0.9% Fe containing alloy. Hence, neutralizing Fe with Mn at
higher level of Fe plays a great role in giving the best fluidity.
ACKNOWLEDGMENT
The authors would like to express grateful acknowledgment
for the financial support from National Board for Higher
Education of Eritrea, and research facilities, Jomo Kenyatta
University of Agriculture and Technology and University of
Nairobi.
REFERENCES
Fig.5 Micrograph of the alloys 5mm from the tip of the fluidity casting, (a)
Base alloy (KA1), (b) strontium modified base alloy, (c) 0.38% Fe containing
base alloy and (d) 0.9% Fe + 0.45% Mn containing base alloy
Haszler A., Vieregge A Recent development in aluminium alloys for
the automotive industry, Materials Science and Engineering, vol.
A280, pp.37- 49.2000.
IJE
RT
Although Sr has a wide range of significant advantages in
comparison with other chemical modifiers such as Na and Sb
in foundry during casting [16]; this study however,
demonstrates that strontium reduces the fluidity of the alloy
developed. Excess amount of Sr leads to porosity, which
among others has been attributed to reduced surface tension of
the melt and increased volumetric shrinkage [17]. Addition of
Sr also leads to segregation of the Cu-containing phases to
regions away from the Al-Si eutectic. This can lead to
blockage of the interdendritic channels if blocky Al2Cu phases
are formed during solidification [18].
[1] Miller W. S., Zhuang L. Bottema J., Wittebrood A. J., De Smet P.
Addition of Fe to this alloy has been observed to produce
various intermetallic phases which have the potential to
reduce the fluidity of the molten metal. In addition, Fig. 5 (c)
shows that a number of Al2Cu phases formed in addition to
eutectic Si and oxides. The microstructure of Fig. 5(d) shows
an increased number of Fe - intermetallics in comparison
with Fig. 5(c). Moreover, the neutralizing effect of Mn is
observed to result in fine Fe intermetallics in the 0.9% Fe
containing alloy. This may explain the higher fluidity reported
in this alloy compared to the 0.38% Fe containing alloy.
Increase in the iron level in an alloy generally leads to an
increase in the amount of insoluble iron-rich bearing phases
which can reduce the alloy's fluidity. The -phases have
largest surface to volume ratio, hence they have the largest
interfacial region with the melt and are likely to be the most
detrimental intermetallic in reducing fluidity [14]. Moreover,
with an increase in size and amount of and phases, there is
usually a concomitant increase in interconnected shrinkage
porosity due to a reduction in the permeability/feeding in
mushy zone.
IJERTV3IS080595
[2] Kuafman J. G. and Rooy E. L., Aluminium alloys casting: Properties,
Processes and Application, ASM International, pp. 69-131, 2004.
[3] Mbuya T. O., Odera B. O. and Nganga S. P.., Influence of iron on
castability and properties of aluminium Silicon alloys, Literature
review, International Journal of Cast Metals Research, vol. 16, no. 5
pp. 451-465, 2003.
[4] Di Sabatini M., Fluidity of aluminium foundry alloys, in PhD
Thesis, Norwegian University of Science and Technology (NTNU),
2005.
[5] Flemings M. C, Solidification processing, Metallurgical and
Materials Trans., vol. 5, no. 10, pp. 2121-2134, 1974.
[6] Di Sabatino M., Syvertsen F., Arnberg L., and Nordmark A., An
improved method for fluidity measurement by gravity casting of spirals
in sand moulds, International Journal of Cast Metals Research, vol.
18, pp. 59-62, 2005.
[7] Di Sabatino M., Arnberg L., A review on the fluidity of Al based
alloys, Metallurgical Science and Technology, Ed. by Teksid
Aluminum, vol. 22, pp. 9-15, 2004.
[8] Ragone D. V., Factors affecting the fluidity of metals, College of
engineering industry program, 1955.
[9] Bouska O., The effect of different casting parameters on the
relationship between flowability, mould filling capacity and cooling
conditions of Al- Si alloys, Metalurgija-Journal of Metallurgy,
AMES, pp. 10-30, 2008.
[10] Sanchez S., Velasco E., P.del C. Zambrano and Cavazos J. L., Effect
of titanium and strontium addition on the fluidity of A319 and A356
aluminium alloys, Materials Science Forum , vol. 509, pp. 159-164,
2006.
[11] Mose B. R., Maranga S. M., Mbuya T. O., Effect of minor elements
on the fluidity of secondary LM25 and LM27 type cast alloys, AFS
Transactions, pp. 93-101, 2009.
[12] Dahle A. K., Tondel P. A., Paradies C. J., and Arnberg L., Effect of
grain refinement on the fluidity of two commerical Al-Si foundry
alloys, Metallurgical and Materials Transactions A, vol. 27A, pp.
2305-2313, 1996.
[13] Sahoo K. L., Sivaramakrishnan C. S., Come studies on Al-8.3Fe0.8V-0.9Si alloy for near net shape casting, Journal of Mat. Proc.
www.ijert.org
(This work is licensed under a Creative Commons Attribution 4.0 International License.)
1507
International Journal of Engineering Research & Technology (IJERT)
ISSN: 2278-0181
Vol. 3 Issue 8, August - 2014
Tech., vol. 135, pp. 253-257, 2003.
[14] Ahmad R., Sadeghi R., Asmael M. B. A, Mohamad H., Harun Z.,
Hasan S., The influence of metallic addition on fluidity of aluminium
(LM6) alloy, Applied Mechanics and Materials, Vols. 465-466, pp.
954-957, 2014.
[15] Gowri S., Samuel F. H., Effect of alloying element on the
solidification characteristics and microstructure of Al-Si-Cu-Mg-Fe
380 alloy, Metall. and Mater. Trans., vol. 25A, pp. 437-448, 1994.
[16] Comalco Aluminium Limited, Modification of foundry Al-Si alloys,
Comalco technical report no.4, 1997.
[17] Li Z, Samuel A.M., Samuel F.H., Ravindran C., Valtierra S., Doty
H.W., Parameters controlling the performance of AA319-type alloys
Part I Tensile Properties, Materials Science and Engineering, vol.
A367, pp. 96-110, 2004.
IJE
RT
[18] Li Z., Samuel A. M., Samuel F. H., Effect of alloying elements on the
segregation and dissolution of CuAl2 phase in Al-Si-Cu 319 alloys,
Journal of Materials Science, vol. 38, pp. 1203-1218, 2003.
IJERTV3IS080595
www.ijert.org
(This work is licensed under a Creative Commons Attribution 4.0 International License.)
1508
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- How To Get The Best From Your: Router CuttersDocument2 pagesHow To Get The Best From Your: Router CuttersArly Demenz'ionNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Gambar Jig LeafDocument9 pagesGambar Jig LeafArly Demenz'ionNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Momentum and ImpulsersDocument15 pagesMomentum and ImpulsersArly Demenz'ionNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Solution Real AnalisisDocument6 pagesSolution Real AnalisisArly Demenz'ionNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- JSF + JPA + JasperReports (Ireport) Part 2 - Ramki Java BlogDocument7 pagesJSF + JPA + JasperReports (Ireport) Part 2 - Ramki Java BlogMartin MurciegoNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Day 3 Polygons Lesson PlanDocument6 pagesDay 3 Polygons Lesson PlanBA RTNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Evaluating of Rutting in Highways & Providing Its Solution in Terms of Stone Matrix Asphalt.Document7 pagesEvaluating of Rutting in Highways & Providing Its Solution in Terms of Stone Matrix Asphalt.IJRASETPublications100% (1)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Gantt ChartDocument4 pagesGantt ChartSyed FaridNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Ball Charge ManagementDocument14 pagesBall Charge ManagementSalud Y SucesosNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- 09.0 Product Description - MAN EcoTorqueDocument2 pages09.0 Product Description - MAN EcoTorquegoginemNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Mod 4 Revision Guide 1 Reaction Kinetics AQA A2 ChemistryDocument5 pagesMod 4 Revision Guide 1 Reaction Kinetics AQA A2 Chemistryviyas07No ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Machine Fault Detection Using Vibration Signal Peak DetectorDocument31 pagesMachine Fault Detection Using Vibration Signal Peak Detectordavison coyNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Tlsiw - Class X - Project Details - 2023-24Document2 pagesTlsiw - Class X - Project Details - 2023-24how toNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Template Question BankDocument11 pagesTemplate Question Bankeshwar_worldNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Module 1 Engineering ScienceDocument38 pagesModule 1 Engineering ScienceLogan JesseNo ratings yet
- 5.3.2 To Sketch Graphs of Trigonometric Functions (Part 2) - SPM Additional MathematicsDocument3 pages5.3.2 To Sketch Graphs of Trigonometric Functions (Part 2) - SPM Additional MathematicsLuke SuouNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Research FinalDocument29 pagesResearch FinalLaw VesperaNo ratings yet
- Evoked Potential Practice Exam - ProProfs QuizDocument23 pagesEvoked Potential Practice Exam - ProProfs QuizAnonymous 9lmlWQoDm8No ratings yet
- DelhiDocument44 pagesDelhiIndia TreadingNo ratings yet
- High Pressure Jet Grouting in TunnelsDocument8 pagesHigh Pressure Jet Grouting in TunnelsSandeep AggarwalNo ratings yet
- GTP For 1CX300sqmmDocument4 pagesGTP For 1CX300sqmmpriyanka236No ratings yet
- Ss 1 Further Mathematics Lesson 4Document7 pagesSs 1 Further Mathematics Lesson 4Adio Babatunde Abiodun CabaxNo ratings yet
- Study of Padmanabhapuram Palace TrivandrumDocument14 pagesStudy of Padmanabhapuram Palace Trivandrumcrustybubbles100% (2)
- Data Cable Containment SizingDocument21 pagesData Cable Containment SizingAngelo Franklin100% (1)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- JVM InternalsDocument55 pagesJVM InternalsAmol ChikhalkarNo ratings yet
- Engg Mechanics Ques BankDocument68 pagesEngg Mechanics Ques BankUtkalNo ratings yet
- Calculus 1: CONTINUITYDocument56 pagesCalculus 1: CONTINUITYMa Lorraine PerezNo ratings yet
- The Guitar in The Sixteenth CenturyDocument16 pagesThe Guitar in The Sixteenth CenturyPat BrandtNo ratings yet
- Pelod Vs Sofa Scoring in PediatricDocument6 pagesPelod Vs Sofa Scoring in PediatricAdrian KhomanNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Phrasal Verbs-Syntactic BehaviorDocument4 pagesPhrasal Verbs-Syntactic BehaviorAntonija KnezovićNo ratings yet
- An FPGA Implementation of A Feed-Back Chaotic Synchronization For Secure CommunicationsDocument5 pagesAn FPGA Implementation of A Feed-Back Chaotic Synchronization For Secure Communicationslaz_chikhi1574No ratings yet
- Epson L6170 Wi-Fi Duplex All-in-One Ink Tank Printer With ADFDocument3 pagesEpson L6170 Wi-Fi Duplex All-in-One Ink Tank Printer With ADFCarl DonaireNo ratings yet
- Hungr Et Al 2005 - Landslide Travel DistanceDocument30 pagesHungr Et Al 2005 - Landslide Travel DistanceJosé Ignacio RamírezNo ratings yet
- Circuit Protective Devices: Learner Work BookDocument41 pagesCircuit Protective Devices: Learner Work BookChanel Maglinao80% (5)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)