Professional Documents
Culture Documents
A Review of Anatomy, Physiology, and Benign Pathology of The Nipple.
Uploaded by
bibliohemerotecaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A Review of Anatomy, Physiology, and Benign Pathology of The Nipple.
Uploaded by
bibliohemerotecaCopyright:
Available Formats
Ann Surg Oncol (2015) 22:32363240
DOI 10.1245/s10434-015-4760-4
ORIGINAL ARTICLE BREAST ONCOLOGY
A Review of Anatomy, Physiology, and Benign Pathology
of the Nipple
Kimberly Stone, MD and Amanda Wheeler, MD
Department of Surgery, Stanford University School of Medicine, Stanford, CA
ABSTRACT The nipple and areola are pigmented areas
of modified skin that connect with the underlying gland of
the breast via ducts. The fairly common congenital
anomalies of the nipple include inversion, clefts, and
supernumerary nipples. The anatomy of the nipple areolar
complex is discussed as a foundation to review anatomical
variants, and the physiologic development of the nipple,
including changes in puberty and pregnancy, as well as the
basis of normal physiologic discharge, are addressed. Skin
conditions affecting the nipple include eczema, which,
while similar to eczema occurring elsewhere on the body,
poses unique aspects in terms of diagnosis and treatment.
This article concludes with discussion on the benign
abnormalities that develop within the nipple, including
intraductal papilloma and nipple adenoma.
Through art, we can appreciate that nipples have
remained relatively constant throughout the centuries
from primitive cave paintings as a simplistic circle with a
dot, to detailed renaissance nude works. Breast physicians
and artists alike are keenly aware of the complexities of the
nipple areolar complex (NAC).
This review attempts to demystify the ubiquitous nipple
and its benign anatomical and physiologic conditions.1
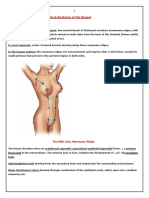
THE NIPPLE AREOLAR COMPLEX (NAC)
Nipples have undergone little, if any, evolutionary
advancement since humans first evolved as they were
essential for mothers to feed their young. Nipples are
Society of Surgical Oncology 2015
First Received: 4 June 2015;
Published Online: 5 August 2015
A. Wheeler, MD
e-mail: wheeler_amanda@yahoo.com
generally located just below the center of the breast and are
smaller in men than women.
Most nipples do not stick straight out but rather are
slightly askew towards the axilla, making it easier to
breastfeed. Sanuki et al. studied the morphologic characteristics of the nipples of 300 women (600 breasts) and
reported a mean diameter of the areola of 4.0 cm, a mean
diameter of the nipple of 1.3 cm, and a mean nipple height
of 0.9 cm. The differences in nipple projection can be
affected by age, race, weight, and hormonal changes.2
The sulcus is a fold at the intersection of the areola and the
rising edge of the nipple. It can often look like a wrinkle,
dimpling, or a smooth curve of skin. The areola is the pigmented circle surrounding the nipple and can range from pink
to red, to dark brown or nearly black. It generally tends to be
paler among people with lighter skin tones and darker among
people with darker skin tones. The areola changes color
during the various stages of sexual arousal and orgasm.3
The surface of the nipple is irregular, with a cobblestone texture and crevices that lead to the duct orifices.
Cellular debris can be found within these crevices and can
form a keratin plug. The pigmented skin of the areola
contains numerous apocrine sweat glands, sebaceous
glands, and hair follicles from the dermal layer of the
skin.4 The skin layer of the areola is usually between 0.5
and 2.0 mm thick and composed of epidermal cells, while
the epidermal skin of the nipple is continuous with the
epithelium of the ducts. It is possible to develop skin tags
on the nipple due to friction. There is little or no fat
between the skin and underlying breast glandular tissue at
the NAC.
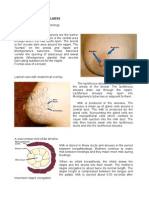
Montgomery glands (also referred to as tubercles) are
below the surface of the areola and may be seen as small
bumps in the skin (Fig. 1). These modified sebaceous
glands are associated with a lactiferous duct that communicates with a rudimentary mammary gland. They provide
lubrication during breastfeeding and are more apparent
during pregnancy. Montgomery glands can become
Anatomy, Physiology, and Benign Pathology of the Nipple
FIG. 1 Montgomery glands
blocked, like pimples, and swell.5 A dermal cyst can also
develop from the dermal layer of the NAC.
The arrector pili muscles are attached to the hair follicles around the areola. Most women have hair follicles in
the areola that appear darker than their hair color. Piloerection of the nipple occurs with cold stimulus, arousal, or
during breastfeeding due to contraction of these muscles.
During the excitement phase of sexual arousal, the nipples
harden and become more erect as the areola swells with
increased blood flow. These muscles can be asymmetric
and lose their contraction over time with pregnancy and
menopause.6,7
At least 50 % of the blood supply to the nipple is located
in the periphery. The small vessels that feed the nipple
arise from the internal mammary (internal thoracic) artery
and lateral thoracic artery.8 Palmer and Taylor reported
that the second intercostal perforator off the internal
mammary artery is the principal perforator that supplies the
NAC 85 % of the time.9 Ligation of this perforator can lead
to nipple necrosis when performing a nipple-sparing
mastectomy.
DUCTAL ANATOMY
Breast milk is released from the nipple via duct orifices
which have tiny sphincters that close to prevent leakage
during breastfeeding. The ducts are lined with myoepithelial cells that help with evacuating the breast milk
during lactation.10,11 Sir Astley Coopers book regarding
the anatomy of the breast is the first well-known report of
ductal anatomy. The greatest number of lactiferous tubes I
have been able to inject, has been twelve, and more frequently from seven to ten. But the greatest number of
3237
FIG. 2 Sagittal section through a nipple with coronal block sections
from a different nipple. The sagittal section illustrates the approximate location of tissue sections. Block sections from a coronallysectioned nipple show differences in morphology with depth. The
duct bundle is outlined in black, and the beginning of the waist can be
seen at the level of the areola. Reprinted from Rusby et al.13, with
permission from Barbara L. Smith, MD, PhD, and with acknowledgment of BioMed Central as the original publisher
orifices I have been able to reckon has been twenty-two:
however, some of these might have been follicles only, and
not open ducts.12
There continue to be discrepancies in the literature on
the number of ductal orifices within the nipple between
different histologic techniques. Love and Barsky found
between five and nine ductal orifices with two to three
ductal openings in the center of the nipple and three to five
arranged around the center.11 A three-dimensional (3D)
reconstruction of one nipple tip demonstrated 29 ducts
arising from 15 orifices.13
The 3D nipple anatomy study conducted by Rusby et al.
has advanced our understanding of the NAC; Fig. 2 is a 3D
re-creation of one of the nipples in their study. Reconstruction and summary data from 25 nipples show a central
duct bundle, with a peripheral duct-free rim, which narrows
to form a waist 2 mm beneath the level of the areola as
the ducts enter the breast parenchyma. The majority of
ducts form a central bundle that occupies 2167 % of the
cross-sectional area of the papilla, forming the central duct
bundle. The authors found that neither duct diameter nor
position predict whether a duct system will terminate close
to the nipple or pass deeper into the breast. The region of
duct widening just beyond the sulcus is referred to as the
ductal ampulla and serves as a reservoir. The main ducts
begin to branch within 8 mm of the areola, into the interlobular ducts.13
PHYSIOLOGIC DEVELOPMENT OF THE NIPPLE
Embryonically, multiple paired areas of ectodermal
thickening occur along the mammary ridges but only one
3238
pair remains to form the breasts. These mammary ridges
are commonly referred to as milk lines. At birth, only the
main lactiferous ducts have developed. Known as witchs
milk, newborn babies secrete fluid from maternal hormonal
stimulation, and between 80 and 90 % of all infants of both
genders have this discharge on the second or third day
following birth.14 Prior to puberty, the male and female
breasts consist of rudimentary ducts with underdeveloped
lobules.
Rapid breast development occurs at puberty, premature
thelarche refers to unilateral early ripening of the breast at
68 years of age, and hormonal stimulus with estrogen
causes connective tissue to elongate and grow, and vascularity and fat deposition to increase. Progesterone
stimulates growth of the terminal ductal lobular unit. The
breast tissue is not considered completely mature or
ripened until pregnancy and lactation occurs, and if there is
no immediate pregnancy the breast tissue fully matures
2 years postmenarche. Breasts are normally asymmetric in
size, with the left breast usually bigger than the right
breast.14,15
Throughout pregnancy the breasts and nipples undergo a
number of changes in preparation for nourishing the infant.
For many women, one of the earliest signs of pregnancy is
breast tenderness and nipple sensitivity, which is caused by
an increase in blood flow to the nipple. Due to increased
progesterone levels, the nipples become plumper and more
prominent. For most women with inverted nipples, the
nipples will evert as pregnancy progresses, and gradual
enlargement and darkening of the areola will be evident.
Montgomery tubercles become more prominent as they
prepare to lubricate the areolar skin during breastfeeding,
and in some cases a secondary areola forms (a ring of
pigmented tissue that forms outside the areolar border).15,16
Nipple changes associated with menopause occur over
time as the connective tissue becomes dehydrated and
inelastic, leading to sagging of the breast. The areola circumference stretches and increases with age, and, as with
other age-related parts, the areolae lose their retraction with
time and become more asymmetric.14,15
Physiologic Nipple Discharge
The painting in Fig. 3 portrays Gabrielle dEstrees,
mistress of King Henry IV of France, sitting nude in a bath,
holding a ring. Her sister sits nude beside her and pinches
her right nipple, showing the King how fertile his mistress
was Reference.17
Squeezing the nipple sends a message to the brain signaling the pituitary gland to respond by making prolactin,
thereby releasing discharge. More discharge is noted at
puberty and just prior to menopause.3 Up to 85 % of
women will have physiologic nipple secretions caused by
K. Stone, A. Wheeler
FIG. 3 Presumed portrait of Gabrielle DEstrees and her sister, the
Duchess of Villars (c. 1594)
the sloughing of epithelial cells. Benign physiologic discharge is usually bilateral, involving multiple ducts and
being nonspontaneous.18
Galactorrhea is defined by milky nipple discharge that is
not associated with pregnancy or a recent history of
breastfeeding. Medications are also known to cause discharge, and include birth control, antihypertensives, and
sedatives, among others. A pituitary tumor can rarely
secrete prolactin indiscriminately, resulting in bilateral
spontaneous nipple discharge.
CONGENITAL ANOMALIES OF NIPPLES
Nipple Inversion
Approximately 1020 % of all women are born with
nipple inversion, referring to when the entire nipple is
pulled inward, whereas retraction implies the nipple only
has an inward slit-like area. The most common causes of
congenital inversion are short ducts or a wide areolar
muscle sphincter.3,19 Other common causes of nipple
inversion include breastfeeding, trauma resulting in fat
necrosis or surgery, ptosis, breast cancer, breast infections,
genetic variation of the nipple shape, pregnancy, sudden
and major weight loss, and tuberculosis.
Nipple Cleft
These ducts are lined by stratified squamous epithelium
near the opening, and the lumens are frequently filled with
desquamated cells. Deeper in the connective tissue, the
ducts acquire a stratified columnar appearance that is really
a cuboidal duct cell sitting on a myoepithelial cell, as in
the sweat gland. This forms what is known as a nipple
cleft.20
Anatomy, Physiology, and Benign Pathology of the Nipple
Supernumerary (Third) Nipple
An accessory nipple (polythelia) can develop anywhere
along the milk line from the axilla to the groin. Accessory
breast and nipple tissue can lactate and develop mastitis,
and is more commonly diagnosed in males at a rate of 1 in
18, whereas only 1 in 50 females are diagnosed with
polythelia.7 Romans regarded a third nipple as a sign of
reinforced femininity; however, during the Salem Witch
Trials an accessory nipple was thought to be used to suckle
the devil and was considered as evidence of being a
witch.4,21
Other congenital anomalies include athelia, absence of
the nipple, and amazia (absence of the development of the
functional breast tissue beneath a normal nipple and areola).
SKIN CONDITIONS OF THE NAC
Nipple eczema is a manifestation of atopic dermatitis
characterized by thickened, cracked, dry, or scaly skin that
can appear raw, inflamed, and swollen from frequent
scratching. The rash appears as small, raised bumps, which
may leak fluid and crust over when scratched (Fig. 4). The
patient typically presents with symptoms of nipple pain,
burning, rash with vesicles, crusting, and erosion.22
These symptoms can present at any point in life, but
may present in a localized form to the NAC during
breastfeeding. Patients with atopic dermatitis typically
have an early onset of symptoms, suffer a chronic and
relapsing course, and have a personal or family history of
asthma and/or hayfever.
The differential diagnosis of an eczematous rash that is
confined to the NAC includes the following benign conditions: allergic contact dermatitis, psoriasis, impetigo,
herpes simplex, or zoster. Eczema of the NAC usually
affects both breasts, often has an intermittent history with
rapid progression of symptoms during flare-ups, can
involve only the areola with sparing of the nipple, and has
an indefinite edge between normal and abnormal tissue.23
If the rash presents as a unilateral, gradually progressive
lesion, one must consider Pagets disease and erosive
adenomatosis (i.e. papillary adenoma) because of their
malignant potential. A punch biopsy is recommended for
FIG. 4 a Eczema of the nipple. b Pagets disease in a male breast
3239
persistent eczematous lesions or in the case of ambiguity in
order to rule out Pagets disease (Fig. 4b).
Pagets disease of the breast is an in situ malignancy
located within the epidermis of the NAC. It presents as
erosion of the nipple tip or base, and persistent itching,
with progression to ulceration as it expands outward over
the areola. Eight-five percent of women who are diagnosed
with Pagets disease will have an associated malignancy
within the same breast, accounting for 0.5 % of all breast
cancers in women. It is usually amenable to a central
lumpectomy in which the NAC is removed.24,25
Erosive adenomatosis is a rare, papillary lesion of the
nipple, often with ulceration. It can be associated with
malignant breast disease in 8 % of cases, although it is not
considered premalignant, and is adequately treated with
local excision of the affected part of the nipple only.23,26
Most people with atopic dermatitis also have Staphylococcus aureus colonization of bacteria on their skin. The
bacteria multiply rapidly when the skin barrier is broken
and fluid is present on the skin. This can occur with
suckling of the nipple. Those with impetigo should be
treated with topical mupirocin if the infection is limited, or
systemic therapy if the infection is extensive.
ABNORMALITIES THAT DEVELOP IN THE
NIPPLE
A plugged duct can lead to a subareolar abscess. The
terminal ducts at the nipple undergo squamous metaplasia
that leads to partial duct obstruction with inspissated
squamous debris and subsequent duct dilatation and stagnation. A secondary infection resulting in an abscess
usually drains at the edge of the areola,27 which can initially be managed with aspiration and antibiotic coverage.
A radial elliptical incision can be performed for a recurrent
abscess, nonhealing fistula, or persistent mass.27 Chronic or
recurrent subareolar abscess can be associated with cigarette smoking.28
A ductal papilloma arising within the terminal ducts
growing outward can also occur, and can sometimes bleed
or seep fluid, causing a watery or bloody discharge from
the nipple. Adequate sampling is recommended to rule out
an occult malignant process.
A nipple adenoma is a type of intraductal papilloma that
arises within the lactiferous ducts. Nipple adenomas most
commonly occur in 30- to 40-year-old women but can also
be diagnosed in men (Fig. 5). They can occur at any age,
including the elderly, in adolescence, and in infants,26 and
can be locally excised without disruption of the nipple.
Florid papillomatosis of the nipple ducts, erosive adenomatosis (as previously described), or a nipple adenoma is
a benign tumor of the ductal epithelium, typically
3240
FIG. 5 Nipple adenoma
presenting as a discrete, palpable tumor of the papilla of the
nipple. Erosion of the nipple can lead to discharge and
bleeding. Histologically, the tumor is characterized by
proliferating ductal structures that invade the surrounding
stroma. A double layer of epithelium lines these ductal
structures and can be further characterized by the presence
of keratin cysts and tiny apical snouts.29
CONCLUSIONS
The human NAC is an anatomical work of art and a
physiologic masterpiece. It has stood the test of time
throughout evolution despite aberrant alterations in its
anatomical structure and benign pathologic conditions, as
reviewed.
ACKNOWLEDGMENT Special thanks to Stefanie Jeffrey, MD,
for her thoughtful edits, as well as Fred Dirbas, MD, and Irene
Wapnir, MD, and to Barbara L. Smith, MD, PhD, for her permission
to use Fig. 2, and her groundbreaking work on nipple anatomy.
DISCLOSURES Kimberly Stone and Amanda Wheeler have no
disclosures to declare.
REFERENCES
1. Wheeler A. Nipples gone bad. The American Society of Breast
Surgeons 16th Annual Meeting; 29 April3 May 2015: Orlando.
2. Sanuki J, Fukuma E, Uchida Y. Morphologic study of nippleareola complex in 600 breasts. Aesthetic Plast Surg.
2009;33:295297.
3. Nicholson BT, Harvey JA, Cohen MA. Nipple areolar complex:
normal anatomy and benign and malignant processes. Radiographics. 2009;29:509523.
4. Kopans DB. Breast anatomy and basic histology, physiology, and
pathology. In: Kopans DB, editor. Breast Imaging. 3rd ed.
Philadelphia: Lippincott Williams & Wilkins; 2007: pp. 743.
5. Da Costa D, Taddese A, Cure ML, Gerson D, Poppiti R Jr,
Esserman LE. Common and unusual diseases of the nipple-areolar complex. Radiographics. 2007;27(Suppl 1):S65S77).
6. Rapini RP, Bolognia JL, Jorizzo JL. Dermatology: 2-volume set.
St. Louis: Mosby; 2007. ISBN 1-4160-2999-0.
7. Levin RJ. The breast/nipple/areola complex and human sexuality.
Sex Relatsh Ther. 2006;21(2):237249.
K. Stone, A. Wheeler
8. Skandalakis J. Breast. In: Skandalakis J, editor. Skandalakis
surgical anatomy: the embryologic and anatomic basis of modern
surgery. Athens: PMP; 2004. pp. 106107.
9. Palmer JH, Taylor GI. The vascular territories of the anterior
chest wall. Br J Plast Surg. 1986;39:28799.
10. Going JJ, Moffat DF. Escaping from flatland: clinical and biological aspects of human mammary duct anatomy in three
dimensions. J Pathol. 2004;203(1):53844.
11. Love SM, Barsky SH. Anatomy of the nipple and breast ducts
revisited. Cancer. 2004;101(9):194757.
12. Cooper A. The anatomy and diseases of the breast. Philadelphia:
Lea and Blanchard; 1845.
13. Rusby JE, Brachtel EF, Michaelson JS, Koerner FC, Smith BL.
Breast duct anatomy in the human nipple: three-dimensional
patterns and clinical implications. Breast Cancer Res Treat.
2007;106:171.
14. Love SM, Lindsey K. The breast and its development. In: Love
SM, Lindsey K. Dr, editor. Susan loves breast book. 2nd ed.
Reading: Addison-Wesley; 1995: pp. 318.
15. Chinyama CN. Normal morphology, physiological changes, and
benign breast disease. In: Dirbas F, Scott-Conner C, editors.
Breast surgical techniques and interdisciplinary management.
New York: Springer; 2011: pp. 6581.
16. Spencer J. Common problems of breastfeeding and weaning. In:
Post TW, editor. UpToDate: Waltham. http://www.uptodate.
com/home. Accessed 14 May 2015.
17. Fontainebleau School. Presumed portrait of Gabrielle DEstrees
and her sister, the Duchess of Villars (c. 1594) [painting]. Musee
Du Louvre, Paris, France. Nipple-Pinching Good Times. Tumblr,
2009. Accessed 29 May 2015.
18. Harris JR, Lippman M, Morrow M, et al. Diseases of the breast.
4th ed. Philadelphia: Lippincott Williams and Wilkins; 2010.
19. Da Costa D, Taddese A, Cure ML, Gerson D, Poppiti R Jr,
Esserman LE. Common and unusual diseases of the nipple-areolar complex. Radiographics. 2007;27 Suppl 1:S65S77.
20. Shah AK, Floyd D. The congenital cleft nipple and its surgical
treatment. J Plast Reconstr Aesthet Surg. 2012;65(4):e75-9.
21. Newson A (2005). Bond villains nipple triple gives clues to
breast cancer treatment. Times Online. Times Newspapers.
22. Barankin B, Gross MS. Nipple and areolar eczema in the
breastfeeding woman. J Cutan Med Surg. 2004;8:126130.
23. Disorders of the nipple and areola. In: Mansel RE, Webster DJT,
Sweetland HM. Hughes, Mansel & Websters benign disorders
and diseases of the breast. 3rd ed. Elsevier; 2009: pp. 195205.
24. Chen CY, Calhoun KE, Anderson BO. Pagets disease of the
breast. In: Dirbas F, editor. Breast surgical techniques and
interdisciplinary management. New York: Springer; 2011. pp.
533534.
25. Giess CS, Raza S, Birdwell RL. Distinguishing breast skin lesions
from superficial breast parenchymal lesions: diagnostic criteria,
imaging
characteristics,
and
pitfalls.
Radiographics.
2011;31(7):195972.
26. MacGrogan G, Moinfar F, Raju U. Intraductal papillary neoplasms. In: Tavassoli FA, Devilee P, editors. World Health
Organization classification of tumours: pathology and genetics of
tumours of the breast and female genital organs. Lyon: IARC
Press; 2003. pp. 7688.
27. Lannin D. Twenty-two year experience with recurring subareolar
abscess and lactiferous duct fistula treated by a single breast
surgeon. Am J Surg. 2004;188:407410.
28. Schafer P, Furrer C, Mermillod B. An association of cigarette
smoking with recurrent subareolar breast abscess. J Epidemiol.
1988;17:810813.
29. Jones DB. Florid papillomatosis of the nipple ducts. Cancer.
1955;8(2):315319.
You might also like
- Breast Diseases: Detection and Physical ExamDocument22 pagesBreast Diseases: Detection and Physical ExamnicewanNo ratings yet
- Theories of Labor Ons-EtDocument19 pagesTheories of Labor Ons-EtHeron BayaninNo ratings yet
- Urinary System For Grade 6Document2 pagesUrinary System For Grade 6Kent Francis Layaguin75% (4)
- Zoology Chapter 29Document100 pagesZoology Chapter 29Erika AquinoNo ratings yet
- 11 Inside Lactating Breast PDFDocument8 pages11 Inside Lactating Breast PDFEdward Arthur IskandarNo ratings yet
- Benign Cervical Lesions and Congenital Anomalies of The Cervix - UpToDateDocument33 pagesBenign Cervical Lesions and Congenital Anomalies of The Cervix - UpToDatecriswesi23No ratings yet
- 1.anatomi MammaeDocument15 pages1.anatomi MammaeKatou Jeffrey ShigehitoNo ratings yet
- Anatomical and Physiological Changes During PregnancyDocument25 pagesAnatomical and Physiological Changes During Pregnancyqaleeq100% (1)
- A Case Study on Breast Cancer Screening and Risk FactorsDocument18 pagesA Case Study on Breast Cancer Screening and Risk FactorsLacangan, Thea YvonneNo ratings yet
- Surgical Anatomy of The Breast T-HAZEM - CompressedDocument28 pagesSurgical Anatomy of The Breast T-HAZEM - CompressedmohamedhazemelfollNo ratings yet
- Path o PhysiologyDocument11 pagesPath o PhysiologyKimm Charmaine RodriguezNo ratings yet
- Maternal Physiology-WilliamsDocument60 pagesMaternal Physiology-WilliamsRegina Marhadisony100% (1)
- Benign Disorders and Breast DXDocument19 pagesBenign Disorders and Breast DXkurniafniatiNo ratings yet
- Surgical Embryology and Anatomy of The Breast and Its Related Anatomic StructuresDocument22 pagesSurgical Embryology and Anatomy of The Breast and Its Related Anatomic StructuresEmilio SanchezNo ratings yet
- Grainger Paediatric AnatomyDocument15 pagesGrainger Paediatric AnatomympNo ratings yet
- Unit 1 - Breast Anatomy and PhysiologyDocument50 pagesUnit 1 - Breast Anatomy and PhysiologyClyde R.OrtegaNo ratings yet
- 3 - Anatomy Presentation NotesDocument43 pages3 - Anatomy Presentation Notesonezwa1992No ratings yet
- Embryonic Breast Development and MalformationsDocument96 pagesEmbryonic Breast Development and MalformationsSalim Mwiti NabeaNo ratings yet
- Anatomical Changes During PregnancyDocument21 pagesAnatomical Changes During Pregnancycurry yumeNo ratings yet
- Breast Edema: Causes, Imaging Findings and ManagementDocument258 pagesBreast Edema: Causes, Imaging Findings and ManagementMartin AmoresNo ratings yet
- 9 Accessory NippleDocument6 pages9 Accessory NippleTri Haryati ParamitaNo ratings yet
- Imperforate Anus and Cloacal MalformationsDocument110 pagesImperforate Anus and Cloacal MalformationsAhmad Abu KushNo ratings yet
- AnatomicallyDocument3 pagesAnatomicallyAjayDeep NallabothulaNo ratings yet
- MRI MAmaDocument67 pagesMRI MAmaManuel Leonardo CaetanoNo ratings yet
- Umbilical Cord Accidents and Legal Implications Edit 14Document6 pagesUmbilical Cord Accidents and Legal Implications Edit 14DanNo ratings yet
- AnatDocument45 pagesAnatRinxas VerinxtNo ratings yet
- Maternal Anatomy Lyra Jean GarciaDocument4 pagesMaternal Anatomy Lyra Jean GarciaLyra Jean Velo GarciaNo ratings yet
- Female Genital Tract and The Pelvic BonesDocument37 pagesFemale Genital Tract and The Pelvic Bonesعبد الله الحربيNo ratings yet
- Breast Anatomy and Examination GuideDocument11 pagesBreast Anatomy and Examination GuideKrishna Faith P. DelaraNo ratings yet
- Delivery of PlacentaDocument8 pagesDelivery of PlacentaAbdullahi Suleiman MakaNo ratings yet
- OB Definition of TermsDocument9 pagesOB Definition of TermsWarrenSandovalNo ratings yet
- Breast anatomy overviewDocument59 pagesBreast anatomy overviewgina2535100% (1)
- Master Degree in Plastic Surgery ThesisDocument117 pagesMaster Degree in Plastic Surgery ThesisMohamed Ahmed El-RoubyNo ratings yet
- Care of The Umbilicus and Management of Umbilical Disorders - UpToDateDocument31 pagesCare of The Umbilicus and Management of Umbilical Disorders - UpToDateJulio Leal100% (1)
- Breast: 1 EtymologyDocument13 pagesBreast: 1 EtymologynathanNo ratings yet
- Detecting Ectopic Pregnancies with UltrasoundDocument30 pagesDetecting Ectopic Pregnancies with UltrasoundgarciajulioNo ratings yet
- 2013 Maternal and Child Health Nursing Reviewer CompleteDocument40 pages2013 Maternal and Child Health Nursing Reviewer CompleteHarley Justiniani Dela CruzNo ratings yet
- Embryology and Functional Anatomy of The BreastDocument20 pagesEmbryology and Functional Anatomy of The BreastseidkeNo ratings yet
- Clin TG Abnormalities of The Placenta & CordDocument13 pagesClin TG Abnormalities of The Placenta & CordTami Selvi100% (2)
- Congenital Abnormalities of The UterusDocument7 pagesCongenital Abnormalities of The Uterusمحمود الموسويNo ratings yet
- Mammary Gland - Histology - Nto, Nto JohnsonDocument22 pagesMammary Gland - Histology - Nto, Nto JohnsonJessy OnahNo ratings yet
- Menstrual Disorders: Endometriosis, Dysmenorrhea and PMDDDocument37 pagesMenstrual Disorders: Endometriosis, Dysmenorrhea and PMDDOka ChintyaNo ratings yet
- Paediatric AnatomyDocument5 pagesPaediatric AnatomyRisma PertiwiNo ratings yet
- Rabbit Spay and Neuter TechniquesDocument4 pagesRabbit Spay and Neuter TechniquesSebastián Ordóñez RamírezNo ratings yet
- Nursing Process for C-Section RecoveryDocument8 pagesNursing Process for C-Section Recoverymarkie917No ratings yet
- 4 1Document265 pages4 1sillypoloNo ratings yet
- Introduction to Breast Diseases and Nipple DischargesDocument10 pagesIntroduction to Breast Diseases and Nipple DischargessintayehuNo ratings yet
- Breast ExaminationDocument16 pagesBreast Examinationjackfruit5887No ratings yet
- Case Study - CS With BTLDocument16 pagesCase Study - CS With BTLAJ Roze Holmes100% (2)
- 3 - Anatomy, Abdomen and Pelvis - Female Internal Genitals - StatPearls - NCBI BookshelfDocument10 pages3 - Anatomy, Abdomen and Pelvis - Female Internal Genitals - StatPearls - NCBI BookshelfmimatechcontabilidadNo ratings yet
- Conception and Diagnosis of Early PregnancyDocument48 pagesConception and Diagnosis of Early PregnancyjerrydanfordfxNo ratings yet
- ANATOMY, BLOOD SUPPLY AND LYMPHATIC DRAINAGE OF THE BREASTDocument62 pagesANATOMY, BLOOD SUPPLY AND LYMPHATIC DRAINAGE OF THE BREASThussain AltaherNo ratings yet
- Giant Omphalocele With OEIS Complex - A Case ReportDocument3 pagesGiant Omphalocele With OEIS Complex - A Case ReportIOSRjournalNo ratings yet
- Mammary Gland Additional NotesDocument36 pagesMammary Gland Additional NotesjdfNo ratings yet
- How To Measure Cervical LengthDocument5 pagesHow To Measure Cervical LengthcarlosNo ratings yet
- Female Vulva Anatomy GuideDocument18 pagesFemale Vulva Anatomy Guidefujimeister0% (1)
- Buhimschi 2004 Endocrinology of LactationDocument17 pagesBuhimschi 2004 Endocrinology of LactationCarolina GómezNo ratings yet
- 2.anatomy and Histology of The CervixDocument37 pages2.anatomy and Histology of The Cervixmuhammadnurul asmiNo ratings yet
- 5 6057345046456304562Document490 pages5 6057345046456304562DK DeepakNo ratings yet
- Anatomy and Embryology of Umbilicus in Newborns: A Review and Clinical CorrelationsDocument7 pagesAnatomy and Embryology of Umbilicus in Newborns: A Review and Clinical Correlationsbayu pamungkasNo ratings yet
- Anatomical and Physiological ChangesDocument25 pagesAnatomical and Physiological ChangespoojasolNo ratings yet
- Men's Complete Health Guide: Expert Answers to the Questions Men Don't Always AskFrom EverandMen's Complete Health Guide: Expert Answers to the Questions Men Don't Always AskNo ratings yet
- Dr. Sak Indriyani, Spa, Mkes: Department of Child Health Rsu MataramDocument48 pagesDr. Sak Indriyani, Spa, Mkes: Department of Child Health Rsu MataramMuhammad Bilal Bin AmirNo ratings yet
- Facial Action Coding SystemDocument13 pagesFacial Action Coding SystemBarkha ShahNo ratings yet
- Article - ECG Vs MCGDocument7 pagesArticle - ECG Vs MCGpaul_calburean7899No ratings yet
- ReportDocument10 pagesReportSems AlcozbaryNo ratings yet
- Pathomorphology Final ExamDocument262 pagesPathomorphology Final ExamMann SarwanNo ratings yet
- LingalaDocument4 pagesLingalaFabio Ando Filho0% (1)
- Parkinson S DiseaseDocument2 pagesParkinson S DiseaseRamesh RajNo ratings yet
- hemiplegia دكتور عزونيDocument4 pageshemiplegia دكتور عزونيRaed AlhnaityNo ratings yet
- Ageless Secrets EbookDocument13 pagesAgeless Secrets Ebookbonifacesilveira0% (1)
- NO Nama Alat Jumlah Kondisi Baik RusakDocument6 pagesNO Nama Alat Jumlah Kondisi Baik RusakRumah Sakit Umum KartiniNo ratings yet
- The Ghost Map NotesDocument9 pagesThe Ghost Map NotesLuke CybulskiNo ratings yet
- Lab 09 HandoutDocument8 pagesLab 09 HandoutHyenaNo ratings yet
- Russel Body - Cause of Cancer and DeathDocument12 pagesRussel Body - Cause of Cancer and DeathAndré AmorimNo ratings yet
- PrefixDocument6 pagesPrefixbingkydoodle1012No ratings yet
- Cellular ComponentsDocument2 pagesCellular ComponentsWangSheng TanNo ratings yet
- Innate ImmunityDocument16 pagesInnate ImmunityikhaNo ratings yet
- Trauma y Memoria Procedural 2015 Paper Peter LevineDocument18 pagesTrauma y Memoria Procedural 2015 Paper Peter LevineMaría Regina Castro CataldiNo ratings yet
- Structure of Blood Vessels - StationsDocument6 pagesStructure of Blood Vessels - StationsRamya MalariniNo ratings yet
- Review Article Hijamah (Cupping Therapy) - A Comprehensive ReviewDocument8 pagesReview Article Hijamah (Cupping Therapy) - A Comprehensive ReviewMohamed ShiffaNo ratings yet
- Microsyllabus BSC 301Document9 pagesMicrosyllabus BSC 301Anwita JhaNo ratings yet
- Frog Dissection (Remote)Document21 pagesFrog Dissection (Remote)Mohammad NiyaifarNo ratings yet
- Age Appropriate Orthodontics Overview HO 2013Document31 pagesAge Appropriate Orthodontics Overview HO 2013javi222222No ratings yet
- Laser removal of lower lip mucoceleDocument5 pagesLaser removal of lower lip mucoceleDevi AlfianiNo ratings yet
- Physical BackgroundDocument24 pagesPhysical BackgroundmarkNo ratings yet
- Immunity in parasitic diseasesDocument17 pagesImmunity in parasitic diseasesAfdaliaNo ratings yet
- Before Diving Make A Complete Dive Plan Together & Estimate Sea ConditionsDocument8 pagesBefore Diving Make A Complete Dive Plan Together & Estimate Sea ConditionsMike LuckyNo ratings yet
- Fungsi Otot-OtotDocument32 pagesFungsi Otot-OtothansenpanjaitanNo ratings yet
- The Breath of Yoga & Martial ArtsDocument15 pagesThe Breath of Yoga & Martial Artskarynchin100% (2)