Professional Documents
Culture Documents
A Stability Function For Explicit Evaluation of The Mullins-Sekerka Interface Stability Criterion
Uploaded by
Kamel HebbacheOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A Stability Function For Explicit Evaluation of The Mullins-Sekerka Interface Stability Criterion
Uploaded by
Kamel HebbacheCopyright:
Available Formats
JOURNAL
OF
APPLIED
PHYSICS
VOLUME
36,
NUMBER
JANUARY
1965
A Stability Function for Explicit Evaluation of the Mullins-Sekerka
Interface Stability Criterion
R. F.
SEKERKA.
Westinghouse Research Laboratories, Pittsburgiz, Pennsylmnia
(Received 23 January 1964; in final form 3 August 1964)
.\ stability function S for explicit evaluation of the Mullins-Sekerka interface stability criterion is introduced and tabulated. Their criterion is then written in terms of S and compared with the constitutional
supercooling criterion for interface stability. From these results, experimental data can be analyzed and a
conclusive test of the stability theory can be made.
a previous paper by Mullins and Sekerka (called
I NM-S),1
it was shown that during the unidirectional
solidification of a dilute binary alloy at constant velocity, a planar solid-liquid interface is stable when
(1)
and unstable when the inequality is reversed. The notation here is the same as used bv 1\1 ullins and Sekerka;
it is summarized in Appendix i. We shall refer to (1)
as the M-S stability criterion; h is given by the expression
h= Imaxf(w) I,
We thus confine attention to the case for which A < 1.
Let us introduce a stability function S defined by the
equation
(4)
From the above considerations, it follows that
(5)
Then (1) becomes, for stability (note reversal of the inequality caused by multiplication by -1)
(2)
where
(6)
Introducing the dimensionless variables
y=(TMr/mGc)w2~O and tJ=.J-kU
(3)
Examination of Eqs. (2) and (3) shows that the determination of h by straightforward application of the
calculus involves the solution of a cubic equation in w.
But due to the cumbersome form of the analytic solution of a cubic equation, it is difficult to assess the M-S
criterion. It is the purpose of this paper to develop explicit expressions for h and to define and tabulate a related quantity S as a function of certain dimensionless
parameters. In this way, the predictions of the M-S
theory can be readily evaluated and compared with
other theories and with experiment; the use of dimensionless parameters alleviates the need to specify constants characteristic of a given material (often poorly
known) in order to give a complete theoretical analysis.
and defining a function (y) by the equation
A:(y) =
few)
2k
mC;,
(1 +tJy)!-l +2k'
---= y+-------
it follows that "c(y) has a minimum which obeys the
inequality
o~ [min(y)J~ 1
(8)
and that
s= l-[min(y)].
(9)
To find the value y= y", where "c(y) has its minimum
value we set
8(y)/
---
8y
INTRODUCTION OF A STABILITY FUNCTION S
=0
y~ljm
This condition yields
It was shown in M-S that for w~O
{}k
(1) f(w)~O,
(1+tJYm)l=~---_----.
[(1+tJYm)I-1 + 2kJ2
(2) O~ Imaxf(w) I ~mGc (note mGc~O),
(3) few) has one and only one maximum,
(4) for A ~ 1, where A is the dimensionless parameter,
k2
(7)
rv
TilT
rv
TM V
- - - - - - - - - - = k--- - - ,
(1-k) D (-mk"
Since (1+tJYm)L1+2k>O (for k>O) one can take the
square root of both sides of (10) without ambiguity of
sign. Introducing the substitution r= (1 +tJYm)t leads
readily to the following cubic equation for r:
D mGcD
r~+ (2k-l)r-
h = mG c and instability is impossible.
(10)
(2k/.ll) = O.
(11)
Writing (11) in the form
-----
W. W. Mullins and R. F. Sekerka, J. Appl. Phys. 35, 444
(1964).
1
264
r3= (2k/ A 1)+(1- 2k)r,
(12)
]\[ F L L I 1'\ S - S EKE R K A
I)J" T
E !{ F ACE S L<\ B r LIT Y C R I T E RIO N
one can plot the left and right sides of Eq. (12) as we
have done for three representative cases in Fig. 1. Xote
that the straight line (2k.'.1 i)+(1-2k)r always has a
positive intercept on the ordinate for positive A and k
while its slope may be positive or negative and depends
only on k. Hence, corresponding to k ~ 0, there exists
one and only one root r>O of Eq. (11); it is this positive
root which corresponds to positive w. Henceforth in this
paper, we shall refer to r as the real positive root of (11).
Obviously, from (11), r is a function of two dimensionless variables, A and 1<, and since
y",=(r L
(13)
ll(A/4k),
FIG.
1. Plot of
Efj. (11) as a function of r for (a) case
1-1 positin root, 2
conjugate imaginary
roots, (b) case II-l
positive root, 2 equal
negative roots, and
Ic) case III-l posi
tive root, 2 unequal
negative roots.
--
it follows from (9) that S is also a function of only "1
and k. Using (7), (9), (10), and (11) we readily find
.1
3Al
S(A,k)= 1+- - ---r 4k
2
EVALUATION OF
~+(1-2k)r
A'12
Roots
.4(1-2k)
---~r2.
(14)
4k
,~(A,k)
t1. Using (1-1-) and
Before treating the case of general A and k, we shall
present three approximate analytic expressions for
SeA ,k) corresponding to: (a) the limiting case A --> 0,
(b) the limiting case k --> 0, and (c) the special case
k=!. We consider these cases in turn.
Case (a). For verv small A (such that 2k/ A 11)
it is clear from Oi) that r1 and, in particular,
r3(2k-1)r. A useful approximation amounts to neglecting the linear term in (11) to get
(2k)1/3
(15)
r~--
A-+ll
265
A 1 If,
and using (14)
(1-2k)
,\)(.1,k) ~ 1-t(2k.1)!----(2kA)i+A ...O
4k
41,
(16)
or
SeA ,k) ~ I-H2kA)t,
(17)
4 ... 0
where (17) is the leading term of (16).
Case (b). In the mathematical limit 1?=O, we have
r=l; accordingly for kl we set r=l+~, where ~1
and proceed to do a simple perturbation calculation to
first order in small quantities. Thus (11) becomes
approxima tel y
(18)
S(A,k)
(19) then gives finally
~
k.... O
(1+A-2.1t)(I-k).
(20)
Case (c). For k=!, the linear term in (11) is zero so
r(A,!)=A-1I6. Then (14) readily yields
S(A,!)= 1-!A 1+..1/2.
(21)
Note that (17) agrees with the leading term of (21) for
1?=!, while (16) reduces exactly to (21) in that case.
For general A and k it is expedient to program the
calculations for computation by an IBM 7090 computer. Since only the positive root of (11) is required for
substitution into (14) and since (11) does not contain
a quadratic tern1, it is easier to make use of a straightforward application of the cubic formula than to use a
standard program for the three roots of a general cubic
and then select the positive root. Three cases arise,
depending on the relative sizes of A and k; the resulting
fornlUlas for r are given in Appendix II for reference .
The program was run for 16 values of k and for A
ranging from 10- 10 to 1. Using these results, Figs. 2 and
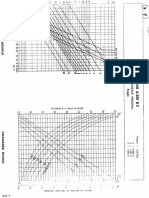
3 were constructed; in Fig. 2, S is plotted as a function
of A. for various k, while in Fig. 3 S is plotted against
log A. Together the plots cover the range of .1 where S
is substantially different from or 1.
THE M-S STABILITY CRITERION IN
TERMS OF S (A,k)
According to Eq. (6), stability obtains when
from which
(g'+g)/2mG c > SeA ,k).
~~k(~-l)+k~(~-~-!),
o AI
A! 2.1
(19)
Using the formulas 1
k ...
where we have selected the root of (18) which makes
(6)
K
c",(k-l) L
DkG c
--(g'-g)
(22)
266
R. F. SEKERKA
of material constants which are specified by a choice of
base material and solute and contains all other parameters except the above three variables of operation.
Within the approximation of dilute solutions, the material constant.s are independent of the kind and amount
of solute. "'ith the preceding classification of parameters, let us consider all of the material constants to be
fixed and suppress their explicit appearance in the
stability uiterion. Accordingly, we define a test function ,(G/V,c,,) by the equation
0.9
0.8
0.7
/Sfable
Unstoble
Proceeding t.o suppress the explicit appearance of the
material constants, we call
0.2
0.3
0.6
0.7
0.8
s( VI coo) = SeA ,k),
0.9
FIG. 2. Plot of the stability function S versus the dimensionless
stability parameter A for several values of the solute partition
coefficient k. A smooth interface is stable if the test function 'I'
fans above the appropriate curve.
and the definitions of ~J' and g, (22) can be written in
terms of experimentally measurable quantities as
k [L
GJ--->S(A,k).
D
--+--
2KL
~---
Ks+Kdl-k) 2KL
(25)
where we continue to use the symbol S without ambiguity because the left side of (25) is a function of
only one variable, the ratio (Vic",). Then for stability,
we need the test function to be greater than the stabilit:-" function, i.e.,
,(G/ V,c",) > s( V/ c",,).
(26)
For extremely small values of A where S -+ lone obtains
(23)
V (-mk"
'l(G/V,c",)
> 1,
(27)
A~()
It is interesting to divide the parameters of this
problem into two classes. The first class consists of the
three variables of operation, G, V, CoO' which can be
varied independently of the base material and kind (but
not amount) of solute, while the second class consists
0.9
O.B
0.7
0.6
0.5
0.4
Unstoble
0.3
0.2
dS
J1
> -2KL
- - [LV
--+G --G
0.1
910
which is the modified constitutional supercooling criterion of lVI-So It is very important to note that Eq. (26)
contains three independent variables of operation (constructed from the three fundamental variables, G, V, CoO,
while Eq. (27), as well as the constitutional supercooling
criterion of Tiller et al./ 3 depends on only two independent variables of operation. Indeed, to get the constitutional supercooling criterion for stability, one sets
KL=K,~ and L=O in (24) and uses (27). Thus, the inclusion of capillarity (S= 1 without capillarity) breaks
the ratio G/V, so to speak, and makes G, V, c'" individually important. It is for this reason that one can
have stability despite the presence of constitutional
supercooling, as pointed out by lVI-So
Let us cast Eq. (23) in still another form where it can
be easily compared with the constitutional supercooling
criterion. Using the notation 4 "gradient of constitutional supercooling," dS/dx=mGc-G, along with Eq.
(22) and reversing the sense of the inequality in (23)
we find that the :M-S theory predicts instability when
-9
-8
-7
-6
-5
-4
-3
Log,oA
FIG. 3. Plot of the ~tability function S versus logA for several
values of k. A smooth interface is stable if the test function 'I' falls
above the appropriate curve.
lix
K 8 +K L 2KL
(28)
2 W. A. Tiller, K. A. Jackson, J. W. Rutter, and B. Chalmers,
Acta Met. 1, 428 (1953).
3 J. W. Rutter and B. Chalmers, Can. J. Phys. 31, 15 (1953).
4 W. A. Tiller, J. App!. Phys. 33,3106 (1962).
1\1 U L LIN S- S EKE R K A
1 NT E R FA C EST A B I LIT Y
or
(29)
whereas the constitutional supercooling criterion predicts instability when
(30)
dS/dx>O.
Equations (29) and (30) show that for instability to
occur, not only must constitutional supercooling exist
but a definite gradient of constitutional supercooling must
exist. Indeed, as $ ----> 0, the amount of constitutional
supercooling needed for instability increases, finally
becoming infinite at $=0 which is the onset of the
absolute stability considered by 1\1-S.
CONCLUSIONS
We have explicitly evaluated the M-S stability criterion in terms of a stability function S and have compared the M-S theory with the theory of constitutional
supercooling. We have seen that the theories are somewhat similar for the case S~l but differ appreciably for
S substantially different from 1. We shall not attempt at
this time to make a detailed comparison with experiment. However, let us note that most experiments to
date have apparently been conducted in the range where
A1, and consequently [see Eq. (17)J S-1. The usual
procedure is then to plot G/V vs Coo and, according to
(24) and (27), get a straight line whose slope is related
to the diffusion coefficient D. Hence, since diffusion coefficients of liquid metals are often poorly known, it may
be useful to begin experiments in the region of low velocities and high solute content so that A --j- 0 and from
the slope of a straight line plot of G/V and c"" determine
D. Then one can proceed to higher velocities and lower
concentrations and use the determined value of D to
evaluate A.
ACKNOWLEDGMENTS
This work was partially supported by the U. S. Air
Force Office of Scientific Research under Contract
AF 49(638)-1029. The author would like to thank Dr.
W. W. Mullins, Department of Metallurgical Engineering, Carnegie Institute of Technology, and Dr. W. A.
Tiller, Crystallogenics Section, Westinghouse Research
Laboratories, for their many helpful suggestions and
for their help in preparing the manuscript.
eRr T E RIO N
267
TjJ = melting point of the pure solvent (OK)
L=latenl heat of fusion per unit volume of the pure
sol ven t (erg/ cm 3)
K L= thermal conductivity of the liquid (erg/cmsec-deg)
K 8= thermal conductivity of the solid (ergs/ cm-sec-deg)
K=(K L +K s )/2=average thermal conductivity of
the system (ergs/cm-sec-deg)
g=G(KL/K)=generalized gradient in the liquid
(deg/cml
g'=Gs(KsiK)=generalized gradient in the solid
(degcm)
Coo = concentration of solute in the liquid far from the
interface (wt.%)
1<= partition coefficient of solute in solven t (dimensionless)
m= slope of liquidus line on phase diagram (deg/wt.%)
D= diffusion coefficient in the liquid (cm2/sec)
Gc =average 5 solute gradient in the liquicl (wt.%/cm)
'Y=solid-liquid surface free energy/unit area (erg/
cm2)
1'= '}'/L = capillarity constant (cm)
w= 27r per wavelength of sinusoidal perturbation of
interface (em-I)
k 2 rv TM
rv 1'11'1 V
A=--------=k--(1-k) D (-m)c",
D mGeD
= stability parameter (dimensionless),
APPENDIX II
A straightforward application of the cuhic formula 6
leads to the following expression for r, the positive root
of (11):
(Al)
r=a+l1,
where a and f3 are given below for the three cases which
occur. Define
k2
(2k-l)3
27
F=-+---.
(A2)
Then Case (1)
If F?;O and (F)!~k/A~,
a= [ kl + (F)!]t
."4 '
(A3a)
APPENDIX I
(A3b)
v = average" velocity of freezing
(em/sec)
temperature gradient III the liquid
G=average 5
(deg/cml
Gs =average 5 temperature
(deg/cm)
gradient
In
the
solid
" Means an li\erage O\er a cross section of the sample at the
interface.
6Handbook 0/ Chemistry and Physics (Chemical Rubber
Company, Cleyeland, Ohio, 1961), 43rd ed., p. 318.
You might also like
- Akoz - The Mixed Finite Element Solution of Circular Beam On Elastic FoundationDocument9 pagesAkoz - The Mixed Finite Element Solution of Circular Beam On Elastic FoundationNguyen Ngoc TuanNo ratings yet
- Department of Physics, Yale University, New Haven, CT. 06520, USA andDocument4 pagesDepartment of Physics, Yale University, New Haven, CT. 06520, USA andEnrique EscalanteNo ratings yet
- Analysis of the Structural Properties of the Solutions to the Speed Gradient Traffic Flow ModelDocument7 pagesAnalysis of the Structural Properties of the Solutions to the Speed Gradient Traffic Flow Modelskr2010No ratings yet
- H.K. Moffatt - Viscous Eddies Near A Sharp CornerDocument8 pagesH.K. Moffatt - Viscous Eddies Near A Sharp CornerYokdmNo ratings yet
- Perturbative Yang-Mills Ground State in The Temporal GaugeDocument7 pagesPerturbative Yang-Mills Ground State in The Temporal GaugeCroco AliNo ratings yet
- Piezoresistance Effect of SiliconDocument9 pagesPiezoresistance Effect of SiliconTavoNo ratings yet
- ZimmerDocument17 pagesZimmerKhai NqNo ratings yet
- 20 Kardom QJMAMDocument13 pages20 Kardom QJMAMmlouredocasadoNo ratings yet
- Rigidity matrices for layered mediaDocument15 pagesRigidity matrices for layered mediaHernan Kennedy Ricaldi PorrasNo ratings yet
- Chow 1962Document17 pagesChow 1962M.SerranoNo ratings yet
- Snoek Effect PDFDocument5 pagesSnoek Effect PDFhamed soleymaniNo ratings yet
- National Academy of SciencesDocument10 pagesNational Academy of SciencesSubhajit KarNo ratings yet
- A Consistent Co-Rotational Formulation For Non-Linear, Three-Dimensional, Beam-ElementsDocument20 pagesA Consistent Co-Rotational Formulation For Non-Linear, Three-Dimensional, Beam-Elements홍성근No ratings yet
- Brief Review On Tight-Binding Model: ~ kα ~ k i~ k· (~ R+~ r) α αDocument10 pagesBrief Review On Tight-Binding Model: ~ kα ~ k i~ k· (~ R+~ r) α αRobert WexlerNo ratings yet
- PhysRevB 22 4950Document10 pagesPhysRevB 22 4950Martín FigueroaNo ratings yet
- Phase Diagrams - Progress in Solid State Chemistry (10) 1975 PeltonDocument37 pagesPhase Diagrams - Progress in Solid State Chemistry (10) 1975 PeltonJunior Lima100% (1)
- Turgeman (Ub 1982)Document20 pagesTurgeman (Ub 1982)Paramita BhattacharyaNo ratings yet
- 1 s2.0 001600326190148X MainDocument8 pages1 s2.0 001600326190148X MainIrani GonçalvesNo ratings yet
- Tables of Coefficients for the Analysis of Triple Angular Correlations of Gamma-Rays from Aligned NucleiFrom EverandTables of Coefficients for the Analysis of Triple Angular Correlations of Gamma-Rays from Aligned NucleiNo ratings yet
- Spherical tensor operators and the Wigner-Eckart theoremDocument8 pagesSpherical tensor operators and the Wigner-Eckart theoremFidel YoNo ratings yet
- Recitation Class No. 7 in Quantum Mechanics 2 (77605) : 1 The LRL VectorDocument5 pagesRecitation Class No. 7 in Quantum Mechanics 2 (77605) : 1 The LRL VectordanielNo ratings yet
- Approximation Techniques in Complex Reaction Kinetics: DannyDocument18 pagesApproximation Techniques in Complex Reaction Kinetics: DannyDeep GhoseNo ratings yet
- Theoretical Solutions For Turbulence Generated by Vibrating Grids and by Wall Flows Using The KDocument10 pagesTheoretical Solutions For Turbulence Generated by Vibrating Grids and by Wall Flows Using The Kjohannesjanzen6527No ratings yet
- Incremental Displacement Algorithms For Nonlinear ProblemsDocument6 pagesIncremental Displacement Algorithms For Nonlinear ProblemsOscar Ortiz MéndezNo ratings yet
- Frequency To Time ConversionDocument4 pagesFrequency To Time ConversionThakur Jitender PundirNo ratings yet
- Appendix A. Second QuantizationDocument24 pagesAppendix A. Second QuantizationAgtc TandayNo ratings yet
- Iterated Crossed Box Diagram in The Complex Angular Momentum Plane and Bethe-Salpeter EquationDocument15 pagesIterated Crossed Box Diagram in The Complex Angular Momentum Plane and Bethe-Salpeter EquationAnonymous FigYuONxuuNo ratings yet
- Cylindrically Orthotropic Plates with Large DeflectionsDocument11 pagesCylindrically Orthotropic Plates with Large DeflectionsyasameenNo ratings yet
- Quadrature Methods For Integral Equations of The Second Kind Over Infinite IntervalsDocument13 pagesQuadrature Methods For Integral Equations of The Second Kind Over Infinite IntervalsFizzerNo ratings yet
- Of Exact: Response The State ADocument9 pagesOf Exact: Response The State APatrícia GonçalvesNo ratings yet
- 1 s2.0 0020746266900242 MainDocument14 pages1 s2.0 0020746266900242 MainIrani GonçalvesNo ratings yet
- Graetz ProblemDocument13 pagesGraetz ProblemBelialVKWWNo ratings yet
- Anastasia Doikou - A Note On The Boundary Spin S XXZ ChainDocument12 pagesAnastasia Doikou - A Note On The Boundary Spin S XXZ ChainPo48HSDNo ratings yet
- Additivity and Multiplicativity Properties of Some Gaussian Channels For Gaussian InputsDocument9 pagesAdditivity and Multiplicativity Properties of Some Gaussian Channels For Gaussian InputsgejikeijiNo ratings yet
- On The Low Frequency Elastic Response of A Spherical ParticleDocument8 pagesOn The Low Frequency Elastic Response of A Spherical ParticlesteveNo ratings yet
- Received 30 September 1992Document7 pagesReceived 30 September 1992Hanna ChingNo ratings yet
- Reissner - 1976 Thick PlatesDocument5 pagesReissner - 1976 Thick PlatesIoannisPerseusAntypasNo ratings yet
- Dijk Graaf 1991Document22 pagesDijk Graaf 1991e6e7e8f4g2No ratings yet
- Some Modified Matrix Eigenvalue: E 2 (X), (A I) A (X) - (9) ) - A (A) O'i (A O'2 (A A, (A) 21 (A) 22 (A) 2, (A)Document17 pagesSome Modified Matrix Eigenvalue: E 2 (X), (A I) A (X) - (9) ) - A (A) O'i (A O'2 (A A, (A) 21 (A) 22 (A) 2, (A)Satyavir YadavNo ratings yet
- Chapter 1 Structure and Properties of Metals and Alloys 1995 Studies in Surface Science and CatalysisDocument66 pagesChapter 1 Structure and Properties of Metals and Alloys 1995 Studies in Surface Science and CatalysisHenrique SouzaNo ratings yet
- Add Eta Singular WojDocument13 pagesAdd Eta Singular Woj123chessNo ratings yet
- Introduction:The Stability and Instability of Structural Elements in Offshore StructuresDocument6 pagesIntroduction:The Stability and Instability of Structural Elements in Offshore Structuressudipta_chakraborty_78No ratings yet
- Effective Numerical Treatment of Boundary Integral Equations. J.C Lachat and WatsonDocument15 pagesEffective Numerical Treatment of Boundary Integral Equations. J.C Lachat and Watsonrahim.sihadjmohandNo ratings yet
- Lorentz Invariance Vs Temperature QFTDocument8 pagesLorentz Invariance Vs Temperature QFTJosé JiménezNo ratings yet
- Discretization To Avoid Singularities in Vibration-Rotation Hamiltonians: Bisector Embedding For AB2 TriatomicsDocument12 pagesDiscretization To Avoid Singularities in Vibration-Rotation Hamiltonians: Bisector Embedding For AB2 TriatomicsPassammNo ratings yet
- Effective Length Factors Across CodesDocument21 pagesEffective Length Factors Across CodesKtk ZadNo ratings yet
- Graph and MatricesDocument17 pagesGraph and Matricessanjay975No ratings yet
- Orthotropic Skew Slab - Coull PDFDocument13 pagesOrthotropic Skew Slab - Coull PDFjnshreyNo ratings yet
- Cylindrical SOVDocument9 pagesCylindrical SOVAlex StevensonNo ratings yet
- Comparison Between Shear Deformable and Kirchhoff Theories For Bending, Buckling and Vibration of Antisymmetric Angle-Ply Laminated PlatesDocument14 pagesComparison Between Shear Deformable and Kirchhoff Theories For Bending, Buckling and Vibration of Antisymmetric Angle-Ply Laminated Plateswallisonkennedy014No ratings yet
- Quantenoptik Vorlesung6 PDFDocument11 pagesQuantenoptik Vorlesung6 PDFErinSuttonNo ratings yet
- As Dira Cop Rs Vector PotentialsDocument4 pagesAs Dira Cop Rs Vector Potentials123chessNo ratings yet
- Straten 75 RotationalDocument10 pagesStraten 75 RotationalHimansu Sekhar SahuNo ratings yet
- A Generalized Theory of GravitationDocument5 pagesA Generalized Theory of GravitationJAZNARFNo ratings yet
- Examples Quantum ChemistryDocument7 pagesExamples Quantum ChemistryEnitiNo ratings yet
- Fibonacci and Related Sequences in Periodic Tridiagonal MatricesDocument9 pagesFibonacci and Related Sequences in Periodic Tridiagonal MatricesCharlie PinedoNo ratings yet
- An Axiomatization of The Lattice of Higher Relative Commutants of A SubfactorDocument19 pagesAn Axiomatization of The Lattice of Higher Relative Commutants of A SubfactorHua WangNo ratings yet
- Journal of Computational Physics (1970) : 6, (k2), rSI (KZ) P (k2) 6g' (k2) I8jz' (k2)Document4 pagesJournal of Computational Physics (1970) : 6, (k2), rSI (KZ) P (k2) 6g' (k2) I8jz' (k2)apooladiNo ratings yet
- M. Axenides Et Al - Q RingsDocument4 pagesM. Axenides Et Al - Q RingsPomac232No ratings yet
- Difference Equations in Normed Spaces: Stability and OscillationsFrom EverandDifference Equations in Normed Spaces: Stability and OscillationsNo ratings yet
- Monitoring Deformations of Istanbul Metro Line Stations Through Sentinel 1 and Levelling ObservationsDocument10 pagesMonitoring Deformations of Istanbul Metro Line Stations Through Sentinel 1 and Levelling ObservationsKamel HebbacheNo ratings yet
- Correlation Between Agrotechnical Properties of Selected Soil Types and Corresponding GPR ResponseDocument7 pagesCorrelation Between Agrotechnical Properties of Selected Soil Types and Corresponding GPR ResponseKamel HebbacheNo ratings yet
- Geotechnical and Geological Investigation of Landslide in West Arsi Zone, EthiopiaDocument17 pagesGeotechnical and Geological Investigation of Landslide in West Arsi Zone, EthiopiaKamel HebbacheNo ratings yet
- Lin2021 Article SurfaceWaterQualityAssessmentBDocument16 pagesLin2021 Article SurfaceWaterQualityAssessmentBKamel HebbacheNo ratings yet
- 10.1002@nsg.12135 2Document15 pages10.1002@nsg.12135 2Kamel HebbacheNo ratings yet
- 10.1002@nsg.12135 2Document15 pages10.1002@nsg.12135 2Kamel HebbacheNo ratings yet
- Holmes 2020Document15 pagesHolmes 2020Kamel HebbacheNo ratings yet
- Soltani 2020Document4 pagesSoltani 2020Kamel HebbacheNo ratings yet
- Moazeni-Noghondar2021 Article StudyOnSoilWaterRetentionAndInDocument18 pagesMoazeni-Noghondar2021 Article StudyOnSoilWaterRetentionAndInKamel HebbacheNo ratings yet
- Hwang-Kim2021 Article OccurrenceOfUranopolycraseInPeDocument11 pagesHwang-Kim2021 Article OccurrenceOfUranopolycraseInPeKamel HebbacheNo ratings yet
- AlQahtani2021 Article ElectricalResistivityAndRefracDocument9 pagesAlQahtani2021 Article ElectricalResistivityAndRefracKamel HebbacheNo ratings yet
- Orenday-Tapia2021 Article RelationBetweenTheVelocityPropDocument10 pagesOrenday-Tapia2021 Article RelationBetweenTheVelocityPropKamel HebbacheNo ratings yet
- Depountis2021 Article ClassificationOfSoilsContaminaDocument10 pagesDepountis2021 Article ClassificationOfSoilsContaminaKamel HebbacheNo ratings yet
- Case History A 3-D Resistivity Investigation of A Contaminated Site at Lernacken, SwedenDocument9 pagesCase History A 3-D Resistivity Investigation of A Contaminated Site at Lernacken, SwedenKamel HebbacheNo ratings yet
- Duan2021 Article EffectsOfWaterContentAndSaltCoDocument15 pagesDuan2021 Article EffectsOfWaterContentAndSaltCoKamel HebbacheNo ratings yet
- Duan2021 Article EffectsOfWaterContentAndSaltCoDocument15 pagesDuan2021 Article EffectsOfWaterContentAndSaltCoKamel HebbacheNo ratings yet
- Detecting Seasonal Flow Pathways in Road StructureDocument13 pagesDetecting Seasonal Flow Pathways in Road StructureKamel HebbacheNo ratings yet
- Air Bus A 300 B2Document1 pageAir Bus A 300 B2Kamel HebbacheNo ratings yet
- Duan2021 Article EffectsOfWaterContentAndSaltCoDocument15 pagesDuan2021 Article EffectsOfWaterContentAndSaltCoKamel HebbacheNo ratings yet
- Bederina 2007Document7 pagesBederina 2007Kamel HebbacheNo ratings yet
- 1 s2.0 S0098300410002128 MainDocument13 pages1 s2.0 S0098300410002128 MainKamel HebbacheNo ratings yet
- Influence of Fibre Reinforcement On Selected Mechanical Properties of Dental CompositesDocument8 pagesInfluence of Fibre Reinforcement On Selected Mechanical Properties of Dental CompositesKamel HebbacheNo ratings yet
- Electrical Resistivity Data Sets That Provide Optimum Subsurface InformationDocument20 pagesElectrical Resistivity Data Sets That Provide Optimum Subsurface InformationKamel HebbacheNo ratings yet
- Hwang-Kim2021 Article OccurrenceOfUranopolycraseInPeDocument11 pagesHwang-Kim2021 Article OccurrenceOfUranopolycraseInPeKamel HebbacheNo ratings yet
- 1 s2.0 S2090997715000449 Main PDFDocument10 pages1 s2.0 S2090997715000449 Main PDFEka HarrisNo ratings yet
- Effects of Machinery Trafficking in An Agricultural Soil Assessed by Electrical Resistivity Tomography (ERT)Document9 pagesEffects of Machinery Trafficking in An Agricultural Soil Assessed by Electrical Resistivity Tomography (ERT)Kamel HebbacheNo ratings yet
- Electrical Methods: Induced PolarizationDocument2 pagesElectrical Methods: Induced PolarizationKamel HebbacheNo ratings yet
- Electrical Resistivity Tomography Using Wenner BDocument7 pagesElectrical Resistivity Tomography Using Wenner BKamel HebbacheNo ratings yet
- 02 04 Ali Et Al MeroeDocument10 pages02 04 Ali Et Al MeroeKamel HebbacheNo ratings yet
- Tunnel v2 FC v3 GBDocument2 pagesTunnel v2 FC v3 GBKamel HebbacheNo ratings yet
- Udel PSU Design Guide - EN v5.0 - 0 - 0 PDFDocument84 pagesUdel PSU Design Guide - EN v5.0 - 0 - 0 PDFReza Pencinta AL HadadNo ratings yet
- Essay 2Document3 pagesEssay 2Norhaida NdNo ratings yet
- Consolidated List of Fdcs Licensed by Slas But Not Permitted by DCG (I)Document8 pagesConsolidated List of Fdcs Licensed by Slas But Not Permitted by DCG (I)NAVNEET BAGGANo ratings yet
- Assignment 5: Unit 7 - Week 5Document1 pageAssignment 5: Unit 7 - Week 5atulNo ratings yet
- X17Crni 16-2: C: 0,19 - 0,22 CR: 15,5 - 17,0 Ni: 1,5 - 2,5Document2 pagesX17Crni 16-2: C: 0,19 - 0,22 CR: 15,5 - 17,0 Ni: 1,5 - 2,5Aadhya engineering ServicesNo ratings yet
- DST 60 IFT MeasurementDocument12 pagesDST 60 IFT Measurement김동완No ratings yet
- Ejercicios PDFDocument4 pagesEjercicios PDFserepoesia10No ratings yet
- As NZS 1865 1997 Aluminium and Aluminium Alloys Drawn Wire Rod Bar and StripDocument7 pagesAs NZS 1865 1997 Aluminium and Aluminium Alloys Drawn Wire Rod Bar and StripYasser Hammad MohamedNo ratings yet
- On-Line Monitoring of Bacterial and Pathogen Load Through Adenosine TriphosphateDocument8 pagesOn-Line Monitoring of Bacterial and Pathogen Load Through Adenosine TriphosphateKhoiril NNo ratings yet
- DurstDocument7 pagesDurstRahul KatreNo ratings yet
- DisinfectionDocument54 pagesDisinfectionShari KNo ratings yet
- Chapter 1 - Chemical Reactions and Equations: Class - X ScienceDocument9 pagesChapter 1 - Chemical Reactions and Equations: Class - X ScienceMiten Gandhi100% (1)
- Tank Cleaning Vacuum Residue AsphaltDocument2 pagesTank Cleaning Vacuum Residue AsphaltSushilNo ratings yet
- Expandable PU Foam Sealant for Filling GapsDocument1 pageExpandable PU Foam Sealant for Filling GapsAmit SinghNo ratings yet
- Effect of geotextiles on low plastic sandy clayDocument9 pagesEffect of geotextiles on low plastic sandy clayRajesh Prasad ShuklaNo ratings yet
- Mineral PropertiesDocument3 pagesMineral PropertiesAji SuhadiNo ratings yet
- Coal Preparation Technologies Sep 2019 Indpnesia (JCOAL)Document23 pagesCoal Preparation Technologies Sep 2019 Indpnesia (JCOAL)ErwinLBudiNo ratings yet
- Unit-Ii Notes .Sme: ThermodynamicsDocument19 pagesUnit-Ii Notes .Sme: Thermodynamicssthavir punwatkarNo ratings yet
- Crystalline Structures AssignmentDocument6 pagesCrystalline Structures AssignmentNouman1203No ratings yet
- UV Coating Flooring - Radtech 2003 - D. Bontinck M. IdacageDocument6 pagesUV Coating Flooring - Radtech 2003 - D. Bontinck M. IdacageJuan Antonio Tito Esp CalNo ratings yet
- Ultimate Cheatcode CematconDocument82 pagesUltimate Cheatcode CematconJulian Deleon100% (1)
- Fouling of Heat Exchangers by Dairy Fluids - A Review: B. BansalDocument9 pagesFouling of Heat Exchangers by Dairy Fluids - A Review: B. BansalfikerykNo ratings yet
- Carboxyl GroupDocument13 pagesCarboxyl GroupMuhammad Syazwan SapirenNo ratings yet
- MSDS Neopelex FS KAO RevisiDocument7 pagesMSDS Neopelex FS KAO RevisiIbnu SalehNo ratings yet
- Heat Transfer and Variable Loads Sept 2017 Rev 6 PresentationDocument49 pagesHeat Transfer and Variable Loads Sept 2017 Rev 6 PresentationFritzel OyaoNo ratings yet
- SMAW LessonDocument8 pagesSMAW LessonOJ DogplaceNo ratings yet
- Waste Incineration Heat Recovery at Roche IrelandDocument12 pagesWaste Incineration Heat Recovery at Roche IrelandidrieshamidiNo ratings yet
- CNT Neutron Porosity ToolDocument2 pagesCNT Neutron Porosity ToolAurora Duran LiraNo ratings yet
- Bio Energiser Hair Boost Caffeine Power Tonic MSDS October 2016Document3 pagesBio Energiser Hair Boost Caffeine Power Tonic MSDS October 2016Alexander JefferyNo ratings yet
- Book of AbstractsDocument56 pagesBook of AbstractsCojocaru Bogdan EugenNo ratings yet