Professional Documents
Culture Documents
Thermal and concentration boundary layer equations
Uploaded by
Rosalyne Artho-PhanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermal and concentration boundary layer equations
Uploaded by
Rosalyne Artho-PhanCopyright:
Available Formats
Ghosh - 550
Page 1
5/23/2016
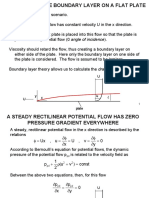
Thermal and Concentration Boundary Layers
Thermal and concentration boundary layers are very similar to the velocity boundary
layers discussed before, except we focus our attention to the temperature and
concentration profiles instead of velocity profiles. Instead of the growth of velocity
from zero to a free stream value, thermal or concentration boundary layers track
the changes in temperature decay or concentration decay. We may discuss all these
boundary layers by using similarity principles as introduced in the next section. Consider
a flow of polluted river water that is brought into a tank for purification. As the flow
enters the bed it may be considered a flow over a flat plate. We would pan heat into the
water to kill germs and apply other methods of pollutant control. The velocity profile,
temperature profile, and concentration profile on the flat plate are all shown in the figure
below:
In this diagram the velocity boundary layer is shown to grow with distance x [marked by
vel(x)], where as the growth of the temperature and concentration profiles are marked as
temp(x) and conc(x). Depending on the transport characteristics of velocity, vorticity,
temperature, and concentration these boundary layers may grow at different rates. When
we speak about velocity changes in the boundary layer the fluid property that influences
them is viscosity, whereas for temperature and concentration boundary layers, the
y
y
u(y)
vel
Flat plate adds heat to flow
T(y)
conc
tem
vel(x
)
CA(y)
conc(x)
temp(x)
TS
C AS
corresponding properties are the convective heat transfer and mass transfer coefficients.
The governing equations for velocity boundary layers, thermal boundary layers, and
concentration boundary layers all follow similar patterns. Rather than deriving the
thermal and concentration boundary layer equation we simply present them below. For a
Ghosh - 550
Page 2
5/23/2016
fluid such as air that may be treated as an ideal, incompressible gas or, for an
incompressible liquid such as water,
T
T
T
T
C u
v
k
q
k
y
x x y y
x
and,
(A)
C
C
C
C
v
N
D
D
x
y
x
x y
y
A
AB
AB
(B)
In the first equation k represents the thermal conductivity of a homogeneous solid, q
represents the rate of heat generation per unit volume and
represents the rate of
viscous dissipation per unit volume, given by:
2
2
u 2 v 2
u v
2 u v
y
y x
3 x y
(C)
Similarly in the equation (B), DAB represents the binary diffusion coefficients and N
represents the rate of generation of the concentration CA. In deriving the above relations
some additional constitutive relations must be recalled. For example if the fluid is an
ideal gas, the gas law gives:
p RT or, p C A RT
A
where, R = Specific gas constant =
MA
R = Universal gas constant
MA = Molecular weight [kg/kmole] of gas, A.
[Q M A C A ]
Fouriers law of heat conduction
Heat flux,
q k
T
y y 0 in
the y-direction where, k = Thermal conductivity of
the wall. But heat convected into the fluid is given by the Newtons law of cooling:
q h (TS T )
where, h = heat transfer coefficient (or, coefficient of heat convection)
TS = Surface temperature = T ( y) y 0
T = Temperature of the ambient fluid
q A =Rate of heat flow into fluid
Thus, Q
S
kA S
T
y y 0
h (TS T )
where, AS = surface area through which heat flows. Therefore the heat transfer
coefficient may be expressed as
T
y y 0
TS T
k
h
(D)
Ghosh - 550
Page 3
5/23/2016
Similar to the heat transfer case the mass transfer constitutive relations are given by
as
Ficks law, which specifies molar flux, N A
D AB
N A
C A
y y 0
where, DAB = Binary diffusion coefficient
But the molar flux coefficient may also be expressed as
h m (C A , S C A , )
N A
where hm = convective mass transfer coefficient
CA,S = Concentration of A at the surface
CA, = Concentration of A in the ambient fluid
Therefore the convective mass transfer coefficient may be expressed as
C A
y y 0
C A,S C A,
D AB
hm
(E)
Remember that h and hm are variables defined by the above laws. For a finite size flat
plate we may define (similar to the overall skin friction coefficient, Cf discussed in the
the velocity boundary layers)
h
1
h dA S
AS A
S
and, h m A h m dA S
S AS
may be related to the molar flux, N A
yielding
The mean flux, n A
n A M A N A h m ( A, S A, )
where, MA = Molecular weight of A
and,
A,S = M A C A, S , etc
The above law shows striking similarity between the velocity boundary layer, thermal
boundary layer, and the concentration boundary layer.
Similarity Rules of Boundary Layers
If you recall the work related to Prandtls analysis in the velocity boundary layer was
derived starting from a non-dimensionalization of the governing equations. The critical
parameter to analyze the velocity boundary layer was the Reynolds number. Similar
relations may be derived in cases of thermal boundary layer and concentration boundary
layer. We shall omit the derivations here. However the set of critical parameters resulting
from these operations must be noted carefully. For engineers, design solutions are
influenced by these numbers encountered everyday. A thorough understanding of these
numbers and their physical significance are essential. Only the non-dimensional numbers
relevant to this course are presented below.
Ghosh - 550
Page 4
5/23/2016
Non-dimensional #
Expression
Physical Meaning
Reynolds No. (ReL)
UL
Inerta force
Viscous force
C p
Viscous Dissipation
Thermal Diffusion
Prandtl No. (Pr)
Biot Number (Bi)
hL
R
Mass Transfer Biot Number (Bim)
hmL
R
Conductive Re sis tan ce
Convective Re sis tan ce (in mass transfer )
Schmidt Number (Sc)
D AB
hmL
Sherwood Number (Sh)
D AB
hL
Rf
Nusselt Number (NuL)
Conductive Re sis tan ce
Convective Re sis tan ce
Viscous Diffusion
Mean Diffusion
Convective Mass Transfer
Surface Mass Transfer
Fluid' s Conductive Re sis tan ce
Convective Re sis tan ce
By the use of the expressions (D) and (E) before, Sh and NuL may be also seen as the
non-dimensional concentration gradient and non-dimensional temperature gradient
respectively. The non-dimensional velocity, temperature, and concentration problems
may be summarized in functional forms as
u* = f1 (x*, y*, ReL, dp*/dx)
T* = f2 (x*, y*, ReL, Pr, dp*/dx)
and,
CA = f3 (x*, y*, ReL, Sc, dp*/dx)
These equations are solved to yield
Cf =
2 u *
|
Re y *
L
y *0
Cf = f4 (x*, ReL)
Ghosh - 550
Nu =
Page 5
hL
k
T *
|
y *
y * 0
5/23/2016
Nu = f5 (x*, ReL, Pr) or, Nu = f6 (ReL, Pr)
and,
h L
Sh =
D
m
AB
C
|
=
y
*
y * 0
Sh = f7 (x*, ReL, Sc) or, Sh = f3 (ReL, Sc)
In other words, when we wish to solve the above problems in practice, we match the
corresponding non-dimensional numbers in parenthesis to solve for the desired physical
variables. In some problems of complex physics it is important to know the relationships
connecting the above non-dimensional parameters. For example, Stanton number (St),
defined as
St =
Vc
=
p
Similarly, Stm =
Nu
Re Pr
h
V
Sh
Re Sc
The fact that Cf /2 = St = Stm is known as the Reynolds Analogy.
This can be applied only if Pr and Sc 1. For wider ranges of these parameters, we use
the modified Reynolds or, Chilton-Colburn analogies
Cf /2 = St . Pr 2/3 = jH ,
0.6 < Pr < 60, and,
Cf /2 = Stm . Pr 2/3 = jm ,
0.6 < Pr < 3000,
where, jH and jm are known as the Colburn j factors for heat and mass transfer.
The problems associated with these areas will now be illustrated.
Continue
You might also like
- ACT Prep Syllabus (Fall 2019)Document7 pagesACT Prep Syllabus (Fall 2019)Fallon HoweNo ratings yet
- Styrene Pressure Drop Tutorial ASPEN PDFDocument10 pagesStyrene Pressure Drop Tutorial ASPEN PDFTomNo ratings yet
- Agitation and Mixing-H4 Class-TKMCEDocument111 pagesAgitation and Mixing-H4 Class-TKMCERaghulal SethumadhavanNo ratings yet
- How Equilibrium Calculations Can Be Applied To Complex SystemsDocument16 pagesHow Equilibrium Calculations Can Be Applied To Complex SystemsOsama HussainNo ratings yet
- Talk Shock TubeDocument25 pagesTalk Shock TubemafrisbeNo ratings yet
- Allen J.R.L.-physical Processes of Sedimentation-GEORGE ALLEN and UNWIN LTD (1980)Document128 pagesAllen J.R.L.-physical Processes of Sedimentation-GEORGE ALLEN and UNWIN LTD (1980)Dwiyana Yogasari100% (1)
- Linear Motion PracticalDocument16 pagesLinear Motion PracticalAlastair TavernerNo ratings yet
- Jig Fix Handbook (Carr Lane)Document438 pagesJig Fix Handbook (Carr Lane)Jobin GeorgeNo ratings yet
- JDA Demand - Focus 2012Document43 pagesJDA Demand - Focus 2012Abbas Ali Shirazi100% (1)
- Internal Forced ConvectionDocument18 pagesInternal Forced ConvectionMohd Jamal Mohd MoktarNo ratings yet
- MC CabeDocument18 pagesMC CabeSata AjjamNo ratings yet
- 3.lecture - 2 Type of Areal PhotgraphDocument34 pages3.lecture - 2 Type of Areal PhotgraphFen Ta HunNo ratings yet
- Solution For Chapter 4 Differential Flow PDFDocument24 pagesSolution For Chapter 4 Differential Flow PDFBowo Yuli PrasetyoNo ratings yet
- Note 5 - Fractional Distillation Using Enthalpy-Concentration MethodDocument30 pagesNote 5 - Fractional Distillation Using Enthalpy-Concentration MethodKaleesh100% (1)
- New BlasDocument13 pagesNew BlasAkash GoyalNo ratings yet
- Ch4-Fluid PDFDocument37 pagesCh4-Fluid PDFShannon HayesNo ratings yet
- Chap3 Differential Eqns ZSJDocument55 pagesChap3 Differential Eqns ZSJNaresh SuwalNo ratings yet
- Assignment 6 With Solutions (2) FM WhiteDocument9 pagesAssignment 6 With Solutions (2) FM WhiteabNo ratings yet
- Asst. Prof. Dr. Hayder Mohammad Jaffal: Homogeneous Two-Phase FlowDocument28 pagesAsst. Prof. Dr. Hayder Mohammad Jaffal: Homogeneous Two-Phase FlowprasanthiNo ratings yet
- Compare and Contrast The Speech Production Models of Pim LeveltDocument6 pagesCompare and Contrast The Speech Production Models of Pim LeveltNick Fletcher100% (1)
- Advanced Fluid Mechanics - Chapter 05 - Boundary Layer TheoryDocument33 pagesAdvanced Fluid Mechanics - Chapter 05 - Boundary Layer Theorysunil481No ratings yet
- Boundary Layer Flat PlateDocument27 pagesBoundary Layer Flat PlateGohar KhokharNo ratings yet
- 20-The SIMPLE Algorithm-BDocument30 pages20-The SIMPLE Algorithm-Balagarg137691100% (1)
- Lid Driven FlowDocument8 pagesLid Driven Flowmanoj0071991No ratings yet
- 3.lecture Chapter 2-Continuity EqDocument21 pages3.lecture Chapter 2-Continuity EqAkmalFadzliNo ratings yet
- Turbulence ModelsDocument5 pagesTurbulence Modelsashoku2100% (1)
- Expansion Waves (Prandtl Meyer Flow)Document19 pagesExpansion Waves (Prandtl Meyer Flow)Hamza AshrafNo ratings yet
- 23-SIMPLEC Algorithm For Colocated MeshesDocument31 pages23-SIMPLEC Algorithm For Colocated Meshesalagarg137691No ratings yet
- 2Document49 pages2ZafirahAhmadFauziNo ratings yet
- Helmholtz TheoremDocument18 pagesHelmholtz Theoremrahpooye313No ratings yet
- Flow Between Parallel PlatesDocument13 pagesFlow Between Parallel Platesbala89krishnanNo ratings yet
- 9.5-Turbulent Boundary LayerDocument15 pages9.5-Turbulent Boundary LayershutdoNo ratings yet
- (McGraw-Hill Series in Mechanical Engineering) Hermann Schlichting-Boundary-layer theory-McGraw-Hill (1979) PDFDocument838 pages(McGraw-Hill Series in Mechanical Engineering) Hermann Schlichting-Boundary-layer theory-McGraw-Hill (1979) PDFGhost_suolNo ratings yet
- Nucleate Boiling and Bubble GrowthDocument9 pagesNucleate Boiling and Bubble GrowthAjinkyaKarlekarNo ratings yet
- Assignment 1Document1 pageAssignment 1Partha SurveNo ratings yet
- The Integer L-Shaped Method For Stochastic Integer Programs With Complete Recourse PDFDocument10 pagesThe Integer L-Shaped Method For Stochastic Integer Programs With Complete Recourse PDFRoger RodríguezNo ratings yet
- Conservation Equations (Cont'd)Document12 pagesConservation Equations (Cont'd)preethamismNo ratings yet
- Chap 02 (Fluid Mechanic) PDFDocument65 pagesChap 02 (Fluid Mechanic) PDFK'roll Azham AvearNo ratings yet
- Lecture Notes On Fluid MechanicsDocument16 pagesLecture Notes On Fluid Mechanicssakura9999No ratings yet
- Vector Calculus ApplicationsDocument4 pagesVector Calculus ApplicationsSamuel HollisNo ratings yet
- 07 Chapter 10 (Compiled)Document86 pages07 Chapter 10 (Compiled)Sofea IzyanNo ratings yet
- Neraca KomponenDocument2 pagesNeraca KomponenSelvy SalfitriNo ratings yet
- Gas Dynamics Lecture on Prandtl-Meyer FlowDocument38 pagesGas Dynamics Lecture on Prandtl-Meyer Flowvandamme789No ratings yet
- Chapter 10Document24 pagesChapter 10Lucy BrownNo ratings yet
- Fluid Friction in Steady One Dimensional FlowDocument38 pagesFluid Friction in Steady One Dimensional FlowAlna LiviaNo ratings yet
- Rayleigh FlowDocument2 pagesRayleigh FlowpraveenrajjNo ratings yet
- NME 416 FINAL EXAM HEAT TRANSFERDocument1 pageNME 416 FINAL EXAM HEAT TRANSFERAaron GamezNo ratings yet
- Thermoviscous Flow ReportDocument20 pagesThermoviscous Flow ReportSoham SahaNo ratings yet
- Chapter - 07 - Dimensional AnalysisDocument51 pagesChapter - 07 - Dimensional AnalysisMuaz MushtaqNo ratings yet
- Stability Analysis of Four Bar Mechanism. Part IDocument9 pagesStability Analysis of Four Bar Mechanism. Part IRizkyArmanNo ratings yet
- Chapter 3 ConSol PPT by E.cusslerDocument39 pagesChapter 3 ConSol PPT by E.cusslerheena_scottNo ratings yet
- Chap 6 Viscous Fluid in Closed ConduitsDocument40 pagesChap 6 Viscous Fluid in Closed ConduitsNg Guan ShengNo ratings yet
- CHF LUT 2006 Lookup TableDocument14 pagesCHF LUT 2006 Lookup Tablefaisal58650No ratings yet
- Chapter 3-Pressure and Fluid Statics.2015-1Document54 pagesChapter 3-Pressure and Fluid Statics.2015-1Elizabeth Navarro De CabanaNo ratings yet
- Chapter - 1Document9 pagesChapter - 1Sahil PatilNo ratings yet
- Transport Phenomena - 2-3 - Vector and Tensor 2 and CoordinatesDocument22 pagesTransport Phenomena - 2-3 - Vector and Tensor 2 and CoordinatesHareritamNo ratings yet
- First Order SystemDocument21 pagesFirst Order SystemNiranjan BeheraNo ratings yet
- Transport PhenomenaDocument21 pagesTransport PhenomenaChristina Joana GuzmanNo ratings yet
- CE-6303 Mechanics of Fluids Two Marks QuestionsDocument25 pagesCE-6303 Mechanics of Fluids Two Marks QuestionsjvinothupendraNo ratings yet
- Concentration Conversions - Neutrium PDFDocument17 pagesConcentration Conversions - Neutrium PDFmurugan1984No ratings yet
- Forced ConvectionDocument16 pagesForced ConvectionAleem AhmedNo ratings yet
- Mass Transfer Coefficient ExplainedDocument37 pagesMass Transfer Coefficient ExplainednivedhithaNo ratings yet
- Cooling TowerDocument11 pagesCooling TowerinstrutechNo ratings yet
- Lec 3Document49 pagesLec 3Krishnan MohanNo ratings yet
- Turbulent Boundary Layer Compressible FlowDocument25 pagesTurbulent Boundary Layer Compressible FlowAnna Lucci de MartinezNo ratings yet
- Chemistry Page 2Document1 pageChemistry Page 2Rosalyne Artho-PhanNo ratings yet
- Chem 202, Experiment 05 Molar Mass of Citric Acid Using TitrationDocument3 pagesChem 202, Experiment 05 Molar Mass of Citric Acid Using TitrationRosalyne Artho-PhanNo ratings yet
- Regular ArticleDocument12 pagesRegular ArticleRosalyne Artho-PhanNo ratings yet
- Hw4 FA13Document2 pagesHw4 FA13Rosalyne Artho-PhanNo ratings yet
- Hw4 FA13Document2 pagesHw4 FA13Rosalyne Artho-PhanNo ratings yet
- Liam Gillick A Game of War Instructions 2011Document1 pageLiam Gillick A Game of War Instructions 2011Rosalyne Artho-PhanNo ratings yet
- CENG 122 Vinyl Chloride Separation Project OptimizationDocument11 pagesCENG 122 Vinyl Chloride Separation Project OptimizationRosalyne Artho-PhanNo ratings yet
- EDocument1 pageERosalyne Artho-PhanNo ratings yet
- An Introduction W.T. Winter 215 Jahn Lab x6876: Wtwinter@syr - EduDocument20 pagesAn Introduction W.T. Winter 215 Jahn Lab x6876: Wtwinter@syr - EduRosalyne Artho-PhanNo ratings yet
- Ceng176 Rubric Written 13jan17Document1 pageCeng176 Rubric Written 13jan17Rosalyne Artho-PhanNo ratings yet
- Writing a Document ExchangeDocument1 pageWriting a Document ExchangeRosalyne Artho-PhanNo ratings yet
- Cancer Drug Discovery and DevelopmentDocument1 pageCancer Drug Discovery and DevelopmentRosalyne Artho-PhanNo ratings yet
- TB R61P13Document16 pagesTB R61P13overlord5555No ratings yet
- SOP For Particle SizingDocument11 pagesSOP For Particle SizingRosalyne Artho-PhanNo ratings yet
- 1 Definitions and History 22sept2016pDocument36 pages1 Definitions and History 22sept2016pRosalyne Artho-PhanNo ratings yet
- What Are The Characteristics of An Ideal Op AmpDocument1 pageWhat Are The Characteristics of An Ideal Op AmpRosalyne Artho-Phan100% (1)
- Heat Transfer in Flow Past ObjectsDocument4 pagesHeat Transfer in Flow Past ObjectsRosalyne Artho-PhanNo ratings yet
- Reading A To ZDocument2 pagesReading A To ZRosalyne Artho-PhanNo ratings yet
- Hydrostatic ForcesDocument9 pagesHydrostatic ForcesM.ThirunavukkarasuNo ratings yet
- Notes On FloodsDocument1 pageNotes On FloodsRosalyne Artho-PhanNo ratings yet
- IJSER TemplateDocument4 pagesIJSER TemplateRosalyne Artho-PhanNo ratings yet
- PressureAltitude Derived PDFDocument4 pagesPressureAltitude Derived PDFrahpooye313No ratings yet
- AER210 2010 Qs 1354920823Document10 pagesAER210 2010 Qs 1354920823Rosalyne Artho-PhanNo ratings yet
- P1.70 (1) FluidsDocument1 pageP1.70 (1) FluidsRosalyne Artho-PhanNo ratings yet
- Introduction To Surveying, To ErrorsDocument129 pagesIntroduction To Surveying, To ErrorsVilluz PHNo ratings yet
- Model Building MethodologyDocument24 pagesModel Building MethodologymtlopezNo ratings yet
- MATH 1003 Calculus and Linear Algebra (Lecture 2) : Albert KuDocument17 pagesMATH 1003 Calculus and Linear Algebra (Lecture 2) : Albert Kuandy15No ratings yet
- An Introduction To Mathematical ModellingDocument34 pagesAn Introduction To Mathematical Modellinghaithamelramlawi7503No ratings yet
- Statistical Methods for Geography - A Student's Guide to Exploring Spatial PatternsDocument26 pagesStatistical Methods for Geography - A Student's Guide to Exploring Spatial PatternsMeenakshi MohanNo ratings yet
- Advancedigital Communications: Instructor: Dr. M. Arif WahlaDocument34 pagesAdvancedigital Communications: Instructor: Dr. M. Arif Wahlafahad_shamshadNo ratings yet
- Pronominal Anaphora Resolution inDocument7 pagesPronominal Anaphora Resolution inijfcstjournalNo ratings yet
- XG BoostDocument5 pagesXG Boostola moviesNo ratings yet
- Sample Paper 10: Class - X Exam 2021-22 (TERM - II) Mathematics BasicDocument3 pagesSample Paper 10: Class - X Exam 2021-22 (TERM - II) Mathematics BasicTanushi GulatiNo ratings yet
- Applied Dynamics - MT 224Document6 pagesApplied Dynamics - MT 224Feker KebereNo ratings yet
- Andrea Steaban Resume June 17Document4 pagesAndrea Steaban Resume June 17api-252743869No ratings yet
- Term1 Revision Core 2019Document6 pagesTerm1 Revision Core 2019Unbox SamuraiNo ratings yet
- 2011 BDMS 4E Prelims 2 AM Paper 2Document25 pages2011 BDMS 4E Prelims 2 AM Paper 2Hui XiuNo ratings yet
- Inter Level - (Xi & Xii STD) Ramanujan Contest-2022Document4 pagesInter Level - (Xi & Xii STD) Ramanujan Contest-2022Anju GuptaNo ratings yet
- Report CVP AnalysisDocument25 pagesReport CVP AnalysisSaief Dip100% (1)
- Effects of Results-Based Financing Intervention On Technical Efficiency of Health Services in AfghanistanDocument10 pagesEffects of Results-Based Financing Intervention On Technical Efficiency of Health Services in AfghanistanNational Graduate ConferenceNo ratings yet
- SAP2000 Demo 2013 PDFDocument24 pagesSAP2000 Demo 2013 PDFLi Yin Ting TerryNo ratings yet
- C Lab Manual Record CSE18R153: Submitted By: T.bindamrutha. 9919004275. A9Document87 pagesC Lab Manual Record CSE18R153: Submitted By: T.bindamrutha. 9919004275. A9John100% (1)
- MPM2D1Document2 pagesMPM2D1anotheroadsideNo ratings yet
- HARDER Developing The Formula 0.5 Ab SIN C For The Area of A TriangleDocument1 pageHARDER Developing The Formula 0.5 Ab SIN C For The Area of A TrianglejulucesNo ratings yet
- FDocument24 pagesFrotenolabsNo ratings yet
- Discrete Phase Modelling - CombustionDocument63 pagesDiscrete Phase Modelling - CombustionFabian Andrey DiazNo ratings yet
- Modeling of The Dynamics of Rotors of An Energy Gas Turbine Installation Using An Analytical Method For Analyzing Active Magnetic Bearing CircuitsDocument6 pagesModeling of The Dynamics of Rotors of An Energy Gas Turbine Installation Using An Analytical Method For Analyzing Active Magnetic Bearing Circuitsshamiul himelNo ratings yet
- Wittgenstein Picture TheoryDocument4 pagesWittgenstein Picture TheoryAlladi Bhadra Rao DevangaNo ratings yet
- Kinematics FREE WORKSHEET/QUESTION BANK BY ANURAG TYAGI CLASSES (ATC)Document3 pagesKinematics FREE WORKSHEET/QUESTION BANK BY ANURAG TYAGI CLASSES (ATC)ANURAG TYAGI100% (2)