Professional Documents
Culture Documents
Ishii 2013
Uploaded by
secateCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ishii 2013
Uploaded by
secateCopyright:
Available Formats
Subscriber access provided by ECU Libraries
Article
Pore Size Determination in Ordered Mesoporous
Materials using Powder X-ray Diffraction
Yosuke Ishii, Yoshiki Nishiwaki, Ayar Al-zubaidi, and Shinji Kawasaki
J. Phys. Chem. C, Just Accepted Manuscript DOI: 10.1021/jp4057362 Publication Date (Web): 06 Aug 2013
Downloaded from http://pubs.acs.org on August 14, 2013
Just Accepted
Just Accepted manuscripts have been peer-reviewed and accepted for publication. They are posted
online prior to technical editing, formatting for publication and author proofing. The American Chemical
Society provides Just Accepted as a free service to the research community to expedite the
dissemination of scientific material as soon as possible after acceptance. Just Accepted manuscripts
appear in full in PDF format accompanied by an HTML abstract. Just Accepted manuscripts have been
fully peer reviewed, but should not be considered the official version of record. They are accessible to all
readers and citable by the Digital Object Identifier (DOI). Just Accepted is an optional service offered
to authors. Therefore, the Just Accepted Web site may not include all articles that will be published
in the journal. After a manuscript is technically edited and formatted, it will be removed from the Just
Accepted Web site and published as an ASAP article. Note that technical editing may introduce minor
changes to the manuscript text and/or graphics which could affect content, and all legal disclaimers
and ethical guidelines that apply to the journal pertain. ACS cannot be held responsible for errors
or consequences arising from the use of information contained in these Just Accepted manuscripts.
The Journal of Physical Chemistry C is published by the American Chemical Society.
1155 Sixteenth Street N.W., Washington, DC 20036
Published by American Chemical Society. Copyright American Chemical Society.
However, no copyright claim is made to original U.S. Government works, or works
produced by employees of any Commonwealth realm Crown government in the course
of their duties.
Page 1 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
Pore Size Determination in Ordered Mesoporous
Materials using Powder X-ray Diffraction
Yosuke Ishii, Yoshiki Nishiwaki, Ayar Al-zubaidi, and Shinji Kawasaki*
AUTHOR ADDRESS
Department of Materials Science and Engineering, Nagoya Institute of Technology, Gokiso-cho,
Showa-ku, Nagoya 466-8555, Japan.
KEYWORDS
Ordered mesoporous materials, Powder X-ray diffraction, Structure determination, Nitrogen
adsorption-desorption isotherm, Transmission electron microscopy
ACS Paragon Plus Environment
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 2 of 42
ABSTRACT
We demonstrated in the present investigation that by analyzing the small-angle powder XRD
pattern, we successfully determined not only the pore-to-pore distance but also the pore diameter
of the mesoporous materials having quasi two dimensional hexagonal pore structure. It was
found that the pore diameter values determined by this method were in good accordance with
those derived from nitrogen gas adsorption measurement (KrukJaroniecSayari method) for
several kinds of mesoporous silicas and carbons. Furthermore, owing to the advantage of this
determination method that it is applicable to the wet sample, we applied this method to the
mesoporous sample immersed in the liquid whose bulk density is known to evaluate the density
of framework materials. The derivation of the theoretical formula of the small-angle XRD
pattern of the mesoporous materials is also described in this paper.
ACS Paragon Plus Environment
Page 3 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
1. Introduction
Ever since the discovery of surfactant-template synthesis methods, ordered mesoporous
materials have generated tremendous interest.1 In addition to the commonly known MCM-41
type silicas, various kinds of mesoporous materials with different structural symmetries210 and
framework compositions1117 have been synthesized over the last twenty years. Because of their
unique and controllable nanostructure, ordered mesoporous materials are expected to be used in
various fields of application, such as catalysts,1921 adsorbents,21, 22 drug derivery,23, 24 sensors,24,
25
and in energy conversion and storage.2631 Needless to say, the structural characterization of
ordered mesoporous materials is essential in researching these applications. Direct observations
using transmission electron microscopy (TEM), gas adsorption-desorption isotherm analysis, and
small-angle powder X-ray diffraction (XRD) are commonly used structural characterization
methods for ordered mesoporous materials. Of these techniques, TEM observation is the most
direct and most commonly used method. In addition to the normal 2D transmission micrographs,
3D structural images can also be obtained using a recently developed electron tomography
technique.29 However, specialized experience is required for exact analysis because TEM
images are strongly affected by various experimental conditions, such as focus settings, sample
thickness, and electromagnetic lens aberrations.32 Furthermore, the TEM method is unsuitable
for statistical analysis, because the images contain only local information from a selected area.
On the other hand, the gas adsorption-desorption method can give bulk pore structure
information. Specific surface areas estimated by the BrunauerEmmettTeller (BET) method33
and pore-size distributions calculated by the BarrettJoynerHalenda (BJH) method34 are often
employ structural information obtained from gas adsorption-desorption isotherm analysis. In
addition to these classical methods, more sophisticated analysis such as non-localized density
ACS Paragon Plus Environment
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 4 of 42
functional theory simulation (NLDFT)3538 and grand canonical Monte Carlo calculations
(GCMC)3941 have also been used in recent years. However, structure parameters obtained by the
gas adsorption-desorption methods are strongly dependent on their calculation models. Moreover,
structural ordering cannot be determined by gas adsorption-desorption methods alone. Smallangle powder XRD is often used to counter this disadvantage of gas adsorption-desorption
methods. From the XRD peak positions, structural symmetry and the distances between adjacent
pores can be determined.
Although structure analysis based on XRD peak positions is a commonly used method
for ordered mesoporous materials, quantitative discussions of XRD peak intensities have not
often been reported. B. P. Feuston et al. reported model structures for MCM-41 silica materials
obtained from molecular dynamics (MD) simulations.42 In their report, XRD pattern simulations
were also performed using optimized atomic coordination obtained from the MD calculations.
They mentioned that the XRD peak intensities are highly dependent on mesopore diameter and
unit cell size. However, no general relationship between XRD peak intensity and mesopore
diameter was discussed in their paper. Furthermore, XRD pattern calculation based on MD
optimization is not suitable for routine analysis, because the MD simulation requires significant
computational expense. Owing to the dramatic improvements of computational technology, the
problem of calculation cost is gradually fading out in recent years. However, the MD-based
method is still not easy to use, because the method requires sophisticated skill and knowledge to
perform molecular calculation. Fortunately, such an elaborate MD simulation process may be not
indispensable for XRD pattern calculation. W. Schmidt demonstrated that reasonable XRD
pattern simulation can be performed even without optimization of the atomic position in the
model structure.43 This means that the detailed atomic coordination does not have a significant
ACS Paragon Plus Environment
Page 5 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
effect on XRD pattern calculation for ordered mesoporous materials. In the meantime, M.
Impror-Clerc et al. demonstrated an XRD pattern simulation for SBA-15 silica materials using a
simpler structural model.44 In their paper, the solid framework structure (amorphous silica
region) was approximated by a uniform electron density matrix instead of by exact atomic
configurations. According to their theory, the XRD peak intensity of hkl lines (
) are
calculated from a unit cell form factor ( ( )) as follows:
| (
where
)|
(1)
is a scaling factor used to adjust the peak intensities to match the experimentally
observed intensities,
is a multiplicity factor for the hkl line,
scattering vector for the hkl line (here,
is the magnitude of the
is calculated using Bragg's law), and
is the
Lorentz factor for powder diffraction. The form factor was calculated from a Fourier transform
of the electron density distribution in the unit cell. Similar uniform electron density matrix model
was also adapted for MCM-41 silica materials by L. A. Solovyov et al.45 Since the XRD data
provide direct structural information without any semi-empirical parameters or assumptions, the
XRD method has been used for discussing the relevance of pore-size distribution data obtained
by gas adsorption-desorption isotherm analysis.46, 47 By modifying the unit cell form factor,
theoretical XRD patterns of more complicated structures, such as a mesoporous material that
contains complex pore-surface structures44, 48 or 3D cage-like mesoporous silicas49 can also be
calculated in the same manner. Furthermore, in situ XRD observations of the gas adoptiondesorption process in ordered mesopores were also performed based on this calculation
method.49, 50 However, we believe that the XRD pattern calculation using equation 1 may be
problematic because the range of pore ordering (i.e., coherent length, crystallite size) was not
ACS Paragon Plus Environment
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 6 of 42
precisely considered. XRD pattern calculation based on equation 1 would be correct only for
infinitely-ordered mesopore samples. Since the XRD peak width, which is indicative of
crystallite size, of mesoporous materials is usually broad (i.e., crystallites are small), such an
infinitely-ordered pore description may be unreasonable. Therefore, we would like to propose a
modified XRD calculation model for ordered mesoporous materials in this paper. In our model,
the degree of interference between unit cells (i.e., crystallite size effect) was treated by
convoluting the unit cell form factor function with a Laue-type interference function. In order to
test the validity of our method, various types of ordered mesoporous samples having 2Dhexagonal symmetry were synthesized, and their XRD patterns were compared with theoretically
calculated patterns.
2. Experimental Methods
2.1 Sample Preparation
2.1.1 Synthesis of SBA-15 Mesoporous Silica
SBA-15 mesoporous silica was synthesized as reported by Zhao et al.51 In this procedure,
we used tetraethoxysilane (TEOS) as a silica precursor, and triblock copolymer Pluronic P123
(EO20PO70EO20; BASF) as a structure directing template. First, 4.0 g of Pluronic P123 was
dissolved in a solution of 30 g of water and 120 g of 0.2 M HCl aqueous solution, and stirred at
60C for 30 min. Then, 8.5 g of TEOS was added to the solution. After stirring the mixture for
10 min, it was kept at 35C for 20 h without stirring. Then, the mixture was aged at 80C for 72
h. The generated solid product was filtered, washed with water and ethanol, and dried in an oven
at 140C for 4 h. To remove the Pluronic P123 template, the sample was heated in an electric
furnace at 550C for 4 h under air. In order to avoid mesostructure collapse, the heating and
ACS Paragon Plus Environment
Page 7 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
cooling rates were fixed at 5C min-1. After the heat treatment, the obtained mesoporous silica
was ground into a fine powder with an agate mortar. For convenience, the obtained product was
designated as MPS1 in this paper.
2.1.2 Synthesis of MCM-41 Mesoporous Silicas
MCM-41 mesoporous silica fiber samples were synthesized using tetrabutoxysilane
(TBOS) as a silica precursor, and cetyltrimethylammonium bromide (CTAB) as a structure
directing template.52 First, 2.25 g of CTAB was dissolved in a solution of 32.5 g of water and
125 g of 5 M HCl aqueous solution, and stirred at room temperature for 30 min. Then, 2.7 g of
TBOS was dripped into the solution, and kept at 50C for 10 days without stirring. The resultant
solid fibers were filtered, washed with water and ethanol, and dried in an oven at 80C for 12 h.
The obtained mesoporous silica was ground into a fine powder with an agate mortar. In order to
remove the CTAB template, two types of post-treatments were performed for the as-synthesized
samples: one was a solvent extraction method, and the other was a combustion method. In the
solvent extraction method, the as-synthesized silica was refluxed with ethanol in a Soxhlet
extractor for 30 h. After the extraction, the sample was dried in an oven at 80C for 12 h. The
obtained product was designated as MPS2. On the other hand, in the combustion method, the assynthesized silica was heated in an electric furnace under O2 flow (50 ccm) at 500C for 5 h. The
heating and cooling rates were fixed at 5C min-1 to avoid mesostructure collapse. The obtained
product was designated as MPS3.
2.1.3 Synthesis of Mesoporous CarbonSilica Composite
The mesoporous carbonsilica composite sample was synthesized using the triconstituent
co-assembly method proposed by Liu et al.53 In this procedure, we used phenolformaldehyde
ACS Paragon Plus Environment
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 8 of 42
resin (resol) as a carbon source, TEOS as a silica precursor, and triblock copolymer Pluronic
F127 (EO106PO70EO106; BASF) as a structure directing template. First, 0.5 g of Pluronic F127
was dissolved in a solution of 32.0 g of ethanol and 4.0 g of 0.2 M HCl aqueous solution, and
stirred for 15 min. Then 8.0 g of TEOS and 20.0 g of 20 wt.% resols ethanolic solution were
added in the solution. After stirring the mixture for 2h, it was transferred into PTFE dishes. The
dishes were maintained at 40C for 24 h to evaporate ethanol. Subsequently they were kept at
100C for 24 h to attain thermo-polymerization. The polymerized product was carbonized by
heating in an electric furnace under N2 flow (30 ccm) at 900 C for 1 h following elimination of
Pluronic F127 by heating at 400C for 2h. The heating and cooling rates were fixed at 1C min-1
to avoid mesostructure collapse. After the carbonization, obtained mesoporous carbonsilica
composite was ground into a fine powder with an agate mortar. The obtained product was
designated as MPCS.
2.2 Characterization
The nanostructures of the obtained samples were observed using a JEOL z2500
transmission electron microscope operated at 200 kV. The removal of surfactant molecules used
as a structure directing template was confirmed by thermogravimetric (TG) analysis using a
Shimadzu TGA-50 instrument. The TG analysis also determined the carbonsilica ratio of the
MPSC sample. Nitrogen adsorption-desorption isotherms at 77 K were measured using a
Shimadzu Gemini 2375 instrument. Prior to the isotherm measurements, the samples were heat
treated at 200C under vacuum (<3 Pa) for at least 1 h to remove adsorbed moisture. The specific
surface areas (
) were estimated using the BrunauerEmmettTeller (BET) method33 and the
adsorption data in the relative pressure (P/P0) range from 0.05 to 0.16. The total pore volumes
(
) were calculated using the Gurvich rule at P/P0 =0.97. The s-plot method was used to
ACS Paragon Plus Environment
Page 9 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
estimate the micropore (
) and mesopore (
) volumes. In the s analysis, adsorption
isotherm data for the LiChrospher Si-1000 silica reported by Jaroniec et al.54 was used as the
reference adsorbent. The pore-size distributions were calculated based on an analysis of the
adsorption branch of the isotherms using the BarrettJoynerHalenda (BJH) method.34 In the
BJH calculation, the Kelvin radius ( ) and statistical film thickness of the N2 adsorbed layer ( )
were estimated using the Kelvin equation (equation 2) and the Halsey equation (equation 3),
respectively.
(
[
where
is expressed in ,
(2)
)
)
(3)
is the surface tension of the liquid N2 (8.88 10-3 J m-2),
molar volume of the liquid N2 (3.468 1025 3 mol-1),
2 K-1 mol-1 m-2), and
is the
is the ideal gas constant (8.8314 1020 J
is the absolute temperature of adsorption (77 K). is expressed in . In
this paper, the primary mesopore diameter determined by the BJH method is designated as
Since the traditional BJH method tends to underestimate mesopore diameter,35, 55 a modified BJH
method proposed by Kruk, Jaroniec, and Sayari (BJHKJS method55) was also used in this study.
In the BJHKJS calculation, a modified Kelvin equation developed for N2 adsorption on silica
samples with cylindrical mesopores (equation 4), and a HarkinsJura type equation (equation 5)
were used to calculate
and , respectively.
(
[
(4)
)
(
(5)
ACS Paragon Plus Environment
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 10 of 42
The primary mesopore diameter estimated by the KJS method is designated as
in this paper.
Powder XRD measurements were carried out using a Rigaku Nano-Viewer
diffractometer, equipped with a two dimensional (2D) imaging plate detector (Rigaku R-AXIS
IV++), using Cu K radiation (=1.54 ) as an incident beam. The XRD measurements were
performed in transmission geometry. Lindemann glass capillary tubes (=0.5 mm) were used as
a sample container, and the typical exposure time was 30 min. For MCM-41 (MPS2 and MPS3)
samples, powder XRD measurements using synchrotron radiation were also performed to obtain
high-quality diffraction patterns. The synchrotron XRD measurements were performed using
beam line BL-18C at the Photon Factory (PF) in the High Energy Accelerator Research
Organization (KEK), Tsukuba, Japan. The synchrotron radiation beams emitted from an electron
storage ring operated at 2.5 GeV were monochromatized by a Si (111) double-crystal
monochromator and collimated by a pinhole collimator 100 m in diameter. The incident X-ray
wavelength for these measurements was =0.613. Each diffraction pattern was collected using
a 2D imaging plate detector (200 mm
250 mm) located 500 mm behind the sample position.
The typical exposure time was 5 min. Lindemann glass capillary tubes (=1.0 mm) were used as
a sample container. Prior to the measurements, the incident X-ray wavelength and the distance
from the sample to the imaging plate detector were calibrated using the XRD peaks of a CeO2
powder by the double cassette method.56 Prior to further analysis, the obtained XRD intensities
(
) were corrected for polarization factor (
detector (
), geometric factor for the 2D flat plate
,57 dark current noise of the detector
background scattering from a blank capillary and air
) (
[ {
, transmission factor, and
) as follows:
}]
(6)
ACS Paragon Plus Environment
10
Page 11 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
where
),
, , and
are the corrected XRD intensity, incident X-ray intensity, X-ray
intensity after transmission through the sample, and X-ray intensity after transmission through
the blank capillary, respectively. All XRD measurements were performed at room temperature.
3. Theoretical Basis
3.1 Theoretical XRD Pattern for Ordered Mesoporous Materials with 2D-Hexagonal
Symmetry
In this section, we theoretically derive powder X-ray diffraction patterns of ordered
mesoporous materials with 2D-alingned cylindrical pores. Here, we assume the structure model
shown schematically in Figure 1(A). In this model, empty cylindrical mesopores (radius: ;
length: ) are hexagonally aligned in a uniform amorphous matrix (density:
; average atomic
X-ray scattering factor: ( )).
The diffraction intensity from a non-oriented powder crystalline sample, ( ), would be
calculated using a unit cell form factor ( ) in the following manner:
( )
where
| ( )| ( )
( )
is the magnitude of the scattering vector (
(7)
),
is the scattering angle,
the X-ray wavelength, ( ) is the Lorentz factor for powder diffraction (
is the multiplicity factor of the hkl diffraction line, and
is
)),
( ) is the Laue function
representing the interference between unit cells. Since the exact form of the Laue function is not
appropriate for numerical calculations,
( ) was approximated by a pseudo Voigt function in
this study. The pseudo Voigt function is the sum of a Gaussian profile and a Lorenz profile, and
can be expressed as
ACS Paragon Plus Environment
11
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
( )
where
) ]
is the full-width-at-half-maximum,
Page 12 of 42
(9)
) ]
is the Gaussian/Lorenz ratio, and
is the
scattering vector at the Braggs angles of the hkl line. Note that such pseudo Voigt type peak
profile functions are often used in Rietveld refinements for powder XRD analysis. In the
meantime, for 2D-hexagonally aligned mesoporous materials, only
(i.e. hk0) diffraction
lines are observed. Therefore, diffraction indices are represented by two digits in the following
parts. In this time, the scattering vector at the Braggs angles of the hk line
can be expressed
as
(10)
where
is a unit cell lattice constant that represents the interval of adjacent mesopore centers.
The multiplicity factors of h0, hh, and hk (
) diffraction peaks become 6, 6, and 12,
respectively.
The form factor ( ) can be calculated on the basis of the structure model shown in
Figure 1(A). In this study, we considered a shifted unit cell (indicated by II in Figure 1(A)),
instead of the commonly used version (indicated by I in Figure 1(A)). As shown in Figure 1(B),
( ) may be expressed as follows:
( )
where
( ) and
( )
( )
( )
( )
(11)
( ) represent structure factors of the uniform matrix and of the
cylinder (radius: , length: ), respectively. The structure factors could be calculated from a
Fourier transform of the atomic (or more principally electron density) distribution. Since the
ACS Paragon Plus Environment
12
Page 13 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
Fourier transform of a constant value, that representing the uniform matrix, becomes a delta
function ( ), the
( ) terms would be replaced by zero, except for in the
case.
Therefore, equation 11 can be rewritten as
( )
( )
( )
(12)
Taking mesoporous silica as an example, ( ) may be expressed as
( )
where
( ) and
( )
(13)
( )
( ) represent the atomic scattering factors of Si and O, respectively.
Although practical values of atomic scattering factors have been tabulated in the International
Tables for Crystallography,58 the ( ) term would be ignored in practical calculations since the
atomic scattering factors of light atoms (such as Si, O, and C) are approximately constant in the
( ) would be expressed as follows:
small-angle region. In the meantime,
( )
where (
(14)
) is the positional vector in the cylinder. Through further calculations, as
described in the supporting information, equation 14 becomes the following expression:
( )
where
is the 1st order Bessel function of the first kind,
the direction of the -axis, and
is the
is the
(15)
component perpendicular to
component parallel to the direction of the -axis.
Because most mesoporous materials have very large
values, the term
) (
behaves like a delta function, ( ). This means that diffraction peaks can be observed only
ACS Paragon Plus Environment
13
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
when
only
(i.e. | |
Page 14 of 42
). This is consistent with the well-known experimental axiom that
diffraction lines are observed in 2D-hexagonal mesoporous materials. In this case,
equation 15 can be further simplified to the following expression:
( )
(16)
Because the mesopore channels in real materials are not perfectly oriented, and their
positions fluctuate as illustrated in Figure S1(A), additional terms are required for practical XRD
pattern fitting. In this work, in order to consider the fluctuation of mesopore positions, equation 7
was multiplied by the Debye-Waller factor ( (
); : mean-square
displacement), assuming a Gaussian displacement of pore center positions. In addition to the
fluctuation in pore position, (i) fluctuation in pore radius R as illustrated in Figure S1(B), and (ii)
deformation of pore structure as illustrated in Figure S1(C) would also be considered as
described in the supporting information (section B and C). Since the mesoporous samples used in
this study have very narrow pore-size distributions and well-formed circular pore-cross-sections,
the factors (i) and (ii) are not considered in the following discussions.
Thus, theoretical XRD patterns of ordered mesoporous materials with 2D-hexagonal
symmetry can be calculated by the following equation:
( )
| ( )
)
|
( ) (
(17)
where is a scaling factor. For example, the theoretical XRD pattern of a mesoporous silica
sample ( =11.0 nm, =3.0 nm) would be calculated as shown in Figure 2.
3.2 Relationship between Lattice Constant and Mesopore Diameter
ACS Paragon Plus Environment
14
Page 15 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
Although theoretical XRD patterns of ordered mesoporous materials can be calculated
using equation 17, the relationship between mesopore structure and the XRD pattern may be
difficult to understand because the equation contains many parameters. In this section, we derive
a more practical expression that represents the relationship between structural features (mesopore
diameter
and lattice constant ) and XRD peak intensities. As discussed in section 3.2, the
diffraction intensity at the hk Braggs angle
| (
is expressed as follows:
) (
(18)
Therefore, the peak intensity ratio of hk to hk can be written:
(
(
)
)
(
(
) (
) (
As shown in the supporting information, the term (
)
)
(
(19)
) in equation 19 may be
expressed as
(
(
)
)
(20)
in the small-angle region. From equation 10,
becomes:
(21)
where
is the mesopore diameter normalized by
). Therefore, if we can
assume that the fluctuation of mesopore position is negligible, equation 19 can be rewritten as:
(22)
ACS Paragon Plus Environment
15
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 16 of 42
(23)
(24)
Equation 22 indicates that the relative diffraction intensities at the Braggs angles are essentially
determined only by the (
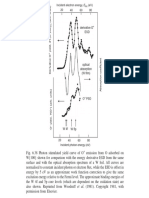
) value. Figure 3 shows the relationship between and the
diffraction peak intensities. In this graph, the intensity of the 10 diffraction line was normalized
to 1. Since the unit cell lattice constant
mesopore diameter
is directly determined from the peak positions, the
can be estimated from the
value determined by analysis of the diffraction
intensity using Figure 3.
Note that if the fluctuation of mesopore position (Debye-Waller factor) is not negligible,
the diffraction peak intensities will decrease with increasing
(diffraction intensity at
value. We should also note that
) is not proportional to the peak area of the hk line
, because
the exact shapes of the diffraction peaks are modulated by the ( ) shape. The difference
between
and
becomes non-negligible when the diffraction peaks are broad. Therefore,
pattern fitting using equation 17 is required for a rigorous structure assessment.
4. Results and Discussion
First, we characterized the nanostructure of the obtained samples using commonly used
procedures. Figure 4 shows TEM images of the MPS1 sample. Hexagonally aligned straight
channel pores were clearly observed. As shown in Figure S2, similar porous structures were
observed in the other samples (MPS2, MPS3, and MPCS) used in this study. Nitrogen
adsorption-desorption isotherms are shown in Figure S3(A). In the MPS1 and MPCS samples,
clear hysteresis loops appeared. Their isotherm shapes were classified as type IV, according to
the IUPAC definition.59 On the other hand, no hysteresis loops were observed in MPS2 or MPS3
ACS Paragon Plus Environment
16
Page 17 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
samples, probably due to their narrow mesopore diameters, which were close to the micropore
range (<2 nm). Such a lack of hysteresis loops feature is often reported for small pore-size
MCM-41 type materials.60, 61 In the adsorption branch of the isotherms, rapid increases of
adsorbed volume, which is characteristic of capillary condensation within mesopores, occurred
around P/P0 = 0.67 (MPS1), 0.32 (MPS2), 0.28 (MPS3), and 0.61 (MPCS). Such a sharp increase
indicates a high uniformity of the mesopores. The narrow distributions of mesopore diameter
were confirmed by pore size distribution curves calculated using the BJH method, as shown in
Figure S3(B). Figures 5 and S4 show powder XRD patterns of the obtained samples. As
illustrated in the figures, more than 4 diffraction peaks were observed in all samples, and these
peaks could be indexed to a 2D-hexagonal symmetry (p6mm). The observed diffraction peak
positions and the peak intensity ratio of MPS1 were quite different from those of MPS2.
Meanwhile, although the peak intensity ratios of MPS2 were close to those of MPS3, the peak
positions of MPS2 were located at lower angles than those of MPS3. The relationship between
mesopore structure and the XRD pattern will be discussed later in this paper. Lattice constant ( )
values of MPS1, MPS2, MPS3, and MPCS, estimated from the positions of 10 diffraction peaks,
were 10.62 nm, 4.56 nm, 4.33 nm, and 11.42 nm, respectively. According to previous reports,
the mesopore diameter (denoted here as
lattice constant
) could be roughly estimated from the unit cell
and mesopore volume
using a simple geometrical calculation, assuming
a pore wall density .55, 63-65 Since the existence of micropores was confirmed by s-plot analysis
(see Figure S5), we used the following equation instead of the previously proposed one.
)}
(25)
ACS Paragon Plus Environment
17
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
where
Page 18 of 42
is the micropore volume, and the densities of SiO2 and C were assumed to
g cm-3 and
g cm-3, respectively. In this equation, we assumed that all of the micropores
are homogeneously dispersed in the wall region located between aligned mesopore channels.
Equation 25 becomes equivalent to the equations presented in previous reports55, 63-65 by
) with
replacing the term (
summarized in Table 1. The
The mismatch between
. The obtained structural parameters are
values were very close to
and
, except for the MPCS sample.
in MPCS may arise from roughness of the mesopore
structure. As shown in the TEM photographs (Figures 4 and S2) and pore size distribution curve
(Figure S3(B)), the structural roughness of the MPCS sample was much higher than in other
samples. In addition to the structural roughness, the differences in surface chemistry between
SiO2 and C may also have affected the pore size estimation, because such differences are not
properly considered in the BJH or KJS calculations in this study. The
value of the MPCS
may also have a problem, because s-analysis should also be affected by the surface properties.
Here we note that the BJH or KJS method has a potential to accommodate surface properties
through the selection of the statistical film thickness for an appropriate surface type. However,
the accommodation is hard for the MPCS due to its complicated chemical composition. In
contrast to the gas adsorption-desorption methods, such information of pore-surface is not
required for the XRD analysis method.
Next, we will discuss the details of the XRD analysis. As mentioned in section 3.2, the
structure parameter
that represents the size ratio between the lattice constant
and the
mesopore diameter
can essentially be estimated from the XRD peak intensities using Figure 3.
For example, for the MPS1 sample, the following relationships were observed in Figure 5: (A)
, (B)
, (C)
. In order to satisfy requirement (A), the
value should be
ACS Paragon Plus Environment
18
Page 19 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
somewhere between 0.58 and 0.73. Furthermore,
satisfy (B) and (C), respectively. Therefore, the
(
and
are also required to
value of MPS1 was estimated to be
). In order to narrow down the estimation range of , XRD
pattern fittings were performed. Equation 17 was used as a model function, and the modified
LevenbergMarquardt method was adopted as a nonlinear least squares fitting algorithm. The
obtained structure parameters are summarized in Table 2. Simulated XRD patterns calculated
from the structural parameters obtained by the fitting are shown in Figure 6. As shown in Figure
6, the simulated patterns well reproduced the experimentally observed patterns. Mesopore
diameters determined by the XRD method (
) were close to
, and were larger than
in all samples. According to previous reports, the traditional BJH method tends to underestimate
mesopore diameter.35, 55 Our XRD analysis data support this underestimation of the BJH method,
without making any semi-empirical assumptions. Furthermore, as shown in Table 2, the
value
of the MPCS sample was larger than those of MPS1, MPS2, and MPS3. This is probably a result
of the abovementioned structural roughness of MPCS.
By comparing Tables 1 and 2, we found that the values of
were smaller than
in
all samples. This means that the observed peak positions were somewhat different from those
simply predicted by Braggs law. This difference seems to arise from the shape of the form
factor term (| ( )| ) in equation 7. We found that | ( )| always monotonically decreases near
the Braggs 10 diffraction angle, independent of
profile (the convolution of | ( )| and
and . Therefore, the net shape of the 10 peak
( )) should be downshifted relative to the pure
( ) peak, which was located at the Braggs angle. The modulation becomes conspicuous with
increasing diffraction peak width. In addition to the 10 peak, other peak positions are also
modulated by the shape of | ( )| . As shown in Figure S6, the degrees of peak modulations are
ACS Paragon Plus Environment
19
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 20 of 42
different with . A similar modulation of XRD peak positions has been reported for singlewalled carbon nanotube (SWCNT) bundles.66 (Note that individual SWCNTs are generally
aggregated due to van der Waals interactions to form a 2D-hexgonally aligned crystal structure
called a bundle. The XRD pattern of SWCNTs can be calculated based on equation 7 by
replacing the form factor with ( )
function of the first kind,
( ) (
), where
is the 0th order Bessel
( ) is the X-ray scattering factor of the carbon atom, and
is the
tube diameter.)
Thus far, we have discussed the XRD patterns of mesoporous materials measured in the
dry state. Let us broaden our scope to include wet conditions. For SWCNTs, it is well known that
the XRD pattern of a water-immersed sample is completely different from that of a dry sample.67
For example, the
ratio of a water-immersed SWCNT is much smaller than that of a dry
SWCNT. However, for mesoporous materials, the peak intensity ratio of a water-immersed
sample was similar to that of a dry sample. Although the XRD peak intensity ratio was the same,
the absolute intensity of a water-immersed wet mesoporous sample was much smaller than that
of a dry sample. We found that the pore wall density can be estimated from the difference in
XRD intensity between dry and wet samples. The XRD patterns of wet mesoporous samples can
be calculated by modifying the unit cell form factor used in equation 11. Assuming that all
mesopores are homogeneously filled with water (density:
factor:
; average atomic X-ray scattering
( )), ( ) may be expressed as follows, except for the
( )
( )
( ( )
( )
( )
( ))
( )
( )
( )
case:
( )
(26)
Since the observed XRD intensity is proportional to | ( )| , the intensity ratio between dry and
wet samples can be expressed as follows:
ACS Paragon Plus Environment
20
Page 21 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
|
where
and
( )
|
( )
(27)
are the XRD intensities of wet and dry samples, respectively. Taking MPS3
as an example, the experimentally observed
was 0.258 (see Figure 7). Assuming that
the density of water in the mesopores is similar to the bulk density (
g cm-3),
was
calculated to be 2.26 g cm-3. This value is reasonable for amorphous SiO2 (about 2.2 g cm-3).65, 68
However, we should note that there is no assurance that the density of water confined in the
mesopore is similar to the bulk density, because there are reports discussing the abnormal
physical properties of molecules confined within nanospaces.67, 69-77 Further research is required
to examine the exact density of water in mesopores. Normally, such a research would be difficult
because we have to distinguish confined water from bulk one. However, we expect that our XRD
method might be useful to probe the density of liquids confined in mesopore, because the XRD
peak intensity of mesoporous samples are selectively affected by the densities of confined liquids.
By comparing XRD intensities of ordered mesoporous samples immersed in various kinds of
liquids, the densities of the liquids confined in mesopores would be evaluated. This research is
currently being carried out, and will be addressed in more detail in a separate publication.
5. Conclusion
In this paper, we derived theoretical XRD patterns of ordered mesoporous materials with
2D-hexagonal symmetry (p6mm). In order to test the validity of our theory, various types of
mesoporous samples with different pore sizes and chemical compositions were synthesized, and
their XRD patterns were compared with theoretically calculations. The theoretically calculated
XRD patterns agreed well with experimentally observed patterns. We found that the exact XRD
peak positions were modulated by the form factor | ( )| , and were different from the positions
ACS Paragon Plus Environment
21
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 22 of 42
calculated using Braggs law. By analyzing the XRD peak intensities carefully, we successfully
determined mesopore diameter and unit cell lattice spacing without support from other methods
such as TEM or N2 adsorption-desorption analysis. We also found that the XRD analysis can be
adopted for water-immersed wet samples, and that the density of the pore wall can be estimated
from the difference between XRD intensities of dry and wet samples.
As discussed above, we demonstrated that the XRD analysis can be performed, even for
samples immersed in water. We believe that this XRD-based method has great potential for in
situ experiments that cannot be performed by TEM or gas-adsorption methods, because the XRD
method does not require any special measurement environments or pretreatments. Therefore, we
expect that the XRD analysis proposed in this study would be used to reveal poorly understood
structural properties of mesoporous materials in various environments (such as high pressures
and high temperatures) in the near future.
ASSOCIATED CONTENT
Supporting Information. Derivation of the structure factor for cylinder. Fluctuation in poreradius or pore-structure. Lorentz factor in small-angle region. Figures S1-S6. This material is
available free of charge via the Internet at http://pubs.acs.org.
AUTHOR INFORMATION
Corresponding Author
*
Shinji Kawasaki (E-mail: kawasaki.shinji@nitech.ac.jp)
ACKNOWLEDGMENT
ACS Paragon Plus Environment
22
Page 23 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
The authors gratefully acknowledge Professor Akio Ohta (Kanazasa University, Japan) for XRD
measurements.
REFERENCES
[1] Davis, M. E. Ordered porous materials for emerging applications. Nature 2002, 417, 813821.
[2] Sakamoto, Y.; Kaneda, M.; Terasaki, O.; Zhao, D. Y.; Kim, J. M.; Stucky, G.; Shin, H. J.;
Ryoo, R. Direct imaging of the pores and cages of three-dimensional mesoporous materials.
Nature 2000, 408, 449-453.
[3] Kaneda, M.; Tsubakiyama, T.; Carlsson, A.; Sakamoto, Y.; Ohsuna, T.; Terasaki, O.; Joo,
S. H.; Ryoo, R. Structural Study of Mesoporous MCM-48 and Carbon Networks
Synthesized in the Spaces of MCM-48 by Electron Crystallography. J. Phys. Chem. B 2002,
106, 1256-1266.
[4] Sakamoto, Y.; Daz, I.; Terasaki, O.; Zhao, D.; Prez-Pariente, J.; Kim, J. M.; Stucky, G. D.
Three-Dimensional Cubic Mesoporous Structures of SBA-12 and Related Materials by
Electron Crystallography. J. Phys. Chem. B 2001, 106, 3118-3123.
[5] Garcia-Bennett, A. E.; Miyasaka, K.; Terasaki, O.; Che, S. Structural Solution of
Mesocaged Material AMS-8. Chem. Mater. 2004, 16, 3597-3605.
[6] Sakamoto, Y.; Kim, T.-W.; Ryoo, R.; Terasaki, O. Three-Dimensional Structure of LargePore Mesoporous Cubic Iaequation imaged Silica with Complementary Pores and Its
Carbon Replica by Electron Crystallography. Angew. Chem. Int. Ed. 2004, 43, 5231-5234.
ACS Paragon Plus Environment
23
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 24 of 42
[7] Garcia-Bennett, A. E.; Kupferschmidt, N.; Sakamoto, Y.; Che, S.; Terasaki, O. Synthesis of
Mesocage Structures by Kinetic Control of Self-Assembly in Anionic Surfactants. Angew.
Chem. Int. Ed. 2005, 44, 5317-5322.
[8] Gao, C.; Sakamoto, Y.; Sakamoto, K.; Terasaki, O.; Che, S. Synthesis and Characterization
of Mesoporous Silica AMS-10 with Bicontinuous Cubic Pn m Symmetry. Angew. Chem.
Int. Ed. 2006, 45, 4295-4298.
[9] Han, Y.; Zhang, D.; Chng, L. L.; Sun, J.; Zhao, L.; Zou, X.; Ying, J. Y. A tri-continuous
mesoporous material with a silica pore wall following a hexagonal minimal surface. Nature
Chem. 2009, 1, 123-127.
[10] Che, S.; Liu, Z.; Ohsuna, T.; Sakamoto, K; Terasaki, O; Tatsumi, T. Synthesis and
characterization of chiral mesoporous silica. Nature 2004, 429, 281-284.
[11] Yang, P.; Zhao, D.; Margolese, D. I.; Chmelka, B. F.; Stucky, G. D. Generalized syntheses
of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 1998, 396,
152-155.
[12] Inagaki, S.; Guan, S.; Ohsuna, T; Terasaki, O. An ordered mesoporous organosilica hybrid
material with a crystal-like wall structure. Nature 2002, 416, 304-307.
[13] Tanaka, S.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Synthesis of ordered mesoporous
carbons with channel structure from an organicorganic nanocomposite. Chem. Commun.
2005, 2125-2127.
ACS Paragon Plus Environment
24
Page 25 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
[14] Zhang, F.; Meng, Y.; Gu, D.; Yan, Y.; Yu, C.; Tu, B.; Zhao, D. A Facile Aqueous Route to
Synthesize Highly Ordered Mesoporous Polymers and Carbon Frameworks with Ia d
Bicontinuous Cubic Structure. J. Am. Chem. Soc. 2005, 127, 13508-13509.
[15] Sun, D.; Riley, A. E.; Cadby, A. J.; Richman, E. K.; Korlann, S. D.; Tolbert, S. H.
Hexagonal nanoporous germanium through surfactant-driven self-assembly of Zintl clusters.
Nature 2006, 441, 1126-1130.
[16] Richman, E. K.; Kang, C. B.; Brezesinski, T.; Tolbert, S. H. Ordered Mesoporous Silicon
through Magnesium Reduction of Polymer Templated Silica Thin Films. Nano Lett. 2008, 8,
3075-3079.
[17] Yamauchi, Y.; Takai, A.; Komatsu, M.; Sawada, M.; Ohsuna, T.; Kuroda, K. Vapor
Infiltration of a Reducing Agent for Facile Synthesis of Mesoporous Pt and Pt-Based Alloys
and Its Application for the Preparation of Mesoporous Pt Microrods in Anodic Porous
Membranes. Chem. Mater. 2008, 20, 1004-1011.
[18] Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in
Catalysis. Chem. Rev. 1997, 97, 2373-2420.
[19] De Vos, D. E.; Dams, M.; Sels, B. F.; Jacobs, P. A. Ordered Mesoporous and Microporous
Molecular Sieves Functionalized with Transition Metal Complexes as Catalysts for
Selective Organic Transformations. Chem. Rev. 2002, 102, 3615-3640.
[20] Taguchi, A.; Schth, F. Ordered mesoporous materials in catalysis. Microporous
Mesoporous Mater. 2005, 77, 1-45.
ACS Paragon Plus Environment
25
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 26 of 42
[21] Hartmann, M. Ordered Mesoporous Materials for Bioadsorption and Biocatalysis. Chem.
Mater. 2005, 17, 4577-4593.
[22] D'Alessandro, D. M.; Smit, B.; Long, J. R. Carbon Dioxide Capture: Prospects for New
Materials. Angew. Chem. Int. Ed. 2010, 49, 6058-6082.
[23] Vallet-Reg, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew.
Chem. Int. Ed. 2007, 46, 7548-7558.
[24] Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for
Drug Delivery and Biosensing Applications. Adv. Funct. Mater. 2007, 17, 1225-1236.
[25] Ndamanisha, J. C.; Guo, L. Ordered mesoporous carbon for electrochemical sensing: A
review. Anal. Chim. Acta 2012, 747, 19-28.
[26] Shalom, M.; Dor, S.; R hle, S.; Grinis, L.; Zaban, A. Core/CdS Quantum Dot/Shell
Mesoporous Solar Cells with Improved Stability and Efficiency Using an Amorphous TiO2
Coating. J. Phys. Chem. C 2009, 113, 3895-3898.
[27] Ji, X.; Lee, K. T.; Nazar, L. F. A highly ordered nanostructured carbonsulphur cathode for
lithiumsulphur batteries. Nature Mater. 2009, 8, 500-506.
[28] Brezesinski, T.; Wang, J.; Tolbert, S. H.; Dunn, B. Ordered mesoporous -MoO3 with isooriented nanocrystalline walls for thin-film pseudocapacitors. Nature Mater. 2010, 9, 146151.
ACS Paragon Plus Environment
26
Page 27 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
[29] Ishii, Y.; Kanamori, Y.; Kawashita, T.; Mukhopadhyay, I.; Kawasaki, S. Mesoporous
carbon-titania nanocomposites for high-power Li-ion battery anode material. J. Phys. Chem.
Solids 2010, 71, 511-514.
[30] Ishii, Y.; Okamura, K.; Matsushita, T.; Kawasaki, S. Origin of High Power Performance of
Mesoporous Carbon-TiO2(B) Nanocomposite Electrodes: An In Situ Synchrotron X-ray
Diffraction Study of TiO2(B) Electrode upon Lithium Insertion. Mater. Express 2012, 2, 2336.
[31] Ishii, Y.; Matsumura, A.; Ishikawa, Y.; Kawasaki, S. White Light Emission from
Mesoporous CarbonSilica Nanocomposites. Jpn. J. Appl. Phys. 2011, 50, 01AF06.
[32] Cowley, J. M.; Moodie, A. F. The scattering of electrons by atoms and crystals. I. A new
theoretical approach. Acta. Cryst. 1959, 10, 609-619.
[33] Brunauer, S.; Emmett, P. H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J.
Am. Chem. Soc. 1938, 60, 309-319.
[34] Barrett, E. P.; Joyner, L. G.; Halenda, P. P. The Determination of Pore Volume and Area
Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem.
Soc. 1951, 73, 373-380.
[35] Lastoskie, C.; Gubbins, K. E.; Quirke, N. Pore size distribution analysis of microporous
carbons: a density functional theory approach. J. Phys. Chem. 1993, 97, 4786-4796.
ACS Paragon Plus Environment
27
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 28 of 42
[36] Neimark, A. V.; Ravikovitch, P. I.; Grn, M.; Schth, F.; Unger, K. K. Pore Size Analysis
of MCM-41 Type Adsorbents by Means of Nitrogen and Argon Adsorption. J. Colloid
Interface Sci. 1998, 207, 159-169.
[37] Ravikovitch, P. I.; Neimark, A. V. Characterization of Micro- and Mesoporosity in SBA-15
Materials from Adsorption Data by the NLDFT Method. J. Phys. Chem. B 2001, 105, 68176823.
[38] Ravikovitch, P. I.; Neimark, A. V.; Density Functional Theory of Adsorption in Spherical
Cavities and Pore Size Characterization of Templated Nanoporous Silicas with Cubic and
Three-Dimensional Hexagonal Structures. Langmuir 2002, 18, 1550-1560.
[39] Pellenq, R. J.-M.; Rousseau, B.; Levitz, P. E. A Grand Canonical Monte Carlo study of
argon adsorption/condensation in mesoporous silica glasses. Phys. Chem. Chem. Phys. 2001,
3, 1207-1212.
[40] Ohkubo, T.; Miyawaki, J.; Kaneko, K.; Ryoo, R.; Seaton, N. A. Adsorption Properties of
Templated Mesoporous Carbon (CMK-1) for Nitrogen and Supercritical
MethaneExperiment and GCMC Simulation. J. Phys. Chem. B 2002, 106, 6523-6528.
[41] Herdes, C.; Santos, M. A.; Medina, F.; Vega, L. F. Pore Size Distribution Analysis of
Selected Hexagonal Mesoporous Silicas by Grand Canonical Monte Carlo Simulations.
Langmuir 2005, 21, 8733-8742.
[42] Feuston, B. P.; Higgins , J. B. Model Structures for MCM-41 Materials: A Molecular
Dynamics Simulation. J. Phys. Chem. 1994, 98, 4459-4462.
ACS Paragon Plus Environment
28
Page 29 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
[43] Schmidt, W. Calculation of XRD patterns of simulated FDU-15, CMK-5, and CMK-3.
Microporous Mesoporous Mater. 2009, 117, 372-379.
[44] Impror-Clerc, M.; Davidson, P.; Davidson, A. Existence of a Microporous Corona around
the Mesopores of Silica-Based SBA-15 Materials Templated by Triblock Copolymers. J.
Am. Chem. Soc. 2000, 122, 11925-11933.
[45] Solovyov, L. A.; Kirik, S. D.; Shmakov, A. N.; Romannikov, V. N. X-ray Structural
modeling of silicate mesoporous mesophase material. Microporous Mesoporous Mater.
2001, 44-45, 17-23.
[46] Jaroniec, M.; Solovyov, L. A. Improvement of the KrukJaroniecSayari Method for Pore
Size Analysis of Ordered Silicas with Cylindrical Mesopores. Langmuir 2006, 22, 67576760.
[47] Morishige, K.; Tateishi, M. Accurate Relations between Pore Size and the Pressure of
Capillary Condensation and the Evaporation of Nitrogen in Cylindrical Pores. Langmuir
2006, 22, 4165-4169.
[48] Sauer, J.; Marlow, F.; Schuth, F. Simulation of powder diffraction patterns of modified
ordered mesoporous materials. Phys. Chem. Chem. Phys. 2001, 3, 5579-5584.
[49] Miyasaka, K.; Hano, H.; Kubota, Y.; Lin, Y.; Ryoo, R.; Takata, M.; Kitagawa, S.; Neimark,
A. V.; Terasaki, O. A. Stand-Alone Mesoporous Crystal Structure Model from in situ X-ray
Diffraction: Nitrogen Adsorption on 3D Cagelike Mesoporous Silica SBA-16. Chem. Eur.
J. 2012, 18, 10300-10311.
ACS Paragon Plus Environment
29
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 30 of 42
[50] Miyasaka, K.; Neimark, A. V.; Terasaki, O. Density Functional Theory of in Situ
Synchrotron Powder X-ray Diffraction on Mesoporous Crystals: Argon Adsorption on
MCM-41. J. Phys. Chem. C 2009, 133, 791-794.
[51] Kruk, M.; Jaroniec, M.; Ko, C. H.; Ryoo, R. Characterization of the Porous Structure of
SBA-15. Chem. Mater. 2000, 12, 1961-1968.
[52] Huo, Q.; Zhao, D.; Feng, J.; Weston, K.; Buratto, S. K.; Stucky, G. D.; Schacht, S. Schth,
F. Room temperature growth of mesoporous silica fibers: A new high-surface-area optical
waveguide. Adv. Mater. 1997, 9, 974-978.
[53] Liu, R.; Shi, Y.; Wan, Y.; Meng, Y.; Zhang, F.; Gu, D.; Chen, Z.; Tu, B.; Zhao, D.
Triconstituent co-assembly to ordered mesostructured polymer-silica and carbon-silica
nanocomposites and large-pore mesoporous carbons with high surface areas. J. Am. Chem.
Soc. 2006, 128, 11652-11662.
[54] Jaroniec, M.; Kruk, M.; Olivier, J. P. Standard Nitrogen Adsorption Data for
Characterization of Nanoporous Silicas. Langmuir 1999, 15, 5410-5413.
[55] Kruk, M.; Jaroniec, M.; Sayari, A. Application of Large Pore MCM-41 Molecular Sieves to
Improve Pore Size Analysis Using Nitrogen Adsorption Measurements. Langmuir 1997, 13,
6267-6273.
[56] Seto, Y; Hamane, D.; Nagai, T.; Sata, N. Development of a Software Suite on X-ray
Diffraction Experiments. Rev. High. Press. Sci. Techn. 2010, 20, 269-276.
ACS Paragon Plus Environment
30
Page 31 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
[57] Skinner, L. B.; Benmore, C. J.; Parise, J. B. Area detector corrections for high quality
synchrotron X-ray structure factor measurements. Nucl. Instr. and Meth. Phys. Res. A 2012,
662, 61-70.
[58] International Union of Crystallography. International Tables for Crystallography; doi:
10.1107/97809553602060000001.
[59] Sing, K. S. W. Reporting physisorption data for gas/solid systems with special reference to
the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603-619.
[60] Branton. P. J.; Hall, P. G.; Sing, K. S. W.; Physisorption of Nitrogen and Oxygen by MCM41, a Model Mesoporous Adsorbent. J. Chem. Soc., Chem. Commun. 1993, 1257-1258.
[61] Kruk, M.; Jaroniec, M. Argon Adsorption at 77k as a Useful Tool for the Elucidation of
Pore Connectivity in Ordered Materials with Large Cagelike Mesopores. Chem. Mater.
2003, 15, 2942-2949.
[62] Kruk, M.; Jaroniec, M.; Sayari, A. Adsorption Study of Surface and Structural Properties of
MCM-41 Materials of Different Pore Sizes. J. Phys. Chem. B 1997, 101, 583-589.
[63] Kruk, M.; Jaroniec, M.; Sayari, A. Structural and surface properties of siliceous and
titanium-modified HMS molecular sieves. Microporous Mater. 1997, 9, 173-183.
[64] Kruk, M.; Jaroniec, M. Relations between Pore Structure Parameters and Their Implications
for Characterization of MCM-41 Using Gas Adsorption and X-ray Diffraction. Chem.
Mater. 1999, 11, 492-500.
ACS Paragon Plus Environment
31
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 32 of 42
[65] Dabadie, T.; Ayral, A.; Guizard, C.; Cot, L.; Lacan, P. Synthesis and characterization of
inorganic gels in a lyotropic liquid crystal medium. Part 2.Synthesis of silica gels in
lyotropic crystal phases obtained from cationic surfactants. J. Mater. Chem. 1996, 6, 17891794.
[66] Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu. C.; Lee, Y. H.; Kim, S.
G.; Rinzler, A. G.; Colbert, D. T.; Scuseria, G. E.; Tomanek, D.; Fischer, J. E.; Smalley, R.
E. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483-487.
[67] Maniwa, Y., Kumazawa, Y., Saito, Y., Tou, H., Kataura, H., Ishii, H., Suzuki, S., Achiba,
Y., Fujiwara, A., Suematsu, H. Anomaly of X-ray Diffraction Profile in Single-Walled
Carbon Nanotubes. Jpn. J. Appl. Phys. 1999, 38, L668-L670.
[68] Sonwane, C. G.; Bhatia, S. K.; Calos, N. Experimental and Theoretical Investigations of
Adsorption Hysteresis and Criticality in MCM-41:Studies with O2, Ar, and CO2. Ind. Eng.
Chem. Res. 1998, 37, 2271-2283.
[69] Koga, K.; Gao, G. T.; Tanaka, H.; Zeng, X. C. Formation of ordered ice nanotubes inside
carbon nanotubes. Nature 2001, 412, 802.
[70] Maniwa, Y.; Kataura, H.; Abe, M.; Suzuki, S.; Achiba, Y.; Kira, H.; Matsuda, K. Phase
Transition in Confined Water Inside Carbon Nanotubes. J. Phys. Soc. Jpn. 2002, 71, 28632866.
[71] Sekhaneh, W.; Kotecha, M.; Dettlaff-Weglikowska, U.; Veeman, W. S. High resolution
NMR of water absorbed in single-wall carbon nanotubes. Chem. Phys. Lett. 2006, 428, 143147.
ACS Paragon Plus Environment
32
Page 33 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
[72] Byl, O.; Liu, J.-C.; Wang, Y.; Yim, W.-L.; Johnson, J. K.; Yates, J. T. Unusual Hydrogen
Bonding in Water-Filled Carbon Nanotubes. J. Am. Chem. Soc. 2006, 128, 12090-12097.
[73] Maniwa, Y.; Matsuda, K.; Kyakuno, H.; Ogasawara, S.; Hibi, T.; Kadowaki, H.; Suzuki, S.;
Achiba, Y.; Kataura, H. Water-filled single-wall carbon nanotubes as molecular nanovalves.
Nature Mater. 2007, 6, 135-141.
[74] Wang, H. J.; Xi, X. K.; Kleinhammes, A.; Wu Y. Temperature-induced hydrophobichydrophilic transition observed by water adsorption. Science 2008, 322, 80-83.
[75] Urita, K.; Shiga, Y.; Fujimori, T.; Iiyama, T.; Hattori, Y.; Kanoh, H.; Ohba, T.; Tanaka, H.;
Yudasaka, M.; Iijima, S.; Moriguchi, I.; Okino, F.; Endo, M.; Kaneko, K. Confinement in
carbon nanospace-induced production of KI nanocrystals of high-pressure phase. J. Am.
Chem. Soc. 2011, 133, 10344-10347.
[76] Kittaka, S.; Ishimaru, S.; Kuranishi, M.; Matsudaa, T.; Yamaguchib, T. Enthalpy and
interfacial free energy changes of water capillary condensed in mesoporous silica, MCM-41
and SBA-15. Phys. Chem. Chem. Phys. 2006, 8, 3223-3231.
[77] Endo, A.; Yamamoto, T.; Inagi, Y.; Iwakabe, K.; Ohmori, T. Characterization of
Nonfreezable Pore Water in Mesoporous Silica by Thermoporometry. J. Phys. Chem. C
2008, 112, 9034-9039.
ACS Paragon Plus Environment
33
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 34 of 42
Table 1. Structural parameters of the obtained materialsa
[nm]
[m2 g-1]
[cm3 g-1]
[cm3 g-1]
[cm3 g-1]
[nm]
[nm]
[nm]
MPS1
10.62
596
0.64
0.43
0.07
6.4
7.3
7.5
MPS2
4.56
962
0.75
0.70
0.02
2.8
3.6
3.7
MPS3
4.33
1156
0.81
0.78
0.00
2.6
3.4
3.6
MPCSb
11.42
365
0.39
0.35
0.03
5.4
6.3
7.6
: lattice constant estimated from apparent position of 10 XRD peak;
: specific surface
area estimated by the BET method;
: total pore volume;
: primary mesopore volume;
: micropore volume;
: primary mesopore diameter estimated by the BJH method;
: primary mesopore diameter estimated by the KJS method;
: mesopore diameter
estimated by geometrical relationship shown in equation 25.
b
C:SiO2 weight ratio estimated by thermogravimetric analysis was 37.5:62.5.
Table 2. Structural parameters determined by non-linear least-square fittings of XRD patterna
[nm]
[nm]
[nm]
) []
MPS1
10.555(1)
6.95( 6)
0.72(2)
0.66
MPS2
4.526(2)
3.60( 7)
0.39(2)
0.79
MPS3
4.292(3)
3.38( 9)
0.40(2)
0.79
MPCS
11.359(2)
6.98(16)
1.04(2)
0.61
: lattice constant;
mesopore position.
: primary mesopore diameter; : mean-square displacement of
ACS Paragon Plus Environment
34
Page 35 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
Figure 1. (A) Schismatic structure of the ordered mesoporous material examined in this study.
Cylindrical mesopores (diameter:
, length: ) are 2D hexagonally aligned, and form an
ordered crystalline nanostructure. The structural backbone (wall region) consists of a uniform
amorphous matrix (density:
). (B) Calculation scheme of the unit cell form factor ( ( )).
ACS Paragon Plus Environment
35
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 36 of 42
Figure 2. (a) Theoretical XRD pattern of an ordered mesoporous material with 2D-hexagonal
symmetry ( =10.0 nm, =3.0 nm). The theoretical XRD profile was calculated by multiplying
(b) the squared unit cell form factor | ( )|
the sum of the peak functions (
| , (c) Lorentz factor ( ), and (d)
( )).
ACS Paragon Plus Environment
36
Page 37 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
Figure 3. Diffraction intensities of (a) 11, (b) 20, (c) 21, and (d) 30 peaks calculated using
equation 22. The peak intensity of the 100 diffraction peak was set to 1. (B) is a magnified view
of (A).
ACS Paragon Plus Environment
37
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 38 of 42
Figure 4. TEM photographs of MPS1 (A) parallel and (B) perpendicular to the channel pore axis.
ACS Paragon Plus Environment
38
Page 39 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
Figure 5. XRD pattern of MPS1.
ACS Paragon Plus Environment
39
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 40 of 42
Figure 6. Comparison between (a) observed and (b) simulated XRD patterns of (A) MPS1, (B)
MPS2, (C) MPS3, and (D) MPCS.
ACS Paragon Plus Environment
40
Page 41 of 42
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
The Journal of Physical Chemistry
Figure 7. XRD patterns of (a) dry and (b) water-immersed MPS3. Diffraction peaks of CeO2
powder used as internal intensity standard (The weight ratio of MPS3:CeO2 was 2:1) also shown.
ACS Paragon Plus Environment
41
The Journal of Physical Chemistry
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
Page 42 of 42
Table of contents (TOC) image
ACS Paragon Plus Environment
42
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Lambda 365 UV/Vis Spectrophotometer With UV Lab Software: Image Not Found Image Not FoundDocument1 pageLambda 365 UV/Vis Spectrophotometer With UV Lab Software: Image Not Found Image Not FoundsecateNo ratings yet
- 8086 Microprocessor Interfacing MCQ SDocument4 pages8086 Microprocessor Interfacing MCQ SDeepak Ahlawat67% (3)
- Kinesiology For The Martial Arts PDFDocument50 pagesKinesiology For The Martial Arts PDFB'MAZ100% (4)
- Surfactant-Assisted Synthesis of Batio Nanoparticles by Micro-Emulsion MethodDocument4 pagesSurfactant-Assisted Synthesis of Batio Nanoparticles by Micro-Emulsion MethodsecateNo ratings yet
- Pancreatic JournalDocument4 pagesPancreatic JournalsecateNo ratings yet
- Je5b00789 Si 001Document7 pagesJe5b00789 Si 001secateNo ratings yet
- AGENDA ITEM: 650-472 Load Combinations 8 Ballot: P Determined in F.4.1Document8 pagesAGENDA ITEM: 650-472 Load Combinations 8 Ballot: P Determined in F.4.1secateNo ratings yet
- Improved Temperature Compensation For in Situhumic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water TypesDocument4 pagesImproved Temperature Compensation For in Situhumic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water TypessecateNo ratings yet
- E-Books: PublicationsDocument4 pagesE-Books: PublicationssecateNo ratings yet
- Extraction Titanium Dioxide (Tio,) From Ilmenite and Titaniferous SlagDocument6 pagesExtraction Titanium Dioxide (Tio,) From Ilmenite and Titaniferous SlagsecateNo ratings yet
- Journal of Colloid and Interface ScienceDocument8 pagesJournal of Colloid and Interface SciencesecateNo ratings yet
- Arsenic in Drinking Water Problems and SolutionsDocument1 pageArsenic in Drinking Water Problems and SolutionssecateNo ratings yet
- Improved Temperature Compensation For in Situ Humic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water Types - Environmental Engineering ScienceDocument2 pagesImproved Temperature Compensation For in Situ Humic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water Types - Environmental Engineering SciencesecateNo ratings yet
- E-Books: PublicationsDocument4 pagesE-Books: PublicationssecateNo ratings yet
- Ab-416 3 enDocument20 pagesAb-416 3 ensecateNo ratings yet
- AN-v213 PDFDocument2 pagesAN-v213 PDFsecateNo ratings yet
- Journal of Colloid and Interface ScienceDocument8 pagesJournal of Colloid and Interface SciencesecateNo ratings yet
- 2222 PDFDocument7 pages2222 PDFsecateNo ratings yet
- Copper in Drinking Water by Anodic Stripping Voltammetry at The Sctrace Gold Using The 946 Portable Va AnalyzerDocument2 pagesCopper in Drinking Water by Anodic Stripping Voltammetry at The Sctrace Gold Using The 946 Portable Va AnalyzersecateNo ratings yet
- Zinc Oxide Nanostructures For Optoelectronic and Energy DevicesDocument3 pagesZinc Oxide Nanostructures For Optoelectronic and Energy DevicessecateNo ratings yet
- Accepted Manuscript: RSC - Li/pccpDocument11 pagesAccepted Manuscript: RSC - Li/pccpsecateNo ratings yet
- 10 1038@nenergy 2017 87Document9 pages10 1038@nenergy 2017 87secateNo ratings yet
- Dsc-Tga SimultaneoDocument1 pageDsc-Tga SimultaneosecateNo ratings yet
- ZnoDocument8 pagesZnosecateNo ratings yet
- Meso-Porous Silicon-Coated Carbon Nanotube As An Anode For Lithium-Ion BatteryDocument8 pagesMeso-Porous Silicon-Coated Carbon Nanotube As An Anode For Lithium-Ion BatterysecateNo ratings yet
- AaaaaaaaaaaaaaaaaaaaaaaaaaaaDocument1 pageAaaaaaaaaaaaaaaaaaaaaaaaaaaasecateNo ratings yet
- File 1424717664Document8 pagesFile 1424717664secateNo ratings yet
- Quattro ESEM DatasheetDocument4 pagesQuattro ESEM DatasheetsecateNo ratings yet
- Personalized PDF Catalog Catalogue Generated September 6, 2017Document4 pagesPersonalized PDF Catalog Catalogue Generated September 6, 2017secateNo ratings yet
- Personalized PDF Catalog Catalogue Generated September 6, 2017Document4 pagesPersonalized PDF Catalog Catalogue Generated September 6, 2017secateNo ratings yet
- Fig. 6.38Document1 pageFig. 6.38secateNo ratings yet
- Sonochemical and Microwave-Assisted Synthesis of Linked Single-Crystalline Zno RodsDocument6 pagesSonochemical and Microwave-Assisted Synthesis of Linked Single-Crystalline Zno RodssecateNo ratings yet
- Manual SIMOTION Rotary Knife V2.0Document140 pagesManual SIMOTION Rotary Knife V2.0Luis Adrian PerezNo ratings yet
- The Alchemist - Book ReviewDocument10 pagesThe Alchemist - Book ReviewNETFLIX ORIGINALSNo ratings yet
- Lista de Paineis LCD Atualizacao 26 04 2013 PDFDocument412 pagesLista de Paineis LCD Atualizacao 26 04 2013 PDFHfssSilva100% (4)
- Manual To KivyDocument2 pagesManual To KivyvalkmaxNo ratings yet
- Lesson Plans For Sped 101Document3 pagesLesson Plans For Sped 101api-271266618No ratings yet
- Analytics For Sustainable BusinessDocument6 pagesAnalytics For Sustainable BusinessDeloitte AnalyticsNo ratings yet
- Sample Summary of TA ConductedDocument1 pageSample Summary of TA ConductedJAN MICHAEL RIPARIPNo ratings yet
- Sample of PHD Thesis Content Format USIM - Computer ScienceDocument6 pagesSample of PHD Thesis Content Format USIM - Computer ScienceKhirulnizam Abd RahmanNo ratings yet
- Ecosytem Report RubricDocument1 pageEcosytem Report Rubricapi-309097762No ratings yet
- Government of Maharashtra: State Common Entrance Test Cell, Maharashtra State, MumbaiDocument1 pageGovernment of Maharashtra: State Common Entrance Test Cell, Maharashtra State, MumbaiDhiraj Jagtap [011]No ratings yet
- Ground Water Brochure of Jhansi District, Uttar Pradesh: Title Page NoDocument19 pagesGround Water Brochure of Jhansi District, Uttar Pradesh: Title Page NoSandeep KumarNo ratings yet
- An Inspector Calls Character Notes Key Quotations Key Language & Structural Features Priestley's IdeasDocument8 pagesAn Inspector Calls Character Notes Key Quotations Key Language & Structural Features Priestley's IdeasPNo ratings yet
- The Menstrual Cycle Remedies Amenorrhea HandoutDocument3 pagesThe Menstrual Cycle Remedies Amenorrhea HandoutRoger AugeNo ratings yet
- Singapore: URA Concept Plan 2011 Focus Group Preliminary RecommendationsDocument7 pagesSingapore: URA Concept Plan 2011 Focus Group Preliminary RecommendationsThe PariahNo ratings yet
- Company Presentation - Company ProfileDocument10 pagesCompany Presentation - Company ProfileNishtha SharmaNo ratings yet
- NamdarDocument38 pagesNamdarthe next miamiNo ratings yet
- Marking Criteria For Paper 2 (PMR)Document7 pagesMarking Criteria For Paper 2 (PMR)nitamansNo ratings yet
- Dorothy Johnson's TheoryDocument23 pagesDorothy Johnson's Theoryarielledy0405No ratings yet
- Aniket CV NewDocument2 pagesAniket CV NewRajreddy ChamawarNo ratings yet
- Cs 1410Document2 pagesCs 1410David DengNo ratings yet
- Customer Relationship Management: Summer Internship ProjectDocument16 pagesCustomer Relationship Management: Summer Internship ProjectSoniNitinNo ratings yet
- CVE 202 Lecture - 28062021Document11 pagesCVE 202 Lecture - 28062021odubade opeyemiNo ratings yet
- BlahDocument5 pagesBlahNaz KierbeyNo ratings yet
- RHCSA Sa1 2 EXAM Questions (1) 1Document23 pagesRHCSA Sa1 2 EXAM Questions (1) 1hosnitmiNo ratings yet
- Demo On Tableau DesktopDocument46 pagesDemo On Tableau DesktopDeepak GuptaNo ratings yet
- Phys101l Ex104Document2 pagesPhys101l Ex104koko BunchNo ratings yet
- P 1075 Basic E 03 - 08Document2 pagesP 1075 Basic E 03 - 08Marco Andres Saldias SagredoNo ratings yet
- English Practice 1: C. Carried D. Sugar B. Underline A. Danger A. CharacterDocument3 pagesEnglish Practice 1: C. Carried D. Sugar B. Underline A. Danger A. CharacterKeisaNo ratings yet