Professional Documents
Culture Documents
Surface Tension (Water Properties), USGS Water Science School
Uploaded by
rajkumarCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Surface Tension (Water Properties), USGS Water Science School
Uploaded by
rajkumarCopyright:
Available Formats
01/09/2015
SurfaceTension(WaterProperties),USGSWaterScienceSchool
TheUSGSWaterScienceSchool
Search...
Search
Backtopreviouspage
SurfaceTensionandWater
The
Surfacetension
cohesive
forces
Thepropertyofthesurface
between
ofaliquidthatallowsitto
liquid
resistanexternalforce,due
molecules
tothecohesivenatureofits
are
molecules.
responsible
forthephenomenonknownassurface
tension.Themoleculesatthesurfaceofa
glassofwaterdonothaveotherwater
moleculesonallsidesofthemand
consequentlytheycoheremorestronglyto
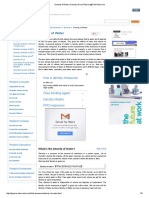
Floatingpaperclipmadeofsteelwithcopper
thosedirectlyassociatedwiththem(inthis
plating.Thehighsurfacetensionhelpsthepaper
case,nexttoandbelowthem,butnot
clipwithmuchhigherdensityfloatonthewater.
above).Itisnotreallytruethata"skin"
Credit:ArminKbelbeck.
formsonthewatersurfacethestronger
cohesionbetweenthewatermoleculesasopposedtotheattractionofthewatermolecules
totheairmakesitmoredifficulttomoveanobjectthroughthesurfacethantomoveit
whenitiscompletelysubmersed.(Source:GSU).
CohesionandSurfaceTension
Thecohesiveforcesbetweenmoleculesinaliquidaresharedwithallneighboring
molecules.Thoseonthesurfacehavenoneighboringmoleculesaboveand,thus,exhibit
strongerattractiveforcesupontheirnearestneighborsonandbelowthesurface.Surface
tensioncouldbedefinedasthepropertyofthesurfaceofaliquidthatallowsittoresistan
externalforce,duetothecohesivenatureofthewatermolecules.
Surfacetensionatamolecularlevel
Watermoleculeswanttoclingtoeachother.Atthesurface,however,therearefewer
watermoleculestoclingtosincethereisairabove(thus,nowatermolecules).Thisresults
inastrongerbondbetweenthosemoleculesthatactuallydocomeincontactwithone
another,andalayerofstronglybondedwater(seediagram).Thissurfacelayer(held
togetherbysurfacetension)createsaconsiderablebarrierbetweentheatmosphereandthe
water.Infact,otherthanmercury,waterhasthegreatestsurfacetensionofanyliquid.
(Source:LakesofMissouri)
Withinabodyofaliquid,amoleculewillnotexperienceanetforcebecausetheforcesby
theneighboringmoleculesallcancelout(diagram).Howeverforamoleculeonthesurface
oftheliquid,therewillbeanetinwardforcesincetherewillbenoattractiveforceacting
fromabove.Thisinwardnetforcecausesthemoleculesonthesurfacetocontractandto
resistbeingstretchedorbroken.Thusthesurfaceisundertension,whichisprobablywhere
thename"surfacetension"camefrom.(Source:WoodrowWilsonFoundation).
Duetothesurfacetension,smallobjectswill"float"onthesurfaceofafluid,aslongasthe
objectcannotbreakthroughandseparatethetoplayerofwatermolecules.Whenanobject
http://water.usgs.gov/edu/surfacetension.html
1/2
01/09/2015
SurfaceTension(WaterProperties),USGSWaterScienceSchool
isonthesurfaceofthefluid,thesurfaceunder
tensionwillbehavelikeanelasticmembrane.
Examplesofsurfacetension
Walkingonwater:
Smallinsectssuch
asthewaterstrider
canwalkonwater
becausetheirweight
isnotenoughto
penetratethe
surface.
Floatinganeedle:Acarefullyplacedsmall
needlecanbemadetofloatonthesurfaceof
watereventhoughitisseveraltimesasdense
aswater.Ifthesurfaceisagitatedtobreakup
thesurfacetension,thenneedlewillquickly
sink.
Don'ttouchthetent!:Commontentmaterialsaresomewhatrainproofinthatthesurface
tensionofwaterwillbridgetheporesinthefinelywovenmaterial.Butifyoutouchthetent
materialwithyourfinger,youbreakthesurfacetensionandtherainwilldripthrough.
Clinicaltestforjaundice:Normalurinehasasurfacetensionofabout66dynes/centimeter
butifbileispresent(atestforjaundice),itdropstoabout55.IntheHaytest,powdered
sulfurissprinkledontheurinesurface.Itwillfloatonnormalurine,butwillsinkifthe
surfacetensionisloweredbythebile.
Surfacetensiondisinfectants:Disinfectantsareusuallysolutionsoflowsurfacetension.This
allowthemtospreadoutonthecellwallsofbacteriaanddisruptthem.
Soapsanddetergents:Thesehelpthecleaningofclothesbyloweringthesurfacetensionof
thewatersothatitmorereadilysoaksintoporesandsoiledareas.
Washingwithcoldwater:Themajorreasonforusinghotwaterforwashingisthatits
surfacetensionisloweranditisabetterwettingagent.Butifthedetergentlowersthe
surfacetension,theheatingmaybeunneccessary.
Whybubblesareround:Thesurfacetensionofwaterprovidesthenecessarywalltensionfor
theformationofbubbleswithwater.Thetendencytominimizethatwalltensionpullsthe
bubblesintosphericalshapes.
SurfaceTensionandDroplets:Surfacetensionisresponsiblefortheshapeofliquiddroplets.
Althougheasilydeformed,dropletsofwatertendtobepulledintoasphericalshapebythe
cohesiveforcesofthesurfacelayer.
Source:GSU
U.S.DepartmentoftheInterior|U.S.GeologicalSurvey
URL:http://water.usgs.gov/edu/surfacetension.html
PageContactInformation:HowardPerlman
PageLastModified:Monday,27Jul201514:42:36EDT
http://water.usgs.gov/edu/surfacetension.html
2/2
You might also like
- IntroductionDocument6 pagesIntroductionleoyong509No ratings yet
- Surface Tension and Water: Cohesive ForcesDocument2 pagesSurface Tension and Water: Cohesive ForcesLydia AjengNo ratings yet
- CHEM - Surface TensionDocument2 pagesCHEM - Surface TensionReyster LalimNo ratings yet
- Brownian Motion and DiffusionDocument4 pagesBrownian Motion and DiffusionANam MUkri100% (3)
- Properties of Water - in ActionDocument2 pagesProperties of Water - in ActionJulia AlconetarNo ratings yet
- Physics ProjectDocument7 pagesPhysics Projectvenkynair0% (1)
- Viscosity and Surface TensionDocument2 pagesViscosity and Surface TensionMichael HeryantoNo ratings yet
- Oden PrintableDocument32 pagesOden PrintableJulalaNo ratings yet
- Cohesion and Adhesion in LiquidsDocument1 pageCohesion and Adhesion in LiquidsjmlagumbayNo ratings yet
- Floating Needle-WPS OfficeDocument2 pagesFloating Needle-WPS OfficeAnnie Valenzona CablasNo ratings yet
- A1.1.3 Cohesion of Water Molecules Due To Hydrogen Bonding and Consequences For OrganismsDocument15 pagesA1.1.3 Cohesion of Water Molecules Due To Hydrogen Bonding and Consequences For Organismstaiga.aisaka14404No ratings yet
- Bio 112 - Activity 4Document3 pagesBio 112 - Activity 4LAGMAY, Daniel Scott G.No ratings yet
- Surface Tension - Chemistry LibreTextsDocument3 pagesSurface Tension - Chemistry LibreTextsmuthamizh rajuNo ratings yet
- Surface TensionDocument2 pagesSurface TensionShashank ChheniyaNo ratings yet
- Cohesive and Adhesive Forces ExplainedDocument3 pagesCohesive and Adhesive Forces ExplainedAhmad RaihanNo ratings yet
- Cohesion (Chemistry) - WikipediaDocument9 pagesCohesion (Chemistry) - WikipediaBashiir NuurNo ratings yet
- Viscosity and Surface TensionDocument15 pagesViscosity and Surface TensionAtul KumarNo ratings yet
- PHYED 131 Last ActivityDocument1 pagePHYED 131 Last ActivityKerby Jean GumallaoiNo ratings yet
- Cohesion and adhesion determine properties of liquidsDocument5 pagesCohesion and adhesion determine properties of liquidsbintang ronauliNo ratings yet
- Chemistry Properties of WaterDocument14 pagesChemistry Properties of WaterKate PerojaNo ratings yet
- Phy 105 - 4 Solids-ADocument6 pagesPhy 105 - 4 Solids-AalakaolamuhammadNo ratings yet
- Investigación EnvironmentalDocument2 pagesInvestigación EnvironmentaleliezerNo ratings yet
- Ministry of Higher Education & Scientific Research Misan University Engineering College Petroleum DepartmentDocument11 pagesMinistry of Higher Education & Scientific Research Misan University Engineering College Petroleum DepartmentMohammad MakeyNo ratings yet
- 03 Module 2 Lesson 1 Physical Properties of WaterDocument13 pages03 Module 2 Lesson 1 Physical Properties of WaterBruce GreenNo ratings yet
- Tema 2 MEDocument5 pagesTema 2 MEMiranda Urbina OlivaresNo ratings yet
- How surface tension allows insects to float on waterDocument1 pageHow surface tension allows insects to float on wateryashsodhaniNo ratings yet
- ProjectDocument2 pagesProjectRauhweltNo ratings yet
- Force Solid LiquidDocument2 pagesForce Solid LiquideliezerNo ratings yet
- Surface TensionDocument126 pagesSurface Tensionaditya2053No ratings yet
- Surface TensionDocument10 pagesSurface Tensionmohnndm82No ratings yet
- PHY1104 PoM Lecture3Document36 pagesPHY1104 PoM Lecture3kirabirasteven81No ratings yet
- Explore The Concept of Surface Tension and Its Effects On Different Liquids.Document6 pagesExplore The Concept of Surface Tension and Its Effects On Different Liquids.Hanks CaineNo ratings yet
- Chem LiquidsDocument2 pagesChem LiquidsHannah Rae S. RuizolNo ratings yet
- Surface TensionDocument15 pagesSurface TensionAnurup ChattopadhyayNo ratings yet
- Untitled PresentationDocument13 pagesUntitled PresentationKristalyn CabreraNo ratings yet
- Surface Tension Allows Insects to FloatDocument1 pageSurface Tension Allows Insects to FloatVictoria CebanNo ratings yet
- Surface TensionDocument21 pagesSurface TensionVarun Sudarsanan80% (5)
- Trees & Capillary Action: Home ProgramsDocument25 pagesTrees & Capillary Action: Home ProgramsRiddhi KatheNo ratings yet
- Gse Solution9Document3 pagesGse Solution9Nayan PaulNo ratings yet
- Solid-Fluid Interaction With Surface-Tension-Dominant ContactDocument12 pagesSolid-Fluid Interaction With Surface-Tension-Dominant ContacthanzNo ratings yet
- FluidDocument3 pagesFluidreannNo ratings yet
- Surface TensionDocument14 pagesSurface TensionAbhishek TyagiNo ratings yet
- Water Conference HandoutDocument11 pagesWater Conference HandoutRichard AwNo ratings yet
- Surface TensionDocument17 pagesSurface TensionVijaya ChintaNo ratings yet
- F KS3 Walking Water WorksheetDocument8 pagesF KS3 Walking Water Worksheetชาเย็น เย็นชาNo ratings yet
- Chem 2Document61 pagesChem 2jvmijaresNo ratings yet
- Toddle - (A1 2Document44 pagesToddle - (A1 2Youssef SamehNo ratings yet
- Fluid Mechanics FundamentalsDocument139 pagesFluid Mechanics Fundamentalslalu prasadNo ratings yet
- Water IBDocument33 pagesWater IBIsadora PereiraNo ratings yet
- Surface TensionDocument22 pagesSurface Tensionvarun_93No ratings yet
- Mini Proyect The E.CDocument2 pagesMini Proyect The E.CLUIS ROJAS SANTANANo ratings yet
- Surface Tension: Practical General ChemistryDocument4 pagesSurface Tension: Practical General Chemistryعمر الرفاعيNo ratings yet
- GROUP 8 (Surface-Tension)Document7 pagesGROUP 8 (Surface-Tension)jRauzzeNo ratings yet
- Chem 02 - Mod - WK1L2Document2 pagesChem 02 - Mod - WK1L22082862No ratings yet
- General ChemistryDocument6 pagesGeneral ChemistrySheryl ValerosoNo ratings yet
- Surface Tension: Cohesive and Adhesive ForcesDocument6 pagesSurface Tension: Cohesive and Adhesive ForcesAbdelrahman Abo AufNo ratings yet
- Science Involved in Floating Paper Clip Science Experiment GuidelinesDocument4 pagesScience Involved in Floating Paper Clip Science Experiment GuidelinesSHIELA RUBIONo ratings yet
- Surface TensionDocument29 pagesSurface Tensionankithns102No ratings yet
- Pressure Control Valves: Hydraulic ComponentsDocument20 pagesPressure Control Valves: Hydraulic ComponentsrajkumarNo ratings yet
- Cutting Tool Materials CompleteDocument15 pagesCutting Tool Materials CompleterajkumarNo ratings yet
- Pressure Relief ValveDocument3 pagesPressure Relief ValverajkumarNo ratings yet
- Casting & Welding Theory & Questions Altogethre 2013Document66 pagesCasting & Welding Theory & Questions Altogethre 2013Stark029No ratings yet
- Density of Water - Density of Ice - Physics@TutorVistaDocument5 pagesDensity of Water - Density of Ice - Physics@TutorVistarajkumarNo ratings yet
- Wireline Logging GuidelinesDocument3 pagesWireline Logging GuidelinesHamdan HamzahNo ratings yet
- Plusco428 Wireline Products 28 Vis Honey Oil With Inhibitor For Pressure ApplicationDocument7 pagesPlusco428 Wireline Products 28 Vis Honey Oil With Inhibitor For Pressure ApplicationebeNo ratings yet
- SR 6898 1 Tevi de Otel PDFDocument1 pageSR 6898 1 Tevi de Otel PDFCRISTIAN SILVIU IANUCNo ratings yet
- MInirhizotron ThecniquesDocument16 pagesMInirhizotron ThecniquesHector Estrada MedinaNo ratings yet
- Sachtleben Technology for Architectural and Decorative PaintsDocument16 pagesSachtleben Technology for Architectural and Decorative PaintswinsonecNo ratings yet
- Fuels Classification and PropertiesDocument57 pagesFuels Classification and PropertiesChinmay LearningNo ratings yet
- Tabela Aço Inox PDFDocument8 pagesTabela Aço Inox PDFjucalele77No ratings yet
- Aggregate Impact Value TestDocument6 pagesAggregate Impact Value Testnadz_fynazNo ratings yet
- Study of Equipment Presses of Cocoa Powder (Theobroma Cacao, L) To Produce Quality Fat Cocoa and Analysis of The ResultingDocument8 pagesStudy of Equipment Presses of Cocoa Powder (Theobroma Cacao, L) To Produce Quality Fat Cocoa and Analysis of The ResultingAlex Ramadhan SabananyoNo ratings yet
- Welcome To This Course Physics 1Document31 pagesWelcome To This Course Physics 1andrewsiby63No ratings yet
- 2018 04 HPDocument149 pages2018 04 HPVS2712No ratings yet
- Powder Coating at HomeDocument9 pagesPowder Coating at Homepakde jongko100% (1)
- Bamboo Reinforced ConcreteDocument14 pagesBamboo Reinforced ConcreteharisankarNo ratings yet
- Questionnaire On Positive Isolation StandardDocument11 pagesQuestionnaire On Positive Isolation StandardBata JenaNo ratings yet
- Southern Company/MHI Ltd. Plant Barry CCS DemonstrationDocument23 pagesSouthern Company/MHI Ltd. Plant Barry CCS Demonstrationrecsco2100% (1)
- Evaluate Mixing Index in Double Cone MixerDocument5 pagesEvaluate Mixing Index in Double Cone MixernithansaNo ratings yet
- Joule Brayton CycleDocument12 pagesJoule Brayton CyclecaptfoleyNo ratings yet
- Coulombs Law PowerpointDocument48 pagesCoulombs Law PowerpointOmar JeCkNo ratings yet
- Schematic For Potable Water SystemDocument17 pagesSchematic For Potable Water Systemmkchy12No ratings yet
- Investigatory Report WewDocument7 pagesInvestigatory Report WewStephen GaleonNo ratings yet
- Membrane ReactorDocument9 pagesMembrane ReactorAzharuddin Ehtesham FarooquiNo ratings yet
- The Nuclear Reactions Involved in The Synthesis of New ElementsDocument3 pagesThe Nuclear Reactions Involved in The Synthesis of New ElementsChristian Isip67% (3)
- Tests For BricksDocument31 pagesTests For BricksTeddy0% (1)
- Characterisation of Polymer With GCDocument104 pagesCharacterisation of Polymer With GCAmit KumarNo ratings yet
- Vitec 5100 Antiscalant DatasheetDocument1 pageVitec 5100 Antiscalant Datasheetdalton2004No ratings yet
- 1577-Research Results-2795-1-10-20191023Document13 pages1577-Research Results-2795-1-10-20191023Nadia fadlNo ratings yet
- Chemistry Aqa A Level AlkenesDocument21 pagesChemistry Aqa A Level AlkenesAttec OinotnaNo ratings yet
- Catálogo SKODADocument14 pagesCatálogo SKODAruben colqueNo ratings yet
- A Dictionary of ColoursDocument528 pagesA Dictionary of ColoursMohammed Mahfouz78% (9)
- CHEM123 Experiment #9 Write UpDocument4 pagesCHEM123 Experiment #9 Write UpKamil KrawczykNo ratings yet