Professional Documents
Culture Documents
Neurology 02
Uploaded by
Ecaterina ChiriacCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Neurology 02
Uploaded by
Ecaterina ChiriacCopyright:
Available Formats
Approach to acute visual loss
11 : 2
Satish Khadilkar, Pramod Dhonde, Mumbai
Introduction
Acute visual loss is common in clinical practice. The symptom is very disturbing to patients and requires
rapid clinical analysis and therapy, as it can be associated with significant morbidity. Etiologies of acute
visual impairment range from retina to the occipital cortex, resulting in a plethora of presentations.
For example in a case of retinal disease patient may feel as if a curtain is rising or dropping and in
cases of occipital lesions, patients may give history of bumping into the objects without realization.
Also, patients tend to mistake diplopia for visual loss, which needs careful analysis.
Acute visual loss could either be monocular or binocular. Monocular loss is often related to local eye
pathologies as glaucoma, uveitis, retinitis or neurological diseases like optic neuritis (ON) and anterior
ischemic optic neuropathy (AION). Binocular diseases are usually associated with lesions affecting
structures such as optic chiasma, lateral geniculate body, occipital radiations and occipital cortex.
Pain is an important association of visual loss. Painful visual impairment is seen in ON, Tolosa Hunt
Syndrome, orbital apex syndrome, pituitary apoplexy and glaucoma. On the other hand, diseases of
the optic tracts, radiations, occipital cortex and AION tend to be painless. The natural history of the
visual impairment is helpful in the differential diagnosis. Transient visual deficit is seen in vascular
diseases, demyelinations, raised intracranial tension and hyper coagulable states. Lasting deficits are
seen with, strokes, tumors and AION.
Following cases will illustrate various clinical situations of acute visual impairment, encountered
by clinicians.
Case 1
32 yrs old female presented with right sided acute onset painful visual loss which progressed within
2-3 hours. Pain was worse with eye movements. On examination, the visual acuity was 6/60 with
cecocentral scotoma on field testing, right sided relative afferent papillary defect (RAPD) on swinging
flash light test, affected colour vision and normal fundus. Investigations revealed P100 latency of
145 ms on visual evoked potential (VEP), MRI Brain with orbital cuts with fat saturation revealed
right optic nerve enhancement with normal MRI spine. CSF study showed normal glucose with mild
raised proteins with pleocytosis. Patient was started on methylprednisolone (MP) for 5 days with
mild recovery over 3 weeks.
Discussion
The diagnosis is of right optic neuritis. Demyelinating optic neuritis is the most common non
glaucomatous optic neuropathy in young people. The incidence of ON is around 5.1 per 100,000 and
a prevalence of 115 per 100,000.1 Females are more commonly affected than males in approximately
3:1 ratio. Symptoms usually start with acute onset vision loss with progression over hours to days.
Pain is often associated with eye movements, but can be continuous. Pain is a consistent feature, seen
in almost 92% of cases which usually distinguishes ON from other optic neuropathies. Painful visual
loss can also be seen with orbital apex syndrome, but associated extraocular muscle involvement
differentiates this from ON. Dyschromatopsia is common, and patients often report that colors,
554
Approach to Acute Visual Loss
Table 1 : Differentiating features between macular and optic
nerve lesions
Characteristics
Macular lesions
Table 2: Differences between papilloedema and papillitis
Papilloedema
Optic nerve lesions
Reduced visual acuity
Yes
Yes
Metamorphosis
Yes
No
Photostress test (bright Slow recovery
light for 10 seconds)
Rapid and significant recovery
Central scotoma
Yes
Reduced colour vision
Papillitis
Visual acuity
Preserved
Visual field
Enlargement of blind spot; Central scotoma
loss of peripheral vision
Reduced
Colour testing
Normal
Loss of red vision
Pupillary reflex Not affected
RAPD
Yes
Eye movements No pain on movements
Pain on movement
Moderate
Often severe
Laterality
Bilateral
Unilateral
Pain
No
Yes
Fundus
Absent of venous pulsation
Venous pulsations present
RAPD
No (Yes if severe)
Yes
normal and may show pallor later in the illness. Papilloedema
and papillitis can be differentiated as follows [Table 2]
The diagnosis of ON is mainly clinical and some ancillary
tests are required for further evaluation and prognostication.
VEP, CSF study and MRI brain with fat saturation images
with contrast with orbital cuts are the initial investigations
required. Sometimes papilloedema is difficult to differentiate
from papillitis. In such cases differences as shown in Table
2 helps. Optic neuritis is commonly the first demyelinating
event in Multiple sclerosis (MS). MRI of the brain can help
confirm the diagnosis of optic neuritis and helps to stratify the
risk of progression to MS. Those with a normal MRI finding
at the time of optic neuritis diagnosis have a 15% risk of
progression to MS at 5 years, 22% at 10 years, and 25% at
15 years; those with an abnormal brain MRI finding have a
42% risk of progression to MS at 5 years, 56% at 10 years,
and 72% at 15 years.3-5 Additionally aquaporin 4 antibody
testing can be considered for diagnosis of neuromyelitis optica
(NMO) as these can present with ON. Also MRI spine in MS
reveals usually short segment involvement while in NMO it
shows long segment involvement. Treatment options include
methylprednisolone for the acute event and interferons in case
of MS and plasmapheresis in case of NMO.
Fig. 1: Swinging flash light test. In this example, the left optic
nerve is not functioning properly, and there is a paradoxic dilation
of the left pupil as the light is directed into it (left relative afferent
pupillary defect).2
Case 2
particularly red, appear less intense in the affected eye. Visual
acuity may range from 6/6 to complete blindness. Visual field
testing varies from enlargement of blind spot to cecocentral
scotoma to complete blindness. RAPD ipsilateral to visual
loss, seen with optic neuropathy or severe retinal disease (in
which case retina looks abnormal on fundus examination) is
the most important clue to differentiate from other diseases.
ON closely mimics maculopathy and can be differentiated as
follows (Table 1).
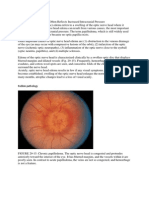
A 74 year old male presented with fever, malaise, weight loss,
progressive headache since 8 weeks, diminution of vision
and inability to open mouth since 5 days. On examination
patient had stable vitals with necrotic patches over scalp, lip
and tongue. Visual acuity revealed only hand movement in
both eyes. Fundus showed left eye anterior ischaemic optic
neuropathy. There was bilateral scalp tenderness with non
pulsatile tender temporal arteries (Fig 1).
RAPD is tested by the swinging flashlight test. This simple
and useful clinical test depicted in Figure 1.
Investigations showed thrombocytosis (8.32 lakhs/cmm), ESR
was 108 mm and C-reactive protein was increased. Temporal
artery biopsy confirmed giant cell arteritis. Discussion
A close clinical differential diagnosis of optic neuritis is
papilloedema. When the disc head is inflamed, the changes
can look close to rise of intracranial pressure. On the other
hand, in cases of retrobulbar neuritis the optic head is often
Optic nerve ischemia occurs most frequently at the optic nerve
head, where structural crowding of nerve fibers and reduction
of the vascular supply may combine to impair perfusion to
a critical degree and produce optic disc edema. The most
555
Medicine Update 2012 Vol. 22
Fig 2: Showing thickened temporal artery with scalp necrosis and AION.
Table 2: Comparison between arteritic and nonarteritic
AION.7
Feature
Arteritic AION
Nonarteritic AION
Age (mean yrs)
70
60
Sex ratio
F>M
M=F
Associated symptoms
Headache, scalp tender- Pain occasionally noted
ness, jaw claudication
Visual acuity
Upto
76%
20/200(6/60)
Disc
Pale > hyperemic oede- Hyperemic
ma
oedema
Cup small
Cup normal
Mean ESR (mm/hr)
70
Natural history
Improvement rare
Improvement in upto
Fellow eye in upto 95% 43%
Fellow eye in < 30%
Treatment
Corticosteroids
females 2 to 6 times more commonly than males. Clinical
criteria most strongly suggestive of giant cell arteritis include
AION, jaw claudication, C-reactive protein above 2.45 mg/
dl, neck pain, and an erythrocyte sedimentation rate of 47
mm/hour or more, in that order. C-reactive protein tend to be
a little more sensitive (100%) than erythrocyte sedimentation
rate (92%) for detection of giant cell arteritis; erythrocyte
sedimentation rate combined with C-reactive protein give the
best specificity (97%).9 American college of rheumatology
(1990) suggested three or more of the following five criteria
to have a specificity of 93.5% and sensitivity of 91.2%.
< Upto 61% > 20/200
(6/60)
>
pale
1. Age of onset greater than 50 years
20-40
2. Onset of new headaches
3. Temporal artery abnormalities (tenderness or reduced
pulsation)
None proved
common such syndrome is AION.6
AION has been divided into arteritic (associated with Giant
cell arteritis) and nonarteritic. Table 2 points out the differences
in the arteritic and non arteritic types.
AION presents with rapid onset of painless, unilateral visual
loss manifested by decreased visual acuity, visual field, or
both. The level of visual acuity impairment varies widely, from
minimal loss to no light perception, and the visual field loss
may conform to any pattern of deficit related to the optic disc.
An altitudinal field defect is most common, but generalized
depression, broad arcuate scotomas, and cecocentral defects
are also uncommonly seen. A relative afferent pupillary defect
invariably is present with monocular optic neuropathy. The
optic disc is edematous at onset, and edema occasionally
precedes visual loss by weeks to months.8
Giant cell arteritis presents in later decades of life, affecting
4. Elevated erythrocyte sedimentation rate (>50 mm/h using the Westergren method)
5. Positive temporal artery biopsy.
Corticosteroids started at the earliest, form the mainstay of
treatment. One may choose not to wait for the biopsy when
visual compromise is seen, as it can lead to permanent visual
loss and neurological complications like strokes. Therapy is
to be continued for at least 1-2 years depending on the clinical
condition. Scalp necrosis seen in the present case is a rare
phenomenon in GCA.
In 9095% of cases, AION is unrelated to temporal arteritis.
The nonarteritic form (NAION) of the disease occurs in a
relatively younger age group (mean age of 60 years) and usually
is associated with less severe visual loss. Frequently, visual
impairment is reported upon awakening, possibly related to
nocturnal systemic hypotension.10 NAION is associated with
556
Approach to Acute Visual Loss
Fig 3: Right occipital infarct
Fig 4: Bilateral occipital subcortical white matter changes suggestive of PRES.
carotid occlusive disease, CNS small vessel disease, diabetes
and hypertension. Diabetic papillopathy characterized by
optic disc edema (unilateral in approximately 60%), only mild
optic nerve dysfunction and type 1 diabetes is of particular
importance in ischaemic optic neuropathies. NAION has
no proven therapy. Lebers hereditary optic neuropathy
(LHON) can also present acutely with vision loss like AION.
LHON progress over a period of time and associated cardiac
abnormalities can be seen with this condition. Fundus in acute
conditions is normal, but presence of telangiectasias favors
LHON. Diagnosis of LHON is achieved by genetic analysis.
Case 3
48 yrs old male, working in a color factory, returned home and
rang the doorbell. When his wife opened the door, he did not
recognize her, apologized and started climbing down. Wife
called him and he immediately recognized her by voice. On
examination his visual acuity was normal, but he was not
able to identify famous personalities. He was not able to mix
and match colors too, which was his job of many years. Final
diagnosis of acute onset prosopagnosia with color agnosia
made. Patient was found to be hypertensive. MRI study
confirmed a right parieto occipital infarct (Fig. 3).
557
Medicine Update 2012 Vol. 22
pressure was 180/110 mm of Hg with normal fundus and
papillary examination. On confrontation she was having left
homonymous hemianopia and MRI Brain was suggestive
of bilateral posterior subcortical white matter changes with
normal angio sequences (Fig 4). Final diagnosis of Antons
syndrome was made as patient was denying blindness as
well as confabulating things, due to posterior reversible
encephalopathy syndrome (PRES). Patient recovered over
2-3 weeks with complete resolution of MRI changes.
Table 3: Cerebral visual loss
Mechanism
Etiologies of vision loss
Vascular
Vertebrobasilar ischemia (PCA territory)
Cerebral anoxia
Cerebral venous thrombosis (superior sagittal sinus)
Hypertensive encephalopathy (posterior reversible encephalopathy syndrome)
Eclampsia
Head trauma
Occipital lobe injury
Occipital mass
Tumor
Abscess
Vascular (aneurysm, arteriovenous malformation)
Hemorrhage
Discussion
PRES is a clinico radiological entity, more prevalent in
women and presents with headache, visual disturbances
(eg, blurry vision, hemianopsia, visual neglect, cortical
blindness), seizures, altered mental status and occasionally
hemiparesis.12 It is usually seen in the setting of moderate
to severe hypertension. Preeclampsia, immunosuppressants
(Tacrolimus, Cyclosporine A), autoimmune diseases (eg,
lupus), sepsis and shock are some of the precursors of
PRES.12-14 Complete recovery of the MRI changes as well as
clinical disability over 2-3 weeks, is common. Compromise
of autoregulatory mechanisms of cerebral blood flow is the
most accepted theory for PRES.14
Demyelinating disease Multiple sclerosis
Infection
Occipital abscess
Meningitis
Progressive multifocal
(PML)
Creuzfeldt-Jacob disease
SSPE
Toxic
Cyclosporine
Tacrolimus
Methanol
Metabolic
Hypoglycemia
Porphyria
Hepatic encephalopathy
leucoencephalopathy
Migraine
Migranous visual aura
Seizure
Occipital lobe seizures [and its causes]
Discussion
In this case, patients inability for face recognition suggested
prosopagnosia. Prosopagnosia is defined as an inability
to recognize faces in the absence of loss of visual acuity
and general intellectual functions. It usually results from
bilateral occipitotemporal infarcts and sometimes right sided
involvement only. Some studies have suggested for object
recognition, left occipitotemporal lobe is activated while for
face recognition, right occipitotemporal lobe is activated.11 In
such cases, prognosis is good with significant recovery over
3-4 months. Cerebral disturbances of vision can be caused
by affection of visual association areas or subcortical white
matter. Alzheimers disease, posterior cortical atrophy and
Creuzfeldt-Jacob disease (Heidenhain variant) can cause
visual agnosias of insidious evolution.
Case 4
A 30 years old pregnant female with history of preeclampsia
on day 3 after uneventful delivery, presented with new onset
headaches and difficulty in vision. She denied blindness
and made up visual sequences [confabulations]. She was
feeling persistence of images after removing her gaze
from objects (palinopsia). She was seeing flashes of lights
(photopsias) and saw relatives in front of her, in their absence
(formed visual hallucinations). On examination her blood
Cortical blindness usually occurs with bilateral PCAocclusions.
Basilar artery thrombosis and vertebral artery dissection tend
to result in such visual loss. Table 3 enumerates etiologies
for cerebral visual loss with underlying mechanisms. The
management of such cases depends on the underlying
etiologies.
Lastly, non organic visual loss, not too uncommon,
needs a special mention.15 There are various clinical and
ophthalmological tests to differentiate it from organic causes.
For example, the patient is instructed to look straight ahead
at the mirror and the mirror is rotated from side to side. Eye
movements indicate that the patient can see his or her own
image in the mirror. Another test is a person who is truly blind
can touch the tips of the fingers properly and a person with
nonorganic visual loss is often unable to touch the fingers
properly. Also positive optokinetic nystagmus indicates
vision of at least 20/400 vision in tested eye.16 Stereopsis,
tangent screen testing and visual evoked potential can aid
in diagnosing nonorganicity. Vision funnels in organic and
tunnels in nonorganic vision loss.
Concluding remarks
As seen from the above discussion, acute vision loss can
be a result of lesions involving anterior or posterior visual
pathways. History and clinical examination are the most
important aspects to help reach the localization. Relevant
hematological tests, followed by neuroimaging help further
differential diagnosis. As visual loss results in decline of
quality of life, early recognition and therapy is of paramount
558
Approach to Acute Visual Loss
importance in the total outlook of recovery.
Examination should start with pin hole testing; if it improves
vision then refractive error is most likely. Visual acuity
testing, confrontation visual testing, pupillary reactions,
ophthalmoscopy, external examination of the eye with a
torchlight and tonometry to measure the intraocular pressure
should be performed in all cases. The following flow diagram
gives the approach towards the visual loss.
Painless
Optic Neuritis
AION
Orbital apex syndrome
Toxic amblyopia
Pituitary apoplexy
LHON
Methanol poisoning
6. ArnoldA.C.:Ischemic optic neuropathies. Ophthalmol Clin North
Am2001;14:83-98.
8. HayrehS.S.:Anterior ischemic optic neuropathy. V. Optic disk
edema an early sign.Arch Ophthalmol1981;99:1030-1040.
Posterior (spares
pupils in most of the
cases)
9. Hayreh SS. Giant cell arteritis: validity and reliability of various
diagnostic criteria. Am J Ophthalmol 1997;123:285-96.
Painful
10. Hayreh SS, Zimmerman BM, Podhajsky PA, Alward WLM. Nocturnal arterial hypotension and its role in optic nerve head and
ocular ischemic disorders. Am J Ophthalmol 1994;117:60324.
9
Painful
5. Optic Neuritis Study Group. Multiple sclerosis risk after optic
neuritis: final optic neuritis treatment trial follow-up. Arch Neurol
2008; 65:72732.
7. Yanoff and Duker Ophthalmology 3rd edition.970.
Approach to acute
vision loss
Anterior (involves
pupils in many
cases)
4. Optic Neuritis Study Group. High- and low-risk profiles for the
development of multiple sclerosis within 10 years after optic neuritis: experience of the optic neuritis treatment trial. Arch Ophthalmol 2003; 121:9449.
Pituitary
9
apoplexy
(Rare, If
postfixed
chiasmal
aection)
Painless
11. Nakamura K, Kawashima R, Sato N et al.Functional delineation
of human occiptotemporal areas related to face and scene processing. A PET study Brain 2000;123:1903-1912.
Hemianopia and
cortical blindness due
to various etiologies
given in Table 3.
12. Hinchey J, Chaves C, Appignani B et al.A reversible posterior leucoencephalopathy syndrome. N Engl J Med 1996;334:494e500.
References
1. Rodriguez M, Siva A, Cross SA, et al. Optic Neuritis, a population-based study in Olmsted County, Minnesota. Neurology
1995;45:24450.
2. Wilhelm H. Neuro-ophthalmology of pupillary function and practical guidelines. J Neurol 1998; 245:57383
3. The 5-year risk of MS after optic neuritis: experience of the optic
neuritis treatment trial. Optic Neuritis Study Group. Neurology
1997; 49:140413.
13. Aranas RM, Prabhakaran S, Lee VH. Posterior reversible encephalopathy syndrome associated with hemorrhage. Neurocrit
Care 2009;10:306e12.
14. Mueller-Mang C, Mang T, Pirker A, et al. Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology 2009;51:373e83.
15. Miller BW. A review of practical tests for ocular malingering and
hysteria. Surv Ophthalmol 1973;17:2416.
16. Biousse V, Newman NJ. Neuro-ophthalmology illustrated. New
York: Thieme; 2009.
559
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Memory Improvement StrategiesDocument3 pagesMemory Improvement StrategiesEcaterina ChiriacNo ratings yet
- (Practical) OphthalmosDocument32 pages(Practical) OphthalmosHoriana BusuiocNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Guidelines On Vertigo and DizzinessDocument59 pagesGuidelines On Vertigo and DizzinessEcaterina ChiriacNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Rapid Diagnosis in Ophthalmology Series - Neuro-Ophthalmology-0323044565Document241 pagesRapid Diagnosis in Ophthalmology Series - Neuro-Ophthalmology-0323044565ahsananwer100% (6)
- AAO Network NeuroOphthalmologyDocument59 pagesAAO Network NeuroOphthalmologySafa Abdualrahaman Ali Hamad100% (1)
- Cerebrospinal Fluid: Anatomy Physiology and Utility of An Examination in Disease StatesDocument52 pagesCerebrospinal Fluid: Anatomy Physiology and Utility of An Examination in Disease StatesChanwit Chaisuriyaphun100% (2)
- NeurosurgeryDocument38 pagesNeurosurgeryakufahaba100% (2)
- Papiledema & Neuritis OpticDocument42 pagesPapiledema & Neuritis OpticNadine Wang0% (1)
- NCP Hydrocephalus With RationaleDocument42 pagesNCP Hydrocephalus With RationaleJulienne Sanchez-Salazar80% (15)
- Neuro OphthalmologyDocument23 pagesNeuro Ophthalmologysk100% (1)
- Twenty Classic Signs in NeuroradiologyDocument11 pagesTwenty Classic Signs in NeuroradiologyEcaterina ChiriacNo ratings yet
- Eye Movement Abnormalities in Stiff Person SyndromeDocument3 pagesEye Movement Abnormalities in Stiff Person SyndromeEcaterina ChiriacNo ratings yet
- Disorders of Optic Nerve and Visual Pathways: Ipek MidiDocument24 pagesDisorders of Optic Nerve and Visual Pathways: Ipek MidiEcaterina ChiriacNo ratings yet
- Cerebral AsymmetryDocument13 pagesCerebral AsymmetryEcaterina ChiriacNo ratings yet
- Dizziness QuestionnaireDocument1 pageDizziness QuestionnaireEcaterina ChiriacNo ratings yet
- Vertigo/Dizziness/Imbalance Questionnaire: Andrew Marlowe, MD, Pa OtolaryngologyDocument2 pagesVertigo/Dizziness/Imbalance Questionnaire: Andrew Marlowe, MD, Pa OtolaryngologyEcaterina ChiriacNo ratings yet
- Neurovascular Coupling in The Normal Brain and in Hypertension, Stroke, and Alzheimer DiseaseDocument8 pagesNeurovascular Coupling in The Normal Brain and in Hypertension, Stroke, and Alzheimer DiseaseEcaterina ChiriacNo ratings yet
- Brain 2010 Mills 3458 69Document12 pagesBrain 2010 Mills 3458 69Ecaterina ChiriacNo ratings yet
- Vertigo/Dizziness/Imbalance Questionnaire: Andrew Marlowe, MD, Pa OtolaryngologyDocument2 pagesVertigo/Dizziness/Imbalance Questionnaire: Andrew Marlowe, MD, Pa OtolaryngologyEcaterina ChiriacNo ratings yet
- A Systematic Review of Vertigo in Primary Care: Karena Hanley, Tom O' Dowd and Niall ConsidineDocument6 pagesA Systematic Review of Vertigo in Primary Care: Karena Hanley, Tom O' Dowd and Niall ConsidineEcaterina ChiriacNo ratings yet
- NC 3 Rs Arrive Guidelines 2013Document2 pagesNC 3 Rs Arrive Guidelines 2013Ecaterina ChiriacNo ratings yet
- Idiopathic Intracranial Hypertension in University Hospital in Saudi Arabia, Retrospective StudyDocument6 pagesIdiopathic Intracranial Hypertension in University Hospital in Saudi Arabia, Retrospective StudyIJAR JOURNALNo ratings yet
- Optic AtrophyDocument40 pagesOptic Atrophypriya0% (1)
- Idiopathic Intracranial Hypertension: Deborah I. Friedman, MD and Daniel M. Jacobson, MDDocument8 pagesIdiopathic Intracranial Hypertension: Deborah I. Friedman, MD and Daniel M. Jacobson, MDNouchan NoupNo ratings yet
- J Neurol Neurosurg Psychiatry 2012 Biousse 488 94Document8 pagesJ Neurol Neurosurg Psychiatry 2012 Biousse 488 94siscaNo ratings yet
- Acute Bacterial MeningitisDocument20 pagesAcute Bacterial Meningitisnight.shadowNo ratings yet
- Neuro-Ophthalmology: Bethlehem Girma (M.D) Assistant Professor of OphthalmologyDocument117 pagesNeuro-Ophthalmology: Bethlehem Girma (M.D) Assistant Professor of Ophthalmologyhenok birukNo ratings yet
- #Complications of Suppurative Otitis MediaDocument8 pages#Complications of Suppurative Otitis MediaameerabestNo ratings yet
- Idiopathic Intracranial Hypertension PresentationDocument16 pagesIdiopathic Intracranial Hypertension PresentationR DruNo ratings yet
- The Optic NerveDocument7 pagesThe Optic NerveEdwin DarmawanNo ratings yet
- Otogonic Intracranial ComplicationsDocument20 pagesOtogonic Intracranial ComplicationsROXTA RAHULNo ratings yet
- Acute Multifocal Placoid Pigment EpitheliopathyDocument8 pagesAcute Multifocal Placoid Pigment EpitheliopathyremotmNo ratings yet
- Neuro-Ophthalmology Review: by Peter Mark G. Chao, MD May 5, 2011Document48 pagesNeuro-Ophthalmology Review: by Peter Mark G. Chao, MD May 5, 2011JL BillsNo ratings yet
- Cefalea LancetDocument2 pagesCefalea LancetJohana Zamudio RojasNo ratings yet
- Idiopathic Intracranial Hypertension (By Dr. Rakshay)Document51 pagesIdiopathic Intracranial Hypertension (By Dr. Rakshay)Rakshay KaulNo ratings yet
- Approach To Space Occupying LesionDocument14 pagesApproach To Space Occupying LesionFatima JamshaidNo ratings yet
- CEFALEA Evaluation of Headache in Adults - UpToDateDocument23 pagesCEFALEA Evaluation of Headache in Adults - UpToDateJOSE ANTONIO ALBARRAN MENDOZANo ratings yet
- Differential Diagnosis of Papilledema - UpToDateDocument28 pagesDifferential Diagnosis of Papilledema - UpToDateDaniel PadillaNo ratings yet
- Clinical Manifestations and Diagnosis of Central Nervous System Tumors in ChildrDocument19 pagesClinical Manifestations and Diagnosis of Central Nervous System Tumors in ChildrNadia BaxNo ratings yet
- Papiledema 3Document27 pagesPapiledema 3nellieauthorNo ratings yet
- Papilloedema and Optic AtrophyDocument27 pagesPapilloedema and Optic AtrophyRobert MontgomeryNo ratings yet
- Fundus Changes in Pregnancy Induced HypertensionDocument4 pagesFundus Changes in Pregnancy Induced HypertensionRamesh BabuNo ratings yet