Professional Documents
Culture Documents
Fibrosing Inflammatory Pseudotumors Involving The Skull Base: MR and CT Manifestations With Histopathologic Comparison
Uploaded by
Nicolas SaputraOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fibrosing Inflammatory Pseudotumors Involving The Skull Base: MR and CT Manifestations With Histopathologic Comparison
Uploaded by
Nicolas SaputraCopyright:
Available Formats
Fibrosing Inflammatory Pseudotumors Involving the Skull Base:
MR and CT Manifestations with Histopathologic Comparison
Moon Hee Han, Je G. Chi, Myung Soon Kim, Kee Hyun Chang, Kwang Hyun Kim, Kyung Mo Yeon, and
Man Chung Han
PURPOSE: To describe the MR and CT features of fibrosing inflammatory pseudotumors of the
skull base region, and to document the MR signal intensity of the lesions with histopathologic
comparison. METHODS: We reviewed the MR and CT studies of five patients with pathologically
proved fibrosing inflammatory pseudotumor involving the skull base. Unenhanced spin-echo T1and T2-weighted and contrast-enhanced T1-weighted MR images were obtained at 0.5 T in three
patients and at 1.5 T in two patients. MR findings were correlated with histopathologic findings in
all five cases, and the enhancement pattern was compared with CT findings in three cases.
RESULTS: In three cases, the cavernous sinus was involved unilaterally, with adjacent extracranial
infiltrative masses. In one case, both orbits, the cavernous sinuses, and the tentorium were involved
with diffuse infiltrative lesions. One patient had an infiltrative nasopharyngeal mass; and in all five
patients, MR images showed localized involvement of the skull base, with bone marrow replaced by
tumor. The soft-tissue lesions were hypointense on T2-weighted images in all five cases and
showed homogeneous contrast enhancement. Histopathologic studies revealed scanty inflammatory cell infiltration with densely fibrotic background in all cases. The hypointensity of the lesions
on T2-weighted images seemed to be related to the degree of fibrosis. CONCLUSION: Fibrosing
inflammatory pseudotumor shows characteristic MR findings of infiltrative lesion with bone destruction and hypointensity on T2-weighted images. The lack of mobile protons due to the fibrotic
background and/or high cellularity of the lesions may be the reason for their hypointensity and
weaker enhancement on MR images.
Index terms: Skull, base; Skull, neoplasms; Head, inflammation
AJNR Am J Neuroradiol 17:515521, March 1996
Inflammatory pseudotumors of the head and
neck are idiopathic, inflammatory lesions that
most commonly involve the orbit (1, 2). They
include a diverse group of lesions characterized
by inflammatory cell infiltration and variable fibrotic responses. In addition to the orbit, other
areas of involvement include the larynx, the
paranasal sinuses, and the soft tissues of the
neck and esophagus (3 6). Similar lesions
have been described in other areas, such as the
lungs (7).
Inflammatory pseudotumor involving the
skull base is rare. The purpose of this article is
to describe the magnetic resonance (MR) and
computed tomographic (CT) features of five
cases of pathologically proved fibrosing inflammatory pseudotumor involving the skull base
and to compare the MR signal intensities of the
lesions with histopathologic findings.
Received April 5, 1995; accepted after revision September 26.
Presented at the annual meeting of American Society of Neuroradiology, Nashville, Tenn, May 1994.
Supported by grant no. 0294 189 from the Seoul National University
Hospital Research Fund.
From the Departments of Diagnostic Radiology (M.H.H., K.H.C.,
K.M.Y., M.C.H.), Pathology (J.G.C.), and Otolaryngology (K.H.K.), Seoul
(Korea) National University College of Medicine; and the Department of
Diagnostic Radiology, Wonju (Korea) Medical College (M.S.K.).
Address reprint requests to Moon Hee Han, MD, Department of Diagnostic Radiology, Seoul National University Hospital, 28 Yeongun-dong,
Chongro-ku, Seoul 110 744, Korea.
Materials and Methods
We reviewed the MR and CT studies of five patients with
pathologically proved fibrosing inflammatory pseudotumor involving the area of the central skull base. Clinical
and radiologic features of the patients are summarized in
the Table. All five patients had long-standing (4 months to
AJNR 17:515521, Mar 1996 0195-6108/96/17030515
q American Society of Neuroradiology
515
516
HAN
AJNR: 17, March 1996
Clinical and radiologic findings in five patients with fibrosing inflammatory pseudotumors of the skull base
Degree of Signal Intensity
on MR Images
Involvement
Case
Age, y/
Sex
Signs and
Symptoms
Imaging
Studies
Region of
Tumor
Bone
38/M
30/M
36/M
57/M
25/M
Ophthalmoplegia
Facial nerve
palsy
Ophthalmoplegia
Trigeminal
neuralgia
Hearing loss
MR (0.5 T),
CT
MR (1.5 T),
CT
MR (0.5 T),
CT
MR (0.5 T)
MR (1.5 T)
Dura
Histopathologic
Findings
T1T2Contrast
Fibrosis Cellularity
Weighted Weighted Enhanced
MCF
Iso
Sl hypo
11
Nasopharynx Clivus
MCF
Iso
Sl hypo
11
11
11
CS, ITF, orbit Clivus
Tentorium
Sl hypo
Mk hypo
11
111
CS, ITF,
brain
CS, ITF
Sphenoid
MCF
Sl hypo
Mk hypo
11
111
Sphenoid
MCF, PC
Mk hypo
Mk hypo
11
CS, ITF
Sphenoid
Note.CS indicates cavernous sinus; ITF, infratemporal fossa; MCF, middle cranial fossa; PC, petroclival; iso, isointensity; sl hypo, slight
hypointensity; mk hypo, marked hypointensity; 1, mild; 11, moderate; and 111, severe.
2 years) signs and symptoms of various cranial nerve
dysfunction. No patient had a history of craniofacial surgery or specific infection; and none had diabetes mellitus
at the time of diagnosis. Patients with histopathologic findings of prominent plasma cell infiltration that were diagnosed as plasma cell granulomas were excluded from the
study.
All five patients were examined with MR imaging at 1.5
T (two cases; Magnetom 1.5, Siemens, Germany, and GE
Signa, General Electric, Milwaukee, Wis) or at 0.5 T (three
cases; Supertech-5000, GoldStar, Korea, and Gyroscan,
Philips, the Netherlands). Spin-echo T1-weighted 500/12
30/4 (repetition time [TR]/echo time [TE]/excitations) and
long-TR/short- and long-TE (2500 3000/30, 90/2) images were obtained. Contrast-enhanced T1-weighted images in axial and coronal planes were obtained after intravenous injection of gadopentetate dimeglumine (0.1
mmol/kg; Magnevist, Schering, Germany) in all cases.
Three of five patients were also examined with CT (GE
9800, General Electric). Contrast-enhanced axial and
coronal scans were obtained with 3- to 5-mm section
thickness.
MR findingsincluding intracranial changes in signal
intensity of the lesions and bonewere described, and
enhancement patterns were compared with CT findings in
three cases. The degree of fibrosis and the extent of inflammatory cell infiltration were evaluated and were
graded relative to findings at histopathologic examination,
and these findings were then compared with MR signal
intensity patterns in all cases.
Results
In four of the five patients studied (cases 1, 3,
4, and 5), the muscles of mastication were involved unilaterally by infiltrative mass lesions
(Fig 1). In three patients (cases 1, 4, and 5), the
cavernous sinus was involved unilaterally with
adjacent extracranial infiltrative masses. In one
patient (case 3), both orbits, the cavernous si-
nuses, and the edges of the tentorium of cerebellum were involved with diffuse and symmetric infiltrative lesions (Fig 2). One patient (case
2) had an infiltrative nasopharyngeal mass involving the stylomastoid foramen (Fig 3).
In all five cases, localized involvement of the
skull base was seen on MR images where tumor
replaced the signal of the fatty bone marrow; in
two of these, the bone lesions were also seen on
CT scans. The greater wing of the sphenoid
bone was involved unilaterally in three patients
(cases 1, 4, and 5; Fig 1), and the clivus was
involved in two patients (cases 2 and 3; Figs 2
and 3). One patient (case 2) also had unilateral
involvement of the mastoid bone (Fig 3).
The soft-tissue mass lesions were hypointense relative to brain on both T1- and T2weighted images in three patients (cases 3, 4,
and 5; Figs 1 and 2). In two patients (cases 1
and 2), the lesions were isointense with brain on
T1-weighted images and hypointense relative
to brain on T2-weighted images (Fig 3). In all
cases, the lesions of the soft tissues and bones
showed homogeneous enhancement on MR images after injection of contrast material. In one
patient with lesions of both orbits (case 3), contrast-enhanced CT scans showed greater enhancement of the retrobulbar soft-tissue lesions
than did the MR images, which showed slight
enhancement (Fig 2).
Thickening and enhancement of the intracranial dural structures adjacent to the lesions were
seen on contrast-enhanced MR images in all
patients. In four patients (cases 1, 2, 4, and 5),
dural involvement was seen in the unilateral
middle cranial fossa, and the tentorium was involved in one patient (case 3). In one patient
AJNR: 17, March 1996
PSEUDOTUMORS
517
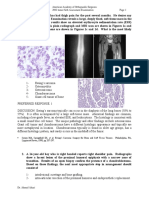
Fig 1. Case 4: 57-year-old man with trigeminal neuralgia.
A, T1-weighted (500/20) axial MR image at the level of the maxillary sinus shows a poorly defined infiltrative lesion with intermediate
intensity in the left infratemporal fossa. Fat planes of the left pterygopalatine fossa and the planes of the muscles of mastication are
obliterated. Posterior wall of the left maxillary sinus is also involved with obliteration of the retromaxillary fat pad.
B, T2-weighted (3000/90) axial MR image shows very low signal intensity of the left infratemporal fossa lesion. The lesion within the
left maxillary sinus has very high signal intensity, suggesting inflammatory mucosal thickening.
C, Contrast-enhanced T1-weighted axial MR image at the same level as A shows diffuse and strong enhancement of the left
infratemporal fossa lesion. Posterior wall of the left maxillary sinus, pterygopalatine fossa, and pterygoid plates are also enhanced.
D, T2-weighted axial MR image at the level of the midorbit shows a localized hyperintense lesion in the medial left temporal lobe of
the brain.
E, Contrast-enhanced T1-weighted axial MR image at the same level as D shows diffuse dural enhancement of anteromedial aspect
of the left middle cranial fossa with a focal nodular enhancement in the left temporal cortex (arrow). An area of hypointensity is seen in
surrounding parenchyma of the left temporal lobe.
(case 4), localized brain swelling, hyperintensity on T2-weighted images, and focal cortical
enhancement were seen in the adjacent anterior
temporal lobe (Fig 1).
Histopathologic specimens were obtained in
all patients by open surgical biopsy of the softtissue mass lesions. Histopathologic examinations revealed lymphocyte and plasma cell infiltration and densely fibrotic changes with
collagen deposition and fibroblastic activity in
all cases (Figs 2E and 3G). The extent of collagen deposition, fibroblastic activity, and inflammatory cell infiltration of the lesions was
variable. The degree of fibrotic changes of the
lesions seemed to vary inversely with the inflammatory cell infiltration. Signal intensity of
the lesions was not closely related to the degree
of fibrosis or cellularity found on histopathologic
specimens (Table). In one patient, in whom
slight enhancement of the lesion was seen on
MR images (case 2), prominent fibrosis and
scanty inflammatory cell infiltration were apparent at microscopic examination (Fig 2E).
Microbiological studies were done during surgery or biopsy in all patients, but no specific
microorganism was isolated. Similarly, no pa-
518
HAN
AJNR: 17, March 1996
Fig 2. Case 3: 36-year-old man with a 4-month history of ophthalmoplegia.
A, Contrast-enhanced CT scan at the level of the midorbit shows total obliteration of the bilateral retrobulbar fat spaces, which are
replaced by homogeneously enhancing lesion. Bilateral cavernous sinuses are involved by the lesion, and the edges of the tentorium of
cerebellum are thickened and enhanced with mild irregularity in the left posterior aspect (arrow).
B, T2-weighted (2500/100) axial MR image shows diffuse hypointensity of the orbital and cavernous sinus lesions.
C, T1-weighted (500/30) sagittal image at the left cavernous sinus plane shows the clivus replaced by hypointense lesion (arrowheads).
D, Contrast-enhanced T1-weighted axial image at the same level as B shows slight enhancement of the orbital, cavernous sinus, and
tentorial lesions. The degree of lesion enhancement appears weaker than that shown by CT, especially on the right side.
E, Histopathologic section shows scanty inflammatory cell infiltration with dense collagen deposition, suggesting severe fibrosis
(hematoxylin-eosin stain, magnification 3100).
tient had evidence of fungal infection at microscopic examination.
After pathologic diagnosis, the patients were
treated with extended steroid therapy for more
than a month without any objective improvement or progression of cranial nerve dysfunctions. In one patient (case 3), a follow-up CT
scan was obtained after 6 months of steroid
therapy and showed no change in the extent of
the lesion.
Discussion
Inflammatory pseudotumor of the head and
neck is most often encountered as a benign,
chronic, inflammatory lesion of the orbit with no
known origin. Although some investigators
have proposed a relationship between adjacent
sinus inflammation and the occurrence of
pseudotumor (8), findings in at least one large
series have failed to document this association
(9). The existence of many synonyms for inflammatory pseudotumor and the varied histopathologic findings of this process suggest
that this is not a single disease entity but rather
an umbrella term for any nonspecific chronic
inflammatory mass lesion (10). So a disease
with similar histopathologic findings of inflammatory pseudotumor located in a different site
is thought to be a different disease with a different clinical course.
In some cases of inflammatory pseudotumor,
temporal progression from acute inflammatory
infiltration to chronic fibrosing process has been
AJNR: 17, March 1996
PSEUDOTUMORS
519
Fig 3. Case 2: 30-year-old man with 1-year history of facial palsy.
A, T1-weighted (500/20) axial MR image at the level of the nasopharynx shows a poorly defined mass with intermediate intensity in
the right nasopharynx. Right parapharyngeal fat is partially obliterated. Note decreased signal intensity of the right mastoid process and
adjacent occipital bone.
B, T2-weighted (3000/90) axial MR image shows hypointensity of the right nasopharyngeal lesion.
C, Contrast-enhanced T1-weighted axial MR image shows diffuse enhancement of the lesion. The right internal carotid artery is
encased by the enhancing lesion and the right stylomastoid foramen is also enhanced (arrow). Right mastoid process and adjacent
occipital bone are enhanced as compared with unenhanced image (A).
D, Contrast-enhanced T1-weighted coronal MR image shows diffuse enhancement of the right nasopharyngeal lesion and the dural
surface of the right middle cranial fossa.
E, Unenhanced T1-weighted sagittal MR image shows localized replacement of the clival bone marrow with adjacent nasopharyngeal
lesion.
F, Axial CT scan with bone window setting shows no significant abnormality in trabecular pattern of the clivus.
G, Histopathologic section shows diffuse inflammatory cell infiltration with a background of fibroblastic activity. Collagen deposition
in this case seems to be less than that of case 3 (Fig 2) and cellularity of the lesion seems much higher (hematoxylin-eosin stain,
magnification 3100).
520
HAN
observed (2, 11, 12). The histopathologic findings
in our patients may reflect a change from inflammatory to chronic pseudotumor, and their long
histories of signs and symptoms may support this
chronicity. However, we found no relationship between the duration of signs and symptoms and
the degree of fibrosis in our series.
In most cases of orbital pseudotumor, the lesion is confined to the orbit without invasion of the
bony walls (13). But in several reported cases of
inflammatory pseudotumor of the maxillary sinus
or infratemporal fossa, CT scans showed localized
bone destruction (3, 4, 14, 15). In cases in which
there is bone destruction, the distinction between
pseudotumor and malignant neoplasm is very difficult without biopsy.
In all our cases, MR images showed extracranial soft-tissue lesions infiltrating the skull base
with intracranial dural involvement. In general,
these MR findings are strongly suggestive of
malignant tumor. CT findings of enlarged muscles with preserved fascial planes between them
has been reported as a suggestive finding of
inflammatory-type lesions (5). In our series,
however, fat planes within involved areas were
obliterated, and this finding was not helpful for
the differential diagnosis (Fig 1). The skull base
changes seen in our cases cannot be distinguished from those of malignant bone invasion,
and malignant tumor must be included in the
differential diagnosis of lesions of the central
skull base.
Changes of adjacent brain parenchyma with
localized enhancement and surrounding edema
were shown on MR images in one patient (case
4) in our series. This is also a finding suggestive
of an aggressive lesion, and may be a reactive
change to an adjacent dural lesion or to direct
parenchymal invasion. In this case, the diagnosis was confirmed by results of an open biopsy
of the infratemporal fossa lesion, and the brain
lesion was not confirmed pathologically. This
case may represent brain invasion by inflammatory pseudotumor.
A common MR finding among our cases was
hypointensity of the lesion on T2-weighted images. T2 hypointensity of a soft-tissue lesion is
thought to be characteristic of fibrosing inflammatory pseudotumor. This hypointensity may
be explained by a relative lack of both free water
and mobile protons within fibrotic lesions. Diffuse fibrotic changes with various degrees of
collagen deposition were seen at microscopic
examination in all our cases. Calcification is a
AJNR: 17, March 1996
possible cause of T2 hypointensity of a lesion,
but we saw no calcification on CT scans or
histopathologic specimens.
On MR images, mobile protons are needed
for enhancement of the lesion, and a fibrotic or
hypercellular lesion enhances weakly. In one
patient in our series (case 3), the lesion showed
weaker enhancement on MR images than on CT
scans, which can be explained by the densely
fibrotic background of the lesion (Fig 2). There
was no apparent difference in the contrast enhancement pattern on MR images and CT scans
in the two other patients who were examined
with both techniques.
It has been reported that high-grade malignant tumors of the parotid gland may appear
hypointense on T2-weighted MR images (16).
This signal characteristic presumably reflects
the lack of serous and mucinous products, the
high mitotic ratio, and the high nuclear-tocytoplasmic ratios that are characteristic of
high-grade malignant lesions of the parotid
gland (16). If such a lesion were to occur at the
skull base, it would be very difficult to distinguish from a lesion with fibrotic background,
like those in our cases. Primary malignant tumors of the central skull base, such as chordomas or chondrosarcomas; direct invasion from
nasopharyngeal carcinomas; and metastatic
malignant tumors from distant primary sites are
the most common malignant tumors of the skull
base. The signal intensity of these lesions is
usually isointense on T1-weighted images and
isointense or slightly hyperintense on T2weighted images.
Postinflammatory or postoperative fibrotic
scars may also appear hypointense on T2weighted images, but these entities usually do
not show mass effects or bone destruction, and
our patients had no history of surgery or specific
inflammation of the head and neck.
Every patient in our series had signs or symptoms of various cranial nerve dysfunctions that
were referable to MR findings. Ophthalmoplegia
in two patients (cases 1 and 3) could be explained by the cavernous sinus involvement
(Fig 2), trigeminal neuralgia (case 4) by an
infiltration of the cranial base (Fig 1), facial
palsy (case 2) by an involvement of the stylomastoid foramen (Fig 3), and hearing loss (case
5) by a thickening of the petroclival dura, including the internal auditory meatus. In every
patient, treatment with oral corticosteroid therapy for more than a month after pathologic
AJNR: 17, March 1996
diagnosis produced no objective improvement
in cranial nerve dysfunction. We do not think
that steroid therapy is helpful to improve dysfunction relating to long-standing fibrotic lesions such as these.
In summary, fibrotic inflammatory pseudotumor with involvement of the skull base may
mimic malignant tumor with skull base invasion. MR imaging shows characteristic findings
of infiltrative soft-tissue lesions, with bone destruction and hypointensity on T2-weighted images. A lack of mobile protons due to the
densely fibrotic background of these lesions
may account for this T2 hypointensity and
weaker enhancement on MR images.
References
1. Chavis RM, Garner AG, Wright JE. Inflammatory orbital pseudotumors. Arch Ophthalmol 1978;96:18721922
2. Heersink B, Ridrigues MR, Flanagan JC. Inflammatory pseudotumor of the orbit. Ann Ophthalmol 1977;9:1729
3. Som PM, Brandwein MS, Maldjian C, Reino AJ, Lawson W. Inflammatory pseudotumor of the maxillary sinus: CT and MR findings in
six cases. AJR Am J Roentgenol 1994;163:689 692
4. Weisman RA, Osguthorpe JD. Pseudotumors of the head and neck
masquerading as neoplasia. Laryngoscope 1988;98:610 614
PSEUDOTUMORS
521
5. Keen M, Conley J, McBride T, Mutter G, Silver J. Pseudotumor of
the pterygomaxillary space presenting as anesthesia of the mandibular nerve. Laryngoscope 1986;96:560 563
6. Hytiroglou P, Brandwein MS, Strauchen JA, Mirante JP, Urken ML,
Biller HF. Inflammatory pseudotumor of the parapharyngeal
space: case report and review of the literature. Head Neck 1992;
14:230 234
7. Berardi RJ, Lee SS, Chen HP, Stines GJ. Inflammatory pseudotumors of the lungs. Surg Gynecol Obstet 1983;156:89 96
8. Eshaghian J, Anderson RL. Sinus involvement in inflammatory
orbital pseudotumor. Arch Ophthalmol 1981;99:627 630
9. Blodi FC, Gass JD. Inflammatory pseudotumor of the orbit. Trans
Am Acad Ophthalmol Otolaryngol 1967;71:303323
10. Seider MJ, Cleary KR, van Tassel P, et al. Plasma cell granuloma
of the nasal cavity treated by radiation therapy. Cancer 1991;67:
929 932
11. Tang TT, Segura AD, Oechler HW, et al. Inflammatory myofibrohistiocytic proliferation simulating sarcoma in children. Cancer
1990;65:1626 1634
12. West SG, Pittman DL, Coggin JT. Intracranial plasma cell granuloma. Cancer 1980;46:330 335
13. Curtin HD. Pseudotumor. Radiol Clin North Am 1987;25:583599
14. Takimoto T, Kathoh T, Ohmura T, Kamide M, Nishimura T,
Umeda R. Inflammatory pseudotumor of the maxillary sinus mimicking malignancy. Rhinology 1990;28:123127
15. Garcia F de M, Aiguabella MAB, Liesa RF, Esteban JR, Aseno
RMBY, Gonzalez EAV. Pseudotumor inflammatorio de senos paranasales. Acta Otorrinolaringol Esp 1990;41:351353
16. Som PM, Biller HF. High-grade malignancies of the parotid
gland: identification with MR imaging. Radiology 1989;173:
823 826
You might also like
- MR Imaging Characteristics of Tuberculous Spondylitis Vertebral OsteomyelitisDocument7 pagesMR Imaging Characteristics of Tuberculous Spondylitis Vertebral OsteomyelitislewienNo ratings yet
- CT and MRI features of spinal tuberculosisDocument5 pagesCT and MRI features of spinal tuberculosisArief RachmanNo ratings yet
- Angioleiomyoma in Soft Tissue of Extremities: MRI FindingsDocument4 pagesAngioleiomyoma in Soft Tissue of Extremities: MRI FindingsLuis Javier del PozoNo ratings yet
- P ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 2000)Document9 pagesP ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 2000)zano_adamNo ratings yet
- 0068KJR - KJR 10 121Document8 pages0068KJR - KJR 10 121Astrid GayatriNo ratings yet
- Primary Extraskeletal Ewing Sarcoma of The Sinonasal Tract - A Rare Case Report and Review of The LiteratureDocument7 pagesPrimary Extraskeletal Ewing Sarcoma of The Sinonasal Tract - A Rare Case Report and Review of The LiteraturemohammadsayfooNo ratings yet
- Disk Displacement, Eccentric Condylar Position, OsteoarthrosisDocument10 pagesDisk Displacement, Eccentric Condylar Position, OsteoarthrosisAlejandro RuizNo ratings yet
- Aims: Tuberculous Infection of The Thoracic Cage Is Rare and Is Difficult To Discern Clinically or OnDocument4 pagesAims: Tuberculous Infection of The Thoracic Cage Is Rare and Is Difficult To Discern Clinically or OnSandy KurniawanNo ratings yet
- Juvenile Ossifying Fibroma of The Mandible: A Case ReportDocument7 pagesJuvenile Ossifying Fibroma of The Mandible: A Case Reportsagarjangam123No ratings yet
- Tumores en CuelloDocument6 pagesTumores en CuellokalixinNo ratings yet
- Ewing'S Sarcoma: Imaging FeaturesDocument9 pagesEwing'S Sarcoma: Imaging FeaturesRachelMayaMalauNo ratings yet
- 1 s2.0 S0720048X16300286Document6 pages1 s2.0 S0720048X16300286Yuda ArifkaNo ratings yet
- MRI findings of TMJ synovial chondromatosisDocument7 pagesMRI findings of TMJ synovial chondromatosiscgarciNo ratings yet
- Radiologic and Clinical Findings in Tuberculous MeningitisDocument6 pagesRadiologic and Clinical Findings in Tuberculous MeningitisAustine OsaweNo ratings yet
- CondyleDocument1 pageCondyleVenkata Ramana Murthy VasupilliNo ratings yet
- Incidental Findings of The Lumbar Spine at MRI During Herniated Intervertebral Disk Disease EvaluationDocument5 pagesIncidental Findings of The Lumbar Spine at MRI During Herniated Intervertebral Disk Disease EvaluationDyah Gaby KesumaNo ratings yet
- MR IntracranIal menIngIomas!!! Vazno!!!Document5 pagesMR IntracranIal menIngIomas!!! Vazno!!!StefanNo ratings yet
- 1369 Full PDFDocument9 pages1369 Full PDFIqbal AbdillahNo ratings yet
- Ameloblastoma of The Jaw and Maxillary Bone: Clinical Study and Report of Our ExperienceDocument7 pagesAmeloblastoma of The Jaw and Maxillary Bone: Clinical Study and Report of Our ExperienceKharismaNisaNo ratings yet
- Cefelometria y ATMDocument7 pagesCefelometria y ATMSharon Tolentino VidalNo ratings yet
- Diagnosis of Brown Sequard SyndromeDocument3 pagesDiagnosis of Brown Sequard SyndromeOktavianus PrayitnoNo ratings yet
- Case Report on Chondrosarcoma of the Temporomandibular JointDocument9 pagesCase Report on Chondrosarcoma of the Temporomandibular JointCatalina Soler LioiNo ratings yet
- CRTC1/MAML2 Fusion Gene in Rare Mandibular CancerDocument6 pagesCRTC1/MAML2 Fusion Gene in Rare Mandibular CancerDanielaNo ratings yet
- 1995 MR Imaging of Solid Cerebellar Tumors in AdultDocument6 pages1995 MR Imaging of Solid Cerebellar Tumors in AdultstaseaditNo ratings yet
- Spinal Chordoma: Radiologic Features: in 14 CasesDocument3 pagesSpinal Chordoma: Radiologic Features: in 14 Casesselvie87No ratings yet
- Tuberkulosis Dinding DadaDocument6 pagesTuberkulosis Dinding DadaSuwandiRahmanNo ratings yet
- Primary Leiomyosarcoma of The Spine: An Analysis of Imaging Manifestations and Clinicopathological FindingsDocument9 pagesPrimary Leiomyosarcoma of The Spine: An Analysis of Imaging Manifestations and Clinicopathological Findingst7xs5mchfzNo ratings yet
- Pictorial Essay: Spectrum of MR Imaging Findings in Spinal TuberculosisDocument5 pagesPictorial Essay: Spectrum of MR Imaging Findings in Spinal TuberculosisAnonymous vnv6QFNo ratings yet
- Spinal Tuberculosis: Imaging Features On MRIDocument6 pagesSpinal Tuberculosis: Imaging Features On MRIEster JuanitaNo ratings yet
- BJR 20140771Document10 pagesBJR 20140771DanaAmaranducaiNo ratings yet
- Ameloblastoma of the Jaw and Maxilla: Clinical Study of 10 CasesDocument7 pagesAmeloblastoma of the Jaw and Maxilla: Clinical Study of 10 CasessriwahyuutamiNo ratings yet
- 10.1007@s11060 004 3390 7Document9 pages10.1007@s11060 004 3390 7AlejitaplafuenteNo ratings yet
- Ghost Cell TumourDocument5 pagesGhost Cell TumourrgrthhnNo ratings yet
- Magnetic Resonance DetectionDocument5 pagesMagnetic Resonance DetectionEoin O'MalleyNo ratings yet
- Collagenous Fibroma (Desmoplastic Fibroblastoma) : Clinical, Pathological and Imaging FindingsDocument41 pagesCollagenous Fibroma (Desmoplastic Fibroblastoma) : Clinical, Pathological and Imaging FindingsAnonymous bE4VegCcNo ratings yet
- Keller Et Al 2011 EpilepsiaDocument10 pagesKeller Et Al 2011 EpilepsiaLaura Gonzalez TamayoNo ratings yet
- 1009 Case 2Document4 pages1009 Case 2willygopeNo ratings yet
- 143 FullDocument8 pages143 FullDokdem AjaNo ratings yet
- Europeanjournalof Radiologyopen: Longitudinal Stress Fracture of The Femur: A Rare PresentationDocument4 pagesEuropeanjournalof Radiologyopen: Longitudinal Stress Fracture of The Femur: A Rare PresentationYusfaIndahNo ratings yet
- Spinal En-Plaque Meningioma Case ReportDocument7 pagesSpinal En-Plaque Meningioma Case ReportArif Susilo RahadiNo ratings yet
- Diagnostics and Complex Treatment of Pain Dysfunction Syndrome of Temporоmandibular JointDocument4 pagesDiagnostics and Complex Treatment of Pain Dysfunction Syndrome of Temporоmandibular JointAcademic JournalNo ratings yet
- Case Report: Coexistence of Ankylosing Spondylitis and Neurofibromatosis Type 1Document4 pagesCase Report: Coexistence of Ankylosing Spondylitis and Neurofibromatosis Type 1Lupita Mora LobacoNo ratings yet
- Jurnal in EnglishDocument5 pagesJurnal in Englishyeni_arnasNo ratings yet
- Li 2015Document4 pagesLi 2015Akmal Niam FirdausiNo ratings yet
- Spinous Process Fractures in Osteoporotic Thoracolumbar Vertebral FracturesDocument4 pagesSpinous Process Fractures in Osteoporotic Thoracolumbar Vertebral FracturesAditya Arya PutraNo ratings yet
- Esti2012 E-0030Document8 pagesEsti2012 E-0030Anonymous p52JDZOdNo ratings yet
- Displasia Cortical de TaylorDocument11 pagesDisplasia Cortical de TaylorOscar F. Ochoa RuizNo ratings yet
- 17 OmerhodzicDocument9 pages17 OmerhodzicFederico MinghinelliNo ratings yet
- Raval PDFDocument5 pagesRaval PDFdebby claudiNo ratings yet
- MRI Findings in Susac SyndromeDocument6 pagesMRI Findings in Susac SyndromeLeonardoBubackNo ratings yet
- Cervical Facet OedemaDocument6 pagesCervical Facet OedemaMarco PeroliNo ratings yet
- AAOS2002 TumorDocument59 pagesAAOS2002 TumorHizkyas KassayeNo ratings yet
- Issn 2 Issn 2 Issn 2 ISSN 2073 073 073 073 - 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. SDocument7 pagesIssn 2 Issn 2 Issn 2 ISSN 2073 073 073 073 - 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. S 9990 East Cent. Afr. J. SAngga Witra NandaNo ratings yet
- Posicion de La CabezaDocument8 pagesPosicion de La Cabezamonyrodriguez.martinezNo ratings yet
- Schick2006 Treatment PDFDocument7 pagesSchick2006 Treatment PDFRifqi FNo ratings yet
- Cystic Lesion of The MaxillaeDocument12 pagesCystic Lesion of The Maxillaegaurav01338599No ratings yet
- 1097-0142 (197402) 33 2 324 Aid-Cncr2820330205 3.0.co 2-U PDFDocument10 pages1097-0142 (197402) 33 2 324 Aid-Cncr2820330205 3.0.co 2-U PDFMeditador OficialNo ratings yet
- Article in Press: Journal of Oral and Maxillofacial Surgery, Medicine, and PathologyDocument6 pagesArticle in Press: Journal of Oral and Maxillofacial Surgery, Medicine, and PathologyOMFS FKG UnimusNo ratings yet
- Chapter 102 Male Anatomy and PhysiologyDocument2 pagesChapter 102 Male Anatomy and PhysiologyNicolas SaputraNo ratings yet
- Acute Appendicitis: Review and UpdateDocument15 pagesAcute Appendicitis: Review and UpdateNicolas SaputraNo ratings yet
- Recent Developments in Diagnosing Pancreatic CancerDocument7 pagesRecent Developments in Diagnosing Pancreatic CancerAgustinus FatollaNo ratings yet
- Clinical Signs and CT Patterns Associated With Intracranial Space-Occupying Lesions in 89 DogsDocument6 pagesClinical Signs and CT Patterns Associated With Intracranial Space-Occupying Lesions in 89 DogsNicolas SaputraNo ratings yet
- International Study ProtocolDocument9 pagesInternational Study ProtocolNicolas SaputraNo ratings yet
- Asthma Guidlines RCPCHDocument28 pagesAsthma Guidlines RCPCHMohamed AmrNo ratings yet
- Intra-Cranial Space Occupying Lesions A Morphological AnalysisDocument5 pagesIntra-Cranial Space Occupying Lesions A Morphological AnalysisNicolas SaputraNo ratings yet
- Unusual Cause of Multiple Cranial Nerve Palsy: Md. Shahriar MahbubDocument1 pageUnusual Cause of Multiple Cranial Nerve Palsy: Md. Shahriar MahbubNicolas SaputraNo ratings yet
- A Case of A Patient With Both Chorea and Restless Legs SyndromeDocument4 pagesA Case of A Patient With Both Chorea and Restless Legs SyndromeNicolas SaputraNo ratings yet
- Gastric Bypass DietDocument9 pagesGastric Bypass DietNicolas SaputraNo ratings yet
- J4Document10 pagesJ4Nicolas SaputraNo ratings yet
- Huntington Chorea: April 21, 2006 Articles Images CME Advanced Search Consumer Health Link To This SiteDocument8 pagesHuntington Chorea: April 21, 2006 Articles Images CME Advanced Search Consumer Health Link To This SiteNicolas SaputraNo ratings yet
- Sleep, Neurobehavioral Functioning, and Behavior Problems in School-Age ChildrenDocument13 pagesSleep, Neurobehavioral Functioning, and Behavior Problems in School-Age ChildrenNicolas SaputraNo ratings yet
- Huntington Chorea: April 21, 2006 Articles Images CME Advanced Search Consumer Health Link To This SiteDocument8 pagesHuntington Chorea: April 21, 2006 Articles Images CME Advanced Search Consumer Health Link To This SiteNicolas SaputraNo ratings yet
- J4Document10 pagesJ4Nicolas SaputraNo ratings yet
- Sleep, Neurobehavioral Functioning, and Behavior Problems in School-Age ChildrenDocument13 pagesSleep, Neurobehavioral Functioning, and Behavior Problems in School-Age ChildrenNicolas SaputraNo ratings yet
- Current Management Strategies for Breast CancerDocument46 pagesCurrent Management Strategies for Breast CancerNicolas SaputraNo ratings yet
- J4Document10 pagesJ4Nicolas SaputraNo ratings yet
- STR File Varun 3Document61 pagesSTR File Varun 3Varun mendirattaNo ratings yet
- Media LiteracyDocument33 pagesMedia LiteracyDo KyungsooNo ratings yet
- Seismic Design Guide (2010)Document102 pagesSeismic Design Guide (2010)ingcarlosgonzalezNo ratings yet
- Otis Brochure Gen2life 191001-BELGIUM SmallDocument20 pagesOtis Brochure Gen2life 191001-BELGIUM SmallveersainikNo ratings yet
- JA Ip42 Creating Maintenance PlansDocument8 pagesJA Ip42 Creating Maintenance PlansvikasbumcaNo ratings yet
- I Am Sharing 'Pregnancy Shady' With YouDocument48 pagesI Am Sharing 'Pregnancy Shady' With YouNouran AlaaNo ratings yet
- Chapter 1 Critical Thin...Document7 pagesChapter 1 Critical Thin...sameh06No ratings yet
- 42U System Cabinet GuideDocument68 pages42U System Cabinet GuideGerman AndersNo ratings yet
- Painter CardDocument1 pagePainter CardPraveen RANANo ratings yet
- Manufacturing Tech-1Document6 pagesManufacturing Tech-1Vikram Rao0% (1)
- Frozen DessertDocument3 pagesFrozen DessertcsqueilNo ratings yet
- Transistor Amplifier Operating ParametersDocument21 pagesTransistor Amplifier Operating ParametersReddyvari VenugopalNo ratings yet
- SpringDocument4 pagesSpringarun123123No ratings yet
- Mitosis Quiz: Answers Each Question. Write The Answer On The Sheet ProvidedDocument5 pagesMitosis Quiz: Answers Each Question. Write The Answer On The Sheet ProvidedJohn Osborne100% (1)
- CP R77.30 ReleaseNotesDocument18 pagesCP R77.30 ReleaseNotesnenjamsNo ratings yet
- The Ideal Structure of ZZ (Alwis)Document8 pagesThe Ideal Structure of ZZ (Alwis)yacp16761No ratings yet
- We Generally View Objects As Either Moving or Not MovingDocument11 pagesWe Generally View Objects As Either Moving or Not MovingMarietoni D. QueseaNo ratings yet
- LAU Paleoart Workbook - 2023Document16 pagesLAU Paleoart Workbook - 2023samuelaguilar990No ratings yet
- Interview Question SalesforceDocument10 pagesInterview Question SalesforcesomNo ratings yet
- Thank you for purchasing your remap from HDI Tuning LtdDocument2 pagesThank you for purchasing your remap from HDI Tuning LtdMaks LebanNo ratings yet
- Chapter-5 Contract ManagementDocument43 pagesChapter-5 Contract Managementprem kumarNo ratings yet
- VANSINA, Jan. Art History in AfricaDocument250 pagesVANSINA, Jan. Art History in AfricaRaphaelTim100% (1)
- TiONA 592 PDS - ENDocument1 pageTiONA 592 PDS - ENQuang VANo ratings yet
- HIS 101 Home Work 2Document10 pagesHIS 101 Home Work 2Nabil HussainNo ratings yet
- EJC H2 Math P1 With Solution PDFDocument23 pagesEJC H2 Math P1 With Solution PDFKipp SohNo ratings yet
- BRKSPG-2904-2904 - Cisco Live Session - v2-CL PDFDocument182 pagesBRKSPG-2904-2904 - Cisco Live Session - v2-CL PDFMohamed SamirNo ratings yet
- 1 API 653 Exam Mar 2015 MemoryDocument12 pages1 API 653 Exam Mar 2015 MemorymajidNo ratings yet
- Karate Writing AssessmentDocument2 pagesKarate Writing AssessmentLeeann RandallNo ratings yet
- Assignment No.7Document2 pagesAssignment No.7queen estevesNo ratings yet
- 2022 - J - Chir - Nastase Managementul Neoplaziilor Pancreatice PapilareDocument8 pages2022 - J - Chir - Nastase Managementul Neoplaziilor Pancreatice PapilarecorinaNo ratings yet