Professional Documents
Culture Documents
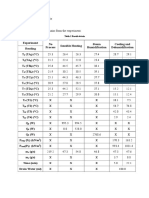
Tiaf (°C) Tfaf (°C) Taf (°C) Tik (°C) TFK (°C) TK (°C) Tiac (°C) Tfac (°C) Tac (°C) Qaf (Cal) QK (°C) Qac (°C) K
Uploaded by
Ulises Martinez FriasOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tiaf (°C) Tfaf (°C) Taf (°C) Tik (°C) TFK (°C) TK (°C) Tiac (°C) Tfac (°C) Tac (°C) Qaf (Cal) QK (°C) Qac (°C) K
Uploaded by
Ulises Martinez FriasCopyright:
Available Formats
Resultados
Parte 1. Determinacin de la capacidad trmica del
calormetro
Masa del agua fra (mf)= 100g
Masa del agua caliente (mc)= 100g
Capacidad trmica especfica del agua (Cagua)= 1cal/gC
Temperatura del agua fra (Tf)=17C
Temperatura del agua caliente (Tc)= 90.5C
Tiaf(C
)
Tfaf(C
)
Taf(C)
TiK(C
)
TfK(C
)
TK(C
)
17
42.
5
41.
6
41.
6
41.
5
41.
5
41.
4
41.
4
41.
4
25.5
90.
5
90.
5
90.
5
90.
5
90.
5
90.
5
90.
5
90.
5
42.
5
41.
6
41.
6
41.
5
41.
5
41.
4
41.
4
41.
4
-48
48.9
48.9
49.0
49.0
49.1
49.1
49.1
17
17
17
17
17
17
17
24.6
24.6
24.5
24.5
24.4
24.4
24.4
Tiac(C
)
Tfac(C
)
Tac(C)
Qaf(cal
)
QK(C
)
Qac(C
)
90.5 42.5
-48
225
0
4800
90.5 41.6
48.9
48.9
49.0
49.0
49.1
49.1
49.1
255
0
246
0
246
0
245
0
245
0
244
0
244
0
244
0
243
0
4890
46.8p
8
49.69
243
0
4890
49.69
245
0
4900
50.00
245
0
4900
50.00
247
0
4910
49.69
247
0
4910
49.69
247
0
4910
49.69
90.5 41.6
90.5 41.5
90.5 41.5
90.5 41.4
90.5 41.4
90.5 41.4
Parte 2. Determinacin del equivalente calor-trabajo
Voltaje= 122.3 volts
Resistencia= 21.1 ohm
Tia(
C)
18.
Tfa(
C)
22.9
Ta(
C)
4.8
TiK(
C)
TfK(
C)
Tk(
C)
Qa(ca
l)
120
Qk(ca
l)
V(v)
122
V 2 (V 2) R( Pot(W
14957
)
21
)
708.
t(s
)
10
We
(J)
7088.
Qab(cal)
-
.3
.29
.1
88
80
22.
9
29.4
6.5
162
5
122
.3
14957
.29
21
.1
708.
88
20
14177
.80
29.
4
34.4
5.0
125
0
122
.3
14957
.29
21
.1
708.
88
30
21266
.40
34.
4
40.5
6.1
152
5
122
.3
14957
.29
21
.1
708.
88
40
28355
.20
40.
5
46.8
6.3
157
5
122
.3
14957
.29
21
.1
708.
88
50
35444
.00
46.
8
53.3
6.5
162
5
122
.3
14957
.29
21
.1
708.
88
60
42532
.80
53.
3
59.7
6.4
160
0
122
.3
14957
.29
21
.1
708.
88
70
49621
.60
59.
7
65.3
5.6
140
0
122
.3
14957
.29
21
.1
708.
88
80
56710
.40
65.
3
71.8
6.5
162
5
122
.3
14957
.29
21
.1
708.
88
90
63799
.20
71.
8
77.6
5.8
145
0
122
.3
14957
.29
21
.1
708.
88
10
0
70888
.00
77.
6
84.8
7.2
180
0
122
.3
14957
.29
21
.1
708.
88
11
0
77976
.80
Algoritmo de clculo
Determinacin de la constante del calormetro
1. Q ced +Qabs=0
2. Qagua caliente +Qagu afria +Q k =0
3. Qagua caliente =mc T ac
4. Qagua fria=mc T af
5. QK =( Q af +Qac )
6.
K=
Qk
Tk
7088.
80
1417.
80
21266
.40
28355
.20
35444
.00
42532
.80
49621
.60
56710
.40
63799
.20
70888
.00
77976
.00
Determinacin de la relacin calor-trabajo
1. Q ced +Qabs=0
2. W e +Qagua+Q k =0
3. Qk ( cal ) =K T
4. Qagua(cal)=mcTa
2
5.
Pot ( W )=
V
R
6. W e (J )=(Pot )(t )
Conclusiones
You might also like
- HCHEM Notes Specific Heat Capacity 3 2Document21 pagesHCHEM Notes Specific Heat Capacity 3 2leimendozaschoolNo ratings yet
- Experiment 5Document13 pagesExperiment 5bm7gyygjtfNo ratings yet
- Temperature and Heat: Younes SinaDocument31 pagesTemperature and Heat: Younes SinayounessinaNo ratings yet
- Lab Phy 03Document17 pagesLab Phy 03Wan AfiffNo ratings yet
- Changes in Temperature and Phase: Set By:nali MahmodDocument25 pagesChanges in Temperature and Phase: Set By:nali MahmodNali MahmodNo ratings yet
- ME-341A - Heat and Mass TransferDocument34 pagesME-341A - Heat and Mass TransferMukul ChandraNo ratings yet
- Result: Table 1: Result That Were RecordedDocument6 pagesResult: Table 1: Result That Were RecordedAhmad SyamilNo ratings yet
- Bsed-Sci2a - Flores Mark Brian - Conversion of TemperatureDocument6 pagesBsed-Sci2a - Flores Mark Brian - Conversion of TemperatureMark Brian FloresNo ratings yet
- Conduction Heat Transfer Part 2Document8 pagesConduction Heat Transfer Part 2Ali AimranNo ratings yet
- Pepito, Alexis R. - Assignment 2.0-Heat and TemperatureDocument5 pagesPepito, Alexis R. - Assignment 2.0-Heat and TemperaturePEPITO, ALEXIS R.SCINo ratings yet
- Experimen T Reading: Table 1: Result TableDocument9 pagesExperimen T Reading: Table 1: Result Table000No ratings yet
- Lecture No.3 Reversed Carnot & Product LoadDocument22 pagesLecture No.3 Reversed Carnot & Product LoadJohn Edriane AlvarezNo ratings yet
- Temperature (°C) Vs Time (Min)Document2 pagesTemperature (°C) Vs Time (Min)karthikeyanNo ratings yet
- Gas Properties: Molecular WeightDocument2 pagesGas Properties: Molecular WeightDamar WibisonoNo ratings yet
- Module in General Physics 2Document7 pagesModule in General Physics 2Jas De GuzmanNo ratings yet
- Latent Heat of FusionDocument3 pagesLatent Heat of FusionJamiel Catapang100% (1)
- Heat and TemperatureDocument37 pagesHeat and TemperatureFaith VijigaNo ratings yet
- CALORIMETRYDocument6 pagesCALORIMETRYRosally BulauanNo ratings yet
- Introduction To RefrigerationDocument79 pagesIntroduction To RefrigerationN S SenanayakeNo ratings yet
- CalorimetryDocument11 pagesCalorimetryJuliana BarnesNo ratings yet
- Sheet ch1 Heat PDFDocument6 pagesSheet ch1 Heat PDFMohammad Yahya AzabNo ratings yet
- ChemLec - Module 4.1 - 4.3Document23 pagesChemLec - Module 4.1 - 4.3Jerick JasperNo ratings yet
- Lab 3 Latent HeatDocument2 pagesLab 3 Latent HeatBernard PrattNo ratings yet
- Developing and Using Stio Tables NotesDocument27 pagesDeveloping and Using Stio Tables NotesThabangNo ratings yet
- Settler: Komponen Mol (Kmol/jam) CP (Kj/mol K) T (K) H (Kj/jam)Document2 pagesSettler: Komponen Mol (Kmol/jam) CP (Kj/mol K) T (K) H (Kj/jam)agung nugrahaNo ratings yet
- Psychometric Properties and ProcessesDocument40 pagesPsychometric Properties and ProcessesUser140035No ratings yet
- Gas Properties: Molecular WeightDocument2 pagesGas Properties: Molecular WeightDamar WibisonoNo ratings yet
- T Literatur ( C) : B D B DDocument17 pagesT Literatur ( C) : B D B DMuhammad Arief NugrahaNo ratings yet
- AP Chemistry Lab Heat of ReactionDocument4 pagesAP Chemistry Lab Heat of ReactionClaudia Huo100% (2)
- Hematra SolutionDocument7 pagesHematra SolutionDarlene FranciaNo ratings yet
- Calorimetry: Latent Heat of VaporizationDocument4 pagesCalorimetry: Latent Heat of VaporizationIvy GalamitonNo ratings yet
- PhysicsDocument14 pagesPhysicsJake Marcelo-TapatNo ratings yet
- Heat of Neutralization - Lab ReportDocument7 pagesHeat of Neutralization - Lab ReportJasmeetSingh56% (9)
- CNX CollegePhysics SolutionManual Ch13Document24 pagesCNX CollegePhysics SolutionManual Ch13KazaValiShaik100% (3)
- Heat Transfer Lab ManualDocument59 pagesHeat Transfer Lab ManualAreez MalikNo ratings yet
- Lab Report Chm432Document31 pagesLab Report Chm432Aniqah AdliNo ratings yet
- Topic1-1 Thermal PrincipleDocument40 pagesTopic1-1 Thermal PrincipleEdith Carumbana JusayanNo ratings yet
- Akp Cupa FixDocument3 pagesAkp Cupa Fixanis wahyu ningsihNo ratings yet
- Kvpy Calorimetry PDFDocument4 pagesKvpy Calorimetry PDFstudysteps.inNo ratings yet
- ACRE1-Unsteady State Foglers Lec25 - Reactor Safety-RevDocument32 pagesACRE1-Unsteady State Foglers Lec25 - Reactor Safety-RevDeneshVijayNo ratings yet
- Activity in General Chemistry II: Thermochemistry Problem SolvingDocument5 pagesActivity in General Chemistry II: Thermochemistry Problem SolvingGheerah PantojaNo ratings yet
- Heat Lecture NotesDocument62 pagesHeat Lecture NotesAS HUMBLE PIANONo ratings yet
- Calorimetry-1Document15 pagesCalorimetry-1mayaNo ratings yet
- 2 3 Temp ConvDocument18 pages2 3 Temp ConveraNo ratings yet
- Temperature Conversions: Temperature and Thermometric ScaleDocument75 pagesTemperature Conversions: Temperature and Thermometric ScaleMedz MelegritoNo ratings yet
- 2 3 Temp ConvDocument18 pages2 3 Temp ConvJohnny BuyuccanNo ratings yet
- Heat Transfer Lab ManualDocument40 pagesHeat Transfer Lab ManualRachit_Goyal25_10No ratings yet
- Table 1 Result ObtainDocument12 pagesTable 1 Result ObtainShamsul AimanNo ratings yet
- Karen Ann v. BACUS - Activity No.3 - CalorimetryDocument7 pagesKaren Ann v. BACUS - Activity No.3 - CalorimetryKaren Ann V. BACUSNo ratings yet
- Thermal Contact and Thermal Equilibrium. Two Objects Are inDocument40 pagesThermal Contact and Thermal Equilibrium. Two Objects Are in11 AniketNo ratings yet
- Solution 4Document5 pagesSolution 4Anshu Kumar Gupta100% (4)
- EXPERIMENT 4 Ch101 Law of Thermodynamics (s11184888)Document8 pagesEXPERIMENT 4 Ch101 Law of Thermodynamics (s11184888)shyla maniNo ratings yet
- "Calorimetry" Learning Activity #1 I. ObjectivesDocument11 pages"Calorimetry" Learning Activity #1 I. ObjectivesYhazmin Iris IlustrisimoNo ratings yet
- Lab Report (Exp) : CHM213 Physical ChemistryDocument18 pagesLab Report (Exp) : CHM213 Physical ChemistryAfina AnuariNo ratings yet
- Past Exam Problems of ThermodynamicsDocument44 pagesPast Exam Problems of Thermodynamicsromaehab201912No ratings yet
- Homework 6.1 SummerDocument3 pagesHomework 6.1 SummerMartin OdhiamboNo ratings yet
- 2 - ProcessesDocument36 pages2 - ProcessesAljohn Mark ReyesNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Recommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsFrom EverandRecommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsE. F. G. HeringtonNo ratings yet