Professional Documents
Culture Documents
AP Chemistry Practice Quiz 6
Uploaded by
Jeromy Rech0 ratings0% found this document useful (0 votes)
66 views2 pagesSpec
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentSpec
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
66 views2 pagesAP Chemistry Practice Quiz 6
Uploaded by
Jeromy RechSpec
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
AP Chemistry Quiz: Chapter 6
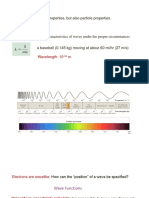
1. Explain in detail (include examples) the wave-particle duality of
electromagnetic radiation.
Electromagnetic radiation exhibits characteristics of both
waves and particles. While travelling through space, ER
exhibits wave characteristics including possession of a
wavelength and frequency. ER exhibits characteristics of
particles when it is absorbed and emitted by matter.
Specifically, ER is only absorbed and emitted in chunks called
quanta.
2. Describe the quantum mechanical model of the atom. Include in your
explanation the contributions of Bohr, de Broglie, Heisenberg, Pauli,
Planck, Rutherford, and Schrdinger.
Massive, positive nucleus (Rutherford)
Electrons in atoms exhibit wave characteristics (de Broglie)
and are only allowed in at certain fixed energies(Bohr)
To move from one energy level to another, electrons must
absorb(to move up) or emit(to drop) energy.(Bohr) This energy
can only be emitted in chunks called quanta.( Planck)
One can only determine the probable location of an electron.
(Heisenberg)
Only 2 electrons, each with opposite spin, are allowed per
orbital.(Pauli)
Orbital shapes are predicted by Schrdingers equation.
(Schrdinger)
3. What is a line spectrum? How was the line spectrum of hydrogen
important to the development of the quantum mechanical model of the
atom?
A line spectrum is a characteristic set of electromagnetic
radiation emitted by an atom when electrons drop from one
energy level to another. It was the line spectrum of the
hydrogen atom upon which Bohr based his theory of
quantized electrons in the atom.
4. For what elements is the Bohr model of the atom valid?
Any one electron system
5. Explain quantum numbers. What are they called? What do they mean?
What are their possible values?
Principal quantum number
o n
o energy level
o + integers starting at 1
Angular momentum quantum number
o l
o shape of orbital
o all integers from 0 to n-1
Magnetic quantum number
o m
o orientation of the orbital in space
o all integers between and including l to +l
Spin quantum number
o S
o spin of the electron
o +1/2 and -1/2 for every value of m
6. List all quantum numbers for the 3rd energy level.
n=3
l= 0, 1, 2
m = 0, -1,0,+1, -2, -1, 0, +1, +2
S = , -1/2 for every value of m
7. Explain in detail the difference between a 5s and 4p orbital.
The electron in the 5s has higher energy, is farther from the
nucleus, and would have a sphere shape and only one
orientation of the shape.
8. Write the orbital notation or electron configuration for
As [Ar] 4s2 3d10 4p3
Cr [Ar] 4s1 3d5
Tl [Xe] 6s2 4f14 5d10 6p1
Zn [Ar] 4s2 3d10
9. How many electrons are in the 3rd energy level and in the 6g orbitals?
18 in both
10.
What orbital shape is associated with L = 2?
Double-peanut
11.
What is the wavelength and energy of electromagnetic radiation
with a frequency of 6.66 x 1014 sec -1?
C = v
_ (3.00e8)/(6.66e14)
_ 4.5045e-7 (10e8)
_ 450 nm
E = hC/
_ (6.626e-34)(3.00e8)/(4.5045e-7)
_ 4.41e-19
12.
Determine the energy and wavelength of the electromagnetic
radiation emitted when an electron drops from n = 6 to n = 2 in a
hydrogen atom.
1=1 (11)
91(nl nh)
nl=2 nh=6
1/ = 2/819 === 409.5nm
You might also like
- Chemistry Made Easy An Illustrated Study Guide For Students To EasilyDocument220 pagesChemistry Made Easy An Illustrated Study Guide For Students To EasilyAnna Vyluschak100% (2)
- Inorganic Chemistry I-VIDocument134 pagesInorganic Chemistry I-VIMurad AlDamen100% (3)
- Thoughts Upon Reading Marko RodinDocument17 pagesThoughts Upon Reading Marko Rodinreflatus13100% (3)
- Psycanics For Conversations With God The Technology To Return To The One PDFDocument240 pagesPsycanics For Conversations With God The Technology To Return To The One PDFAndrei S. Grama100% (1)
- Atomic Structure - Network Solids Part 1 2Document60 pagesAtomic Structure - Network Solids Part 1 2eiwk100% (1)
- Chapter 2.1 - Structure of AtomsDocument71 pagesChapter 2.1 - Structure of Atomsahmad yasinNo ratings yet
- 2007 Electrons in AtomsDocument123 pages2007 Electrons in Atomsapi-293306937No ratings yet
- Electronic Structure of The AtomDocument55 pagesElectronic Structure of The AtomAlekhoy Pakz100% (1)
- (International Series in Pure and Applied Physics) Floyd K. Richtmyer, Earle H. Kennard - Introduction To Modern Physics-McGraw-Hill Book Company, Inc. (1947)Document780 pages(International Series in Pure and Applied Physics) Floyd K. Richtmyer, Earle H. Kennard - Introduction To Modern Physics-McGraw-Hill Book Company, Inc. (1947)jor marketing100% (2)
- Fundamentals of Energy Dispersive X-Ray Analysis: Butterworths Monographs in MaterialsFrom EverandFundamentals of Energy Dispersive X-Ray Analysis: Butterworths Monographs in MaterialsRating: 5 out of 5 stars5/5 (1)
- The Quantum Mechanical Model of An AtomDocument24 pagesThe Quantum Mechanical Model of An AtomKim Christian CombaterNo ratings yet
- School WorkDocument15 pagesSchool Workjdubey4258No ratings yet
- AtomsDocument16 pagesAtomsthinkiitNo ratings yet
- Art L3Document10 pagesArt L3nickmcklin7No ratings yet
- Electron StructureDocument80 pagesElectron StructureCacey Daiwey CalixtoNo ratings yet
- Structure of AtomDocument112 pagesStructure of AtomAnanya SinghNo ratings yet
- Atomic Line Spectra ExplainedDocument4 pagesAtomic Line Spectra Explaineddomer2011No ratings yet
- 5,6,7Document14 pages5,6,7عباسNo ratings yet
- Quantum Mechanics /quantum Numbers: Modern Concepts in Atomic StructureDocument9 pagesQuantum Mechanics /quantum Numbers: Modern Concepts in Atomic StructureManal.N.TNo ratings yet
- Week 1 Quantum Mechanical ModelDocument34 pagesWeek 1 Quantum Mechanical ModelVince PonceNo ratings yet
- Gen Chem 1 - Q2Document55 pagesGen Chem 1 - Q2pingcj9No ratings yet
- Electronic Structure Revised 2015Document42 pagesElectronic Structure Revised 2015Ralph RebugioNo ratings yet
- CH 6 (Cont'd)Document5 pagesCH 6 (Cont'd)PineraserNo ratings yet
- Atom 1Document3 pagesAtom 1GayatriNo ratings yet
- General Chemistry 1: Quarter 2 - Module 1 Quantum Mechanical Description and The Electronic Structure of AtomsDocument11 pagesGeneral Chemistry 1: Quarter 2 - Module 1 Quantum Mechanical Description and The Electronic Structure of AtomsEian InganNo ratings yet
- General Chemistry 1: Quarter 2 - Week 1Document13 pagesGeneral Chemistry 1: Quarter 2 - Week 1Janzelle BorbonNo ratings yet
- Atomic Structure 10feb07Document27 pagesAtomic Structure 10feb07Fredrick MutungaNo ratings yet
- Electronic ConfigDocument22 pagesElectronic ConfigGhienel Surla NaguitNo ratings yet
- Quantum Numbers GENCHEM1 Q2Document2 pagesQuantum Numbers GENCHEM1 Q2Glean EsmileNo ratings yet
- 8 - Atoms and Nuclei PDFDocument25 pages8 - Atoms and Nuclei PDFthinkiit67% (3)
- Structure of AtomDocument3 pagesStructure of AtomvuppalasampathNo ratings yet
- Electronic Structure of The Atom: Charlito R. AligadoDocument32 pagesElectronic Structure of The Atom: Charlito R. AligadoElaine Mata100% (1)
- HL Chemistry: Notes Atomic TheoryDocument3 pagesHL Chemistry: Notes Atomic TheoryKhondokar TarakkyNo ratings yet
- Atomic Structure... (3) PPTDocument32 pagesAtomic Structure... (3) PPTVaibhav KargetiNo ratings yet
- Science Grade 9 Handout 1 Quantum Mechanical ModelDocument6 pagesScience Grade 9 Handout 1 Quantum Mechanical ModelClinton YmbongNo ratings yet
- Hydrogen Spectrum and Atomic Terms NotesDocument32 pagesHydrogen Spectrum and Atomic Terms Notesoyamo markNo ratings yet
- CHEM 111 UNIT 02 - Atomic Structure & PeriodicityDocument48 pagesCHEM 111 UNIT 02 - Atomic Structure & PeriodicitycarlNo ratings yet
- 1A The Shapes and Structures of Molecules Student Handout Part Two 2022.23Document70 pages1A The Shapes and Structures of Molecules Student Handout Part Two 2022.23Music MaestroNo ratings yet
- Atomic Orbitals: Quantum NumbersDocument16 pagesAtomic Orbitals: Quantum NumberslostgirlNo ratings yet
- Ws 2Document4 pagesWs 2Claude CaduceusNo ratings yet
- General Chemistry Lecture 3Document80 pagesGeneral Chemistry Lecture 3Aaron Dela CruzNo ratings yet
- Chapter One Atomic SturctureDocument7 pagesChapter One Atomic SturctureWorld ShortsNo ratings yet
- AU Chemistry Unit-1Document26 pagesAU Chemistry Unit-1Aarush PitlaNo ratings yet
- 101 - Chem. General ChemistryDocument33 pages101 - Chem. General Chemistrygmgmfn dhdNo ratings yet
- Quantum Numbers Describe Atomic OrbitalsDocument16 pagesQuantum Numbers Describe Atomic OrbitalsR-jayVenturilloNo ratings yet
- Electronic Structure of AtomsDocument87 pagesElectronic Structure of AtomsAlbert Jade Pontimayor LegariaNo ratings yet
- Chapter 4: Arrangement of Electrons in Atoms: Chapter 4.1: The Development of A New Atomic ModelDocument2 pagesChapter 4: Arrangement of Electrons in Atoms: Chapter 4.1: The Development of A New Atomic Modelrrr rrrNo ratings yet
- Lecture 1Document5 pagesLecture 1Samuel Barcelo LeronNo ratings yet
- Rutherford's α-particle scattering experiment:: 엠 radioactive sourceDocument6 pagesRutherford's α-particle scattering experiment:: 엠 radioactive sourcerohit chakNo ratings yet
- General Chemistry 1: Quarter 2 - Week 2Document18 pagesGeneral Chemistry 1: Quarter 2 - Week 2Janzelle BorbonNo ratings yet
- General Chemistry I: Atomic Orbitals and Electron ConfigurationDocument96 pagesGeneral Chemistry I: Atomic Orbitals and Electron ConfigurationKatto - Darling in the PianoNo ratings yet
- The Ratio of Mass of Electron To The Mass of Proton: Evidences in Favour of Bohr's TheoryDocument10 pagesThe Ratio of Mass of Electron To The Mass of Proton: Evidences in Favour of Bohr's TheorySukhwinder Singh GillNo ratings yet
- Lec 3Document21 pagesLec 3Rishikesh BobbyNo ratings yet
- Bohr Model and Electron ConfigurationDocument35 pagesBohr Model and Electron ConfigurationJoric MagusaraNo ratings yet
- STPM Chemistry Chapter 2 sem 1Document4 pagesSTPM Chemistry Chapter 2 sem 1Aquila Wong40% (5)
- De Broglie Relation: EinsteinDocument19 pagesDe Broglie Relation: EinsteinMaisha MaimunaNo ratings yet
- Junior ChemistryDocument72 pagesJunior ChemistryjahnavikidambiNo ratings yet
- Electronic Theory of ChemistryDocument43 pagesElectronic Theory of ChemistryMaheshNo ratings yet
- Quantum Numbers and Periodic Trends ExplainedDocument14 pagesQuantum Numbers and Periodic Trends ExplainedMuhammad HarisNo ratings yet
- Quantum theory and the electronic structure of atomsDocument17 pagesQuantum theory and the electronic structure of atomsSalama NaumanNo ratings yet
- 2.2 Electron Configuration 1Document46 pages2.2 Electron Configuration 1Sonali MkNo ratings yet
- S Orbital (L 0) P Orbital (L 1) D Orbital (L 2) : Quantum NumbersDocument2 pagesS Orbital (L 0) P Orbital (L 1) D Orbital (L 2) : Quantum NumbersBittuNo ratings yet
- AtomsDocument60 pagesAtomsRohit Kumar BaghelNo ratings yet
- Elementary Particles: The Commonwealth and International LibraryFrom EverandElementary Particles: The Commonwealth and International LibraryNo ratings yet
- AP Gov FRQDocument2 pagesAP Gov FRQJeromy RechNo ratings yet
- AP Chemistry Practice Quiz 7Document3 pagesAP Chemistry Practice Quiz 7Jeromy RechNo ratings yet
- Woodward Fieser RulesDocument2 pagesWoodward Fieser RulesJeromy RechNo ratings yet
- Exponential Growth/Decay Half Life o Kinetics and Concentrations o Interest o Compounded "K" Times: oDocument4 pagesExponential Growth/Decay Half Life o Kinetics and Concentrations o Interest o Compounded "K" Times: oJeromy RechNo ratings yet
- AP Gov FRQ IIIDocument5 pagesAP Gov FRQ IIIJeromy RechNo ratings yet
- Practice AP Problems For APUSHDocument16 pagesPractice AP Problems For APUSHJeromy RechNo ratings yet
- Cult and Civ Test 6Document8 pagesCult and Civ Test 6Jeromy RechNo ratings yet
- Calc 1 - What Is A LimitDocument7 pagesCalc 1 - What Is A LimitJeromy RechNo ratings yet
- Numerology 14Document1 pageNumerology 14Jeromy RechNo ratings yet
- Cult and Civ Test 4Document4 pagesCult and Civ Test 4Jeromy RechNo ratings yet
- AP Gov FRQ IVDocument5 pagesAP Gov FRQ IVJeromy RechNo ratings yet
- Calc 1 - Major EqsDocument2 pagesCalc 1 - Major EqsJeromy RechNo ratings yet
- AP Gov FRQ IDocument2 pagesAP Gov FRQ IJeromy RechNo ratings yet
- Cult and Civ Test 5Document5 pagesCult and Civ Test 5Jeromy RechNo ratings yet
- # Amendments: Twenty-First AmendmentDocument2 pages# Amendments: Twenty-First AmendmentJeromy RechNo ratings yet
- Cult and Civ Test 5.5Document1 pageCult and Civ Test 5.5Jeromy RechNo ratings yet
- Ethics - Basic TermsDocument1 pageEthics - Basic TermsJeromy RechNo ratings yet
- Cult and Civ Test 3Document5 pagesCult and Civ Test 3Jeromy RechNo ratings yet
- Cult and Civ Test 4.1Document1 pageCult and Civ Test 4.1Jeromy RechNo ratings yet
- Cult and Civ Test 1Document9 pagesCult and Civ Test 1Jeromy RechNo ratings yet
- Common Terms To Know For APUSHDocument2 pagesCommon Terms To Know For APUSHJeromy RechNo ratings yet
- Cult and Civ Test 1.1Document1 pageCult and Civ Test 1.1Jeromy RechNo ratings yet
- Cult and Civ Test 2.1Document1 pageCult and Civ Test 2.1Jeromy RechNo ratings yet
- Calc Review - Series and SequencesDocument1 pageCalc Review - Series and SequencesJeromy RechNo ratings yet
- Cult and Civ Test 2Document5 pagesCult and Civ Test 2Jeromy RechNo ratings yet
- Cult and Civ Test 3.5Document1 pageCult and Civ Test 3.5Jeromy RechNo ratings yet
- CHEM Solutions and Kinetics EquationsDocument2 pagesCHEM Solutions and Kinetics EquationsJeromy RechNo ratings yet
- Basic Excel TutorialDocument20 pagesBasic Excel TutorialJeromy RechNo ratings yet
- Final Exam ReviewDocument19 pagesFinal Exam ReviewJeromy Rech100% (1)
- Elementary Particles: Particle PhysicsDocument22 pagesElementary Particles: Particle PhysicsAshutosh DashNo ratings yet
- Kesan Fotoelektrik 1Document2 pagesKesan Fotoelektrik 1Aleva KongNo ratings yet
- 10 Life Lessons From EinsteinDocument14 pages10 Life Lessons From EinsteinStone HeartNo ratings yet
- IntegralLeadershipReview 3Document8 pagesIntegralLeadershipReview 3Omotayo AkinpelumiNo ratings yet
- R.P. Feynman - A Relativistic Cut-Off For Classical ElectrodynamicsDocument8 pagesR.P. Feynman - A Relativistic Cut-Off For Classical ElectrodynamicsnellopostiNo ratings yet
- Illuminating The UniverseDocument2 pagesIlluminating The UniversePatricia SusckindNo ratings yet
- Takahiro Fukui and Yasuhiro Hatsugai - Topological Aspects of The Quantum Spin-Hall Effect in Graphene: Z2 Topological Order and Spin Chern NumberDocument4 pagesTakahiro Fukui and Yasuhiro Hatsugai - Topological Aspects of The Quantum Spin-Hall Effect in Graphene: Z2 Topological Order and Spin Chern NumberYamcsaNo ratings yet
- Chapter 1 IntroductionDocument6 pagesChapter 1 IntroductionJames Ryan GarmaNo ratings yet
- Perturbation theory explainedDocument24 pagesPerturbation theory explainedDeepak SharmaNo ratings yet
- Bob's Legacy 2Document414 pagesBob's Legacy 2fredekzgredekNo ratings yet
- Photo Luminescence Aplication SDocument10 pagesPhoto Luminescence Aplication SphydiscNo ratings yet
- Quantum Mechanics 01Document50 pagesQuantum Mechanics 01Venugopal ARNo ratings yet
- UntitledDocument489 pagesUntitledEnginNo ratings yet
- Approximation MethodsDocument98 pagesApproximation Methodsbinseung skzNo ratings yet
- Assignment 2 PDFDocument1 pageAssignment 2 PDFManojNo ratings yet
- IndexDocument32 pagesIndexramjiiisNo ratings yet
- ECE Chemistry Review: Key ConceptsDocument49 pagesECE Chemistry Review: Key ConceptsJohnMarcusNo ratings yet
- Hartree FockDocument24 pagesHartree FockJames TruxonNo ratings yet
- S09 NativeWisdomInAQuantumWorld ParryDocument5 pagesS09 NativeWisdomInAQuantumWorld ParryOliduboisNo ratings yet
- Michel Planat and Anne-Celine Baboin - Qudits of Composite Dimension, Mutually Unbiased Bases and Projective Ring GeometryDocument10 pagesMichel Planat and Anne-Celine Baboin - Qudits of Composite Dimension, Mutually Unbiased Bases and Projective Ring GeometryJupweNo ratings yet
- Quantum Mechanics JEST 2012-2017Document30 pagesQuantum Mechanics JEST 2012-2017anup_sky88No ratings yet
- Analyze Paramagnetic Compounds with ESR SpectroscopyDocument27 pagesAnalyze Paramagnetic Compounds with ESR SpectroscopyVeeruNo ratings yet
- Statis Phys, MCQDocument15 pagesStatis Phys, MCQMouaz MahmoudNo ratings yet
- Electromagnetic RadiationDocument5 pagesElectromagnetic RadiationrendakianNo ratings yet
- Laser Physics Course OverviewDocument22 pagesLaser Physics Course OverviewFAKIHA GULZAR BS PhysicsNo ratings yet
- Atom OrbitalDocument4 pagesAtom OrbitalRinaldi SatriaNo ratings yet