Professional Documents
Culture Documents
4 H
Uploaded by
Dee GeeCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
4 H
Uploaded by
Dee GeeCopyright:
Available Formats
February
2, 2016
GEN CAMATO
ERYTHROCYTE STUDIES
HEMATOLOGY 2
I.
II.
III.
IV.
Qualitative evaluation
Quantitative evaluation
Classification of anemias
Common anemias
4.
I. QUALITATIVE EVALUATION
m Blood smear distribution we analyzed the:
o Shape
o Arrangement
o Color of the cells
o Number of the cells that can be found in the
smear
Hb CC Crystals
10
HB SC Crystals
11
*Specimen: WHOLE BLOOD in EDTA tube; Peripheral Blood Smear (CBC)
To check for the NORMAL MORPHOLOGY
*8-20 platelets/field (Oil Immersion Objective)
*Normal color of RBC (if stained) (pink or bluish color) WRIGHTS Stain
*Poikilocytes variations in shape of cells
*Anisocytes variations in size
*Anisochromasia variations in colors
Basics of Qualitative Evaluation of RBC
1.
Developmental variation
Presence of Macrocytes and Microcytes
*Maturation sequence of the RBC or WBC
*The LARGER the CELLS the MORE IMMATURE
2.
Membrane abnormality
Examples:
Echinocytes
aka Crenated RBC
Condition occurs only in RBC that has been placed
1
under Hypertonic NaCl solution (0.9%)
Burr Cell
RBC with Reversible thorn-like projection and
2
somewhat elongated
Achantocytes
aka Thorn cell, Spur cell
found in patient with abetalipoprotein
3
aka Ovaloytes
Elliptocytes
4
found in Pernicious anemia
Stomatocytes
aka Mouth cell
found in Hereditary Stomatocytes, Alcoholism, &
5
Liver diseases
Spur Cell 3
(same with Achantocytes)
Pernicious anemia (Hypersegmented Neutrophils) [5-10 lobes]
3. Trauma formation of:
aka Fragmenting or Disintegrating RBC
Schistocytes
found in Hemolytic Anemia, Patient with sever
6
burns & suffers with Disseminated Intravascular
Coagulation Syndrome (DIC)
Keratocytes of besis aka Triangle cell, Contracted RBC, Helmet cell
7
results after the blistered or ruptured blister cell
aka Pessary cells, Anulocyte
Ghost cell
8
RBC with thin rim of Hb with clear central area
Seen in Iron Deficiency Anemia
aka Sickle cell, C shape, Crescent shape,
Menischocyte
found in Sickle cell anemia, disease
Refers also as Resistant cell, Irreversible reaction
Dark staining hexagonal crystals with blunt ends
inside the cell membrane leaving the cell relatively
free and colorless
Seen in Hemoglobin C disease especially after
Splenectomy
Dark-hued crystals with parallel sides, one blunt,
pointed, protruding end due to condensed
hemoglobin
Washingtons monument
Seen in Hemoglobin SC disease
Dacrocytes Tear drop cell
5.
m Individual morphology of RBC includes:
o Hemoglobin
o Content
o Shape
o Size of RBC
Abnormal Hemoglobin content
Drepanocytes
Inclusion categorized into 3
a. Developmental
Howell-jolly
Pappenheimer
Basophilic stipplings and Retics(Fine
reticulum of RNA)
b. Abnormal Hb precipitation
c. Presence of parasite enlargement of
normal size RBC in the presence of parasite
Howell-jolly
bodies
12
Pappenheimer
13
Basophilic
stippling
14
Heinz bodies
15
Parasite
16
Bar of Gold Crystals Hemoglobin C
Remnant of the Nucleus
Prussian blue stain & Perls reagent
Conditons: severe Hemolytic anemia, Pernicious
anemia, Megaloblastic anemia, Iron deficiency
anemia
aka Siderocytic granules/bodies or Siderocytes
Condtions: Thalassemia, Sideroblastic anemia
aka Punctate Basophilia
Indication of Lead poisoning
Conditions: Megaloblastic anemia, thalassemia &
Alcoholism
(Heinz-erlich bodies)
denatured Hemoglobin
conditions: Sickle cell anemia, G6PD, Hemolytic
anemia, Post spleenectomy
cause by Plasmodium spp. (Malarial parasite)
P. malariae Ziemmans / Christopher dots
P. falciparum Maurers dots
P. ovale James dots
P. vivax Schuffners dots
*other Parasites: Babesia spp. & Trypanosomes
GEN CAMATO
ERYTHROCYTE STUDIES
HEMATOLOGY 2
C. Morphological classification
II. QUANTITATIVE EVALUATION
1.
A. Anemia Due to DECREASE RBC; disorder characterized by O2
B. Erythrocytosis
*Due to:
III. CLASSIFICATION OF ANEMIAS
Anemia disorder characterized by the O2 carrying capacity of the RBC (Decrease
RBC count, Hemoglobin, Hematocrit)
A. Classification of Anemia base on Etiology
1.
2.
3.
4.
Relative anemia (Pregnancy, Menstrual period)
Defective Hb synthesis:
Iron Deficiency Anemia (IDA)
Siderocyte
Non-heme iron deposit Pappenheimer Bodies
Thalassemia
Deficiency in vit.B12 & folic acid(Megaloblastic Anemia)
Normal RBC: 6.2 8.2 m
Giant RBC: > 9 m
Impaired Bone marrow function
Examples:
Aplastic anemia bone marrow injury / drugs (Idiopathic)
2.
3.
4.
Macrocytic
Normochromic
Macrocytic
Hypochromic
Blood loss
(due to: Hemorrhage, Bleeding or any Type of Blood Loss
B. Classification of Anemia base on
Physiology
Ineffective erythropoiesis
If the Reticulocyte Production Index (RPI) is
lower than 2.0
Patient with Hypoproliferative Anemia;
Decrease production of Red cell & maturation disorder
Macrocytic Normochromic
Increase of MCV and MCH but normal MCHC
*Due to:
Pernicious Anemia
Myelophthisic anemia (bone marrow replacement)
Ineffective hematopoiesis(Px with Myelodysplastic Anemia) Young Proliferation of cells
Decrease BM stimulation (Px with Renal Failure; Decrease Release of Blood Cells
5. Macrocytic Hypochromic
Constitutional/ congenital anemia
Increase of MCV but decrease of MCH and
(Diamond Black fan type Anemia Fanconis anemia & Familial Aplastic
Anemia); Under HA
MCHC
Acquired Pure Red Cell Aplasia (PRCA) (Thymoma)
*Due to:
Folate Deficiency Anemia
It has PALE RBC or Hemoglobin distribution
Paroxysmal Nocturnal Hemoglobinuria (PNH)
(Presence of Hemoglobinuria); Pre-leukemic; Under HA
Decreased RBC survival
MCV
MCH
MCHC
2 conditions associated with are:
Normocytic
Normochromic
N
N
N
Intrinsic factor (inside cell)
(ex. Enzyme deficiency & Hemoglobinopathy)
Microcytic Normochromic
N
Extrinsic factor (outside cell)
(Could be due to IMMUNE[Antibody Destruction] &
Microcytic
Hypochromic
2.
Chronic inflammation
Microcytic hypochromic anemia
Decrease in MCV, MCH, MCHC
*Due to ATIS: Anemia of Chronic disease
Thalassemia
Iron Deficiency Anemia
Sideroblastic Anemia
NON-IMMUNE[Hypotonic solution] Mechanism
1.
Microcytic Normochromic anemia
Decrease of MCV and MCH but Normal MCHC
*Due to:
6.
Hemolytic anema
Sickle cell anemia
Hemorrhage
Hemodilution
Aplastic anemia
Hemoconcentration
*Drugs Chloramphenicol(drugs against malaria) or
Too much exposure to Radiation
5.
Normocytic Normochromic anemia
Normal MCV, MCH, MCHC
But there is proportionate decrease in RBC,
hemoglobin and hematocrit
Effective erythropoiesis
If the RPI is greater than 3.0
RBC easily destructed just to compensate RBC.
Cause immediate reaction
Kidneys very sensitive to Hypoxia
Ex. Hemolytic anemia (easily Lyse because of Low pH)
GEN CAMATO
ERYTHROCYTE STUDIES
HEMATOLOGY 2
RBC Indices J
!
I. Aplastic Anemia
Mean Corpuscular Volume (MCV)
W
The description of individual cell size and is the best index for
m Formed due to functional inability of the bone
marrow to replace the RBC
o Normocytic, Normochromic
o Leucopenia
o Thrombocytopenia
o Pancytopenia
m Causes of Aplastic Anemia
o Ionizing Radiation
o Tumor of the Thymus
o Exposure to radicals:
Naphthalene
Arsenical (pesticides)
Benzene
BENZENE in soft drinks has to be
seen in the context of other
environmental exposure.
Taking the worst example found to
date of a soft drink containing 87.9
ppb (Polybrominated biphenyl)
benzene, someone drinking a
350mL (12oz) can would ingest 31g
(microgram) of benzene, almost
equivalent to the benzene inhaled
by a motorist refilling a fuel tank for
3 minutes.
According to the World Health
Organization (WHO), this exposure
can cause Reproductive and
developmental problems, damage
the highest levels of these
compounds are found in soils,
sediments, and food such as dairy
products, meat, fish, and shellfish
Chlordane
m Congenital / Hereditary
o Diamond black fan type
o Viral heap
o Oncogenic virus
m Idiopathic
m Decrease Osmotic Fragility Testing (OFT)
classifying anemias.
W
W
Expresses the volume occupied by a single red cell.
Reference range: 82-92 fl (femtoliter)
Mean Corpuscular Hemoglobin (MCH)
W
A measure of the average weight of hemoglobin in the RBCs.
W
Is of value in diagnosing severely anemic patients
W
RR: 27-32 pg (picogram)
Mean Corpuscular Hemoglobin Concentration (MCHC)
W
The measure of the average concentration of hemoglobin in
W
W
the RBCs.
Most valuable in evaluating the therapy for anemia because
the two most accurate hematologic determinations are used
for calculating this test.
RR: 32-38%
D. 4 Classification of Anemia according to
Cause
1)
Due to decrease RBC production
*Problem in Bone Marrow/Renal disease; APLASTIC ANEMIA
2)
Due to hemolysis of RBC
*HEMOLYTIC ANEMIA observe characteristic of RBC is
SPHEROCYTE(Microcytic cells; No Central Pallor; aka as Fragile cell
3)
Due to nuclear maturation abnormality
4)
Due to cytoplasmic maturation abnormality
*No proper maturation of RB; ex. PERNICIOUS
ANEMIA(Multinucleated/Hypersegmented neutrophil)
*Thalassemia; patient with problem in Iron Deficiency Anemia
Problem in Cytoplasm because of No Proper Maturation
*Poikilocyte problems: Target cell, Bulls eye, Mexican Hat cell,
Codocytes
IV. COMMON ANEMIAS
i.
ii.
iii.
iv.
v.
vi.
vii.
Aplastic Anemia
Hemolytic Anemia
Sickle Cell Anemia
Thalassemia
Severe Iron Deficiency Anemia
Pernicious Anemia
Folic Acid Deficiency Anemia
GEN CAMATO
ERYTHROCYTE STUDIES
HEMATOLOGY 2
B.
II. Hemolytic Anemia
m Formed due to excessive destruction and shortening
of the life-span of the RBC, it can be an acquired or
congenital type
m Causes:
a. Due to the presence of the antibodies
*(Multiple Blood Transfusion)
2)
*Hemolytic disease of the New Born
5)
Chemicals
*Benzene, Lead, Aniline dyes
6)
Mechanicals
*Extraneous exercise
7)
Physical age
Burns, too much exposure
Normocytic, Normochromic
Very high retics count
Increase of NRBC
Increase of Osmotic Fragility Index
Presence of Poikilocytosis
Inclusion bodies: Howell-jolly bodies J
m 2 common types of Hemolytic Anemia
1. PCH (Paroxysmal Cold Hemoglobinuria)
Associated with exposure to cold temperature
due to the presence of cold agglutinin
antibodies also called Donath Landsteiner
hemolysin that fixed in the RBC at cold
temperature and causes lysis in body
temperature.
They exhibit tea colored urine sample
m
m
m
m
m
m
2.
-
Hexose monophosphate
shunt transfer of
glucose to the cell
s Ex. G6PD
Enzyme involve in
methemoglobin
formation Glutathione
synthetase
Infection
Drugs
*Antimalarial drugs such as salicylate &
Chloramphenicol; may lead to HA
Due to toxins/poisons
Due to drugs
Due to inherited intracorpuscular anomaly
affected are the enzymes
m 2 Main Division that Causes HA
A. Intrinsic/Corpuscular defect the problem
is the RBC itself
1) Defect in the cell membrane
o Examples:
o PNH (membrane defect)
o Hereditary spherocytosis
o Achantocytosis
o Elliptocytosis
o Zeives syndrome
2) Defect intracellular enzyme (Nonspherocytic HA) enzyme involve
in:
o Anaerobic glycolysis
s Hexokinase
s Phosphohexose
isomerase
s Phosphoglycerate
kinase
s Pyruvate kinase
o
Paroxysmal Cold Hemolysis (PCH)
*During cold temperature; Lysis of RBC
3)
4)
b.
c.
d.
Extrinsic/Extracorpuscular defect eternal
factor that can cause hemolytic anemia
1) Acquired autoimmune HA
PNH (Paroxysmal Nocturnal Hemoglobinuria)
Associated with complement mediated
hemolysis
Due to a decrease of activity of acetylcholinesterase enzyme that is found in the cell
membrane
Hams Acidified Serum Test
aka Marchiafava-Micheli Syndrome
GEN CAMATO
February 23, 2016
ERYTHROCYTE STUDIES
HEMATOLOGY 2
III. Sickle Cell Anemia
IV. Thalassemia
m due to the presence of HbS variant that causes
sickling of RBC under reduce Oxygen tension
m it may cause vaso-occlusive crisis
m Blood picture:
Presence of sickle cell
Poikilocytes
Thrombocytosis
Neutrophilia
Anisocytosis
Decrease OFI
Inclusion bodies
Normocytic Normochromic
m Due to abnormal production rate of one of the
polypeptide chain of hemoglobin molecule. It can be
the alpha or the beta
m Also known as:
Cooleys anemia
Hereditary leptocytosis (predominant cell Is
target cell)
Mediterranean anemia
m Beta and Alpha thalassemia common in Southeast
Asia
m 2 classification of Thalassemia
Beta thalassemia
Alpha thalassemia
Beta thalassemia
m Cause by point mutation that results in premature
chain termination or abnormal transcription of RNA
that leads to the formation of reduce/absence of
-globin chain
m -thalassemia major predominance of hbF
m -thalassemia minor predominance of HbA2
m Sickle cell can be categorized as:
1. Homozygous SC presence of 2 Hbs
2. Heterozygous SC carrier, they have the sickle
cell trait
Thalassemia major/Homozygous thalassemia
m Results from either a decrease or an absence in chain production by both gene alleles
With a decrease in beta chain production, chain production is high which results in
increased Hb F.
Excess of alpha-chains due to lack of
matching beta chains
Clinical symptoms
m Test for Sickle Cell Anemia
A. Scriver and Waugh method
Simple test for Sickle cell
B.
Daland and DaSilva
Best method
Chemical deoxygenizers
2% Ascorbic Acid
2% Na Methabiosulfate
2% Na dithionite
C.
Shermans method
Uses 2 mL formol saline solution
1 mL mineral oil (to prevent the entrance of
oxygen; for adhesion)
1 mL blood
Stand for 1 hour
% =
#
100
100
HbS + HbA *should not be in the circulation J
INTERPRETATION:
* if 40% is observed after an hour the patient has SC anemia
* 1% of SC after an hour Sickle cell trait
* <40%, wait for 6 hours, then observed again
Jaundice
Splenomegaly
Prominent frontal bones (cheek, jaws)
Chronic marrow hyperplasia
Stunted growth
Delayed puberty
Hemochromatosis
Cardiac failure (major cause of death)
Due to myocardial siderosis by 30 years
of age
* Microcytic/Hypochromic anemia
* Extreme poikilocytosis and anisocytosis (target cells, ovalocytes,
howell-jolly bodies, normoblasts, siderocytes, cabot rings)
* Reticulocyte count is less elevated than what would be expected
b/c of destruction of erythroid precursors in the marrow
* Osmotic fragility test is decrease
* Serum iron is increased
* Indirect bilirubin is increased
* MCV is decreased with an increased RDW
GEN CAMATO
ERYTHROCYTE STUDIES
HEMATOLOGY 2
Thalassemia minor/Heterozygous
-thalassemia
Hb H disease/ Alpha-Thalassemia
Major
m Results from the absence or decrease in beta-chain
production at one gene allele
From severe microcytic/hypochromic
anemia to normal clinical findings
Slight haemolytic jaundice and
splenomegaly
m Hematologic Profile:
RBC count is increased and Hb and Hct are
reduced
RBC indices are below normal and RDW is
increased
Smear shows moderate poikilocytosis,
target cells, and basophili stippling
Osmotic fragility is decreased
Serum iron levels are normal to high
m Thalassemia in which three of the four alpha-globin
genes are absent (--/-)
m Patients who have this disorder have a chronic
haemolytic anemia
m Hematologic characteristics include: decreased MCV
and MCH, hypochromia and target cells on the
blood smear, moderate anisocytosis and
poikilocytosis, reticulocyte count of 4%-5%,
moderate Heinz bodies (Hb H precipitates)
Alpha-Thalassemia Minor
Beta-Thalassemia
m Result from a partial or total decrease in the
production of alpha chains
m Mild alpha-thalassemia, is a deletion of only one
gene; represented as /
m Severe alpha-thalassemia is a deletion of two alpha
globin genes, and is represented as --/
m HYDROPS FETALIS ,with Hb BARTS- most severe
form of alpha-thalassemia. Individuals of this form
have a diploid genotype of --/-- which represents the
absence of all alpha chains
m The absence of all alpha chains is incompatible with
life and infants who have this disorder are stillborn
or die soon after birth
m Display of electrophoretic pattern:
Hb A, Hb F, and Hb A2= NONE
Hb Barts: greater than 80%
m Clinically mild and resembles beta-thalassemia
minor.
m Genetically, patients are lacking two of the four
alpha-globin genes
m --/ or /-
m Patients have mild anemia, microcytosis, and normal
serum iron
m Electrophoretic pattern shows:
I.
Diagnosis is made by finding 5%6% of Hb Barts in cord blood
II.
Adults show no evidence of an Hb
imbalance
m
SILENT CARRIER OF ALPHA-THALASSEMIAS- represents a
disorder that has only one defective alpha-globin gene (/ ) and is not associated with any hematologic
abnormalities
HEMOGLOBIN CONSTANT SPRING (Hb CS) is an alphachain variant with 31 extra amino acids
Alpha chains are functionally normal but
synthesized more slowly, and therefore present
a clinical picture of thalassemia
Hb CS migrates slower than HbA2 at an alkaline
pH
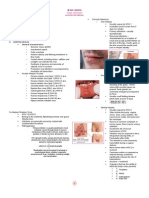
-Thalassemia facial bone abnormalitites. These chsnges include
bossing of the sku;;; hypertrophy of the maxilla, exposing the
upper teeth; depression of nasal bridge; and periorbital puffiness.
-Thalassemia major. Note the pallor, short stature, massive
hepatosplenomegaly, and wasted limbs in this undertransfused
case of -thalassemia major.
March 8, 2016
GEN CAMATO
ERYTHROCYTE STUDIES
HEMATOLOGY 2
m Three Stages of IDA
Iron depletion stage
beginning of iron deficiency. A
negative iron balance develops,
and iron is mobilized from iron
stores. When this process begins,
storage iron decreases, plasma
ferritin decreases, GI absorption
increases to a limited extent, &
amount of plasma ferritin (TIBC)
increases
Iron deficient erythropoiesis stage
occurs when tissue iron stores are
depleted. When transferrin
saturation falls, below 15%, the
percent of marrow sideroblasts
decreases; serum iron is
decreased, & levels of RBC
photoporphyrin increase
V. Severe Iron Deficiency Anemia
m Due to depletion of iron storage in the body, needed
for normal Hemoglobin production
m Causes:
1. Due to poor diet that leads to poor iron intake
2. Due to faulty absorption
3. Excessive iron loss
4. Due to excessive iron demand
m Iron Metabolism-Associated disorders
Most commonly occur as a result of an iron
deficiency but can result from a block in the
enzyme that inserts iron (ferrochelatase)
into the heme ring.
Iron and its metabolism is vital to the body
because a Hb molecule is non functioning
without iron and two thirds or more of the
total body iron is in the RBCs and their
precursors
Each milliliter of RBCs contains 1 mg of iron
Storage iron is present in macrophages or
normoblasts as ferritin or hemosiderin
Recycle Hb is transported to normoblasts by
plasma transferrin
Only 0.9 1.3 mg of iron per day is lost
from the body
m Iron Deficiency Anemia
Results when iron loss exceeds iron intake
for a long time and the bodys iron stores
are depleted.
Iron deficiency develops when there is an
increased need for ironm such as in rapid
growth in infancy, childhood, or during
pregnancy
Most common in children who are between
the ages of 6 and 24 months
Develops when there is excessive chronic
loss of blood
m clinical Signs and Symptoms IDA
Numbness and tingling of extremities
Atrophy of the epithelium of the tongue
with soreness
Cracks or ulcers at the corners of the mouth
Concave or spoon-shaped nails
Pica
m Laboratory Diagnosis IDA
Peripheral Blood:
microcytic/hypochromic RBCs with
marked anisocytosis and slight
poikilocytosis
Reticlocytes are decreased
MCV is low and RDW is increased
Serum iron is decreased; TIBC is
increased (NV: 250-400 mg/dL)
Intracellular protoporphyrin IX is
increased
Bone Marrow:
Normoblasts are smaller than
normal
Storage iron is absent
Sideroblasts are decreased to
lower than 20%
7
By the IDA stage
a clinical anemia becomes
detectable as
normocytic/normochromic anemia,
then gradually progressing to a
microcytic/hypochromic anemia
GEN CAMATO
ERYTHROCYTE STUDIES
HEMATOLOGY 2
m Blood picture:
Microcytic hypochromic
Anisocytosis
Poikilocytosis
Decrease of OFI
Thrombocytosis
Increase Total Iron Binding Capacity (TIBC)
m Primary Familial Hemochromatosis
Condition of iron excess that results from
an abnormality in a gene on chromosome 6
Increase in absorption by intestinal mucosal
cells
Increase serum iron level, increase
transferrin saturation, iron-loading in
macrophages and hepatocytes
m SIDEROBLASTIC ANEMIA
disorder of iron excess that is associated
with defective synthesis of heme because of
multiple enzyme defects, and a resulting
iron overload in the mitochondria of
normoblasts.
Patients RBC morphology: hypochromic
and often microcytic
Increased serum iron levels, decreased
TIBC, and greatly increased transferrin
Increased sideroblasts greater than 50%
Disorder
Serum Iron
Iron
Deciency
Anemia
Decreased
Anemia of
chronic
disorders
Decreased
Sideroblas4c
anemia
Increased
Thalassemia
Normal to
increased
TIBC
% Satura4on
Ferri4n
Bone
Marrow Iron
Increased
Decreased
Decreased
Decreased
Normal to
decreased
Decreased
Normal to
decreased
m UIBC (unsaturated iron binding capacity)
A measure of the reserved iron binding
capacity of transferrin
Formula: TIBC serum iron
m Percent saturation
It is also known as the transferrin
saturation; an index of iron storage
It is the ratio of serum iron to TIBC
Formula:
% =
Normal to
decreased
Normal to
increased
Normal to
increased
Increased
Increased
Increased
Normal to
Increased
Normal to
increased
Normal to
increased
!!"# (!"/!")
100
Increased:
Iron overdose
Hemochromatosis
Sideroblastic anemia
Decreased:
IDA (lowest % saturation)
Malignancy
Chronic infection
Anemia of chronic disease
Reference value:
20 50%
m Serum transferrin
Transferrin is the principal iron transport
protein
Formula:
TIBC (/) x 0.7 = /
It refers to the amount of iron that could be
bound by saturating transferrin and other
minor-iron binding proteins present in the
serum plasma sample
IDA has high TIBC; non-iron anemias has
low TIBC
Anemia of chronic infection has low or
normal TIBC
!"!#$ !"#$ (!"/!")
m TIBC
All TIBC methods require addition of excess
iron to saturate transferrin
Excess iron is then removed by adding
magnesium carbonate to measure the
bound iron
Reference value:
245 425 g/dL (adult male and
female)
10 250 g/dL (>40 years old)
100 250 g/dL (new born and
child)
Formulas: TIBC = UIBC + serum iron
Increased:
IDA
Hepatits
Iron-supplemented pregnancy
Decreased:
Non-IDA
Nephrosis
GEN CAMATO
ERYTHROCYTE STUDIES
HEMATOLOGY 2
VI. Pernicious Anemia
VII. Folic Acid Deficiency Anemia
m Is a conditioned nutritional deficiency of B12
caused by failure of the gastric mucosa to secrete IF
m Also called macrocytic or megalocytic anemia or
Addisonians anemia
m There is 3x increase of RBC production in bone
marrow but the rate of release remains constant
m PA is an inherited disorder, most commonly
occurring in persons older than 40 years.
m 2 types of autoantibodies have been identified in PA
patients
1. Autoantibody that is directed against the
parietal cells
2. Autoantibodies can develop that are
directed against IF or vit. B12 complex
m Most common cause due to:
1. Inadequate dietary intake of folic acid
2. Alcoholism
3. Anorexic patient
4. Those patient who do not eat fresh foods
and vegetables
5. Overcooking of food may attribute to this
deficiency
m Sources. Folate is primarily acquired from the diet
in such food as eggs, milk, leafy vegetables, yeast,
liver, and fruits. A smaller percentage is formed by
intestinal flora.
m Minimum daily dietary requirement has been set at
50 mg. Dietary ingestion just barely meets the
minimum daily requirement. The bodys reserve
lasts for only 3 months.
m Storage. Liver tissue is the main storage site of folic
acid
m Normal serum reference values are 5-21 ug/L, and
150-600 ug/L for RBC folate
m Deficiency in Folic Acid:
Can occur within weeks, as compared with
years for a Vitamin B12 deficiency
A megaloblastic anemia caused by folate
deficiency is most commonly due to
insufficient dietary intake
A womans demand for folate increases
during pregnancy, and pregnant women
should receive folate supplements of
approximately 500 mg/d
Leukopenia and thrombocytopenia; less
common with folate deficiency
m Causes of Deficiency: FOLATE
LIVER DISEASE associated with alcoholism;
differential diagnosis is necessary to
distinguish this from anemia of liver
disease; which has a normal folate but is
macrocytic with a normoblastic marrow
DEFECTIVE ABSORPTION can result from
malabsorption syndromes; adult celiac
disease
INADEQUATE UTILIZATION of folate in the
body can be blocked with chemotheraphy
drugs (methotrexate) which are folic acid
antagonists. In addition to inhibit tumor
growth, chemotherapeutic drugs also
produce megaloblastic anemia.
m Other causes of PA
Gastrectomy
Removes the source of IF and
results in megaloblastic anemia if
vit. B12 supplements are not
provided
Defective absorption of vit. B12
into the intestinal mucosal cell can
result in a secondary vit. B12
deficiency even if dietary intake is
normal
Fish tapeworm
Bacterial infestation in a blind loop
of the intestine
m Blood picture:
Macrocytic Normochromic
Leukopenia
Thrombocytopenia
Pancytopenia
Inclusion bodies
Shift to the right
Anisocytosis
Poikilocytosis
m Laboratory diagnosis base on characteristics
1. Study the peripheral blood smear
2. Consider achlorhydria after histamine
stimulation
3. Megaloblastic dysplasia of the BM
4. Low concentration of Vit. B12 in urine
5. Low conc. Of Vit. B12 in serum
6. BM aspirate, serum, urine are the specimen
needed for the determination of pernicious
anemia
GEN CAMATO
ERYTHROCYTE STUDIES
HEMATOLOGY 2
m There is increased requirement of folate in the
following conditions:
1. Pregnancy
2. Patient with Hemolytic Anemia
3. Patient with exfoliative skin disease
4. Patient who do not meet the normal diet
requirement of folate
m Blood picture:
Similar to pernicious anemia however their
morphological classification is different
Macrocytic Hyperchromic
m LABORATORY PROFILES FOR DNA DISORDERS
DISORDER
CHARACTERISTICS
Megaloblas*c Anemia
-Marrow has enlarged precursor cells
-Blood smear shows: macrocytes
mixed with microcytes, moderate
anisocytosis and poikilocytosis,
dacryocytes, basophilic sJppling,
howell-jolly bodies, cabots rings, and
a few NRBCs
Vitamin B12 Deciency
Folic Acid Deciency
-Pancytopenia
-Low RBC count
-Low haemoglobin, haematocrit
-MCV > 100 fL
-increased to normal MCH
-Leukopenia with hypersegmentaJon
of neutrophils
-Mild thrombocytopenia
-Increased serum iron
-Same characterisJcs as
megaloblasJc anemia
-Serum Vit B12 assay value <100 ng/L
(NV: 200-900 ng/L)
-Increased urinary methylmalonic
acid
-Schilling test* can indicate an IF
dysfuncJon or PA
-Leukopenia and thrombocytopenia
are less common than with Vit B12
-Serum folate <3 ug/L (NV: 5-21 ug/L)
-RBC folate <150 ug/L (NV: 150-600
ug/L)
-Urinary forminomonoglutamic acid
level is increased
10
LABORATORY PROFILE
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Ix. CSF AnalysisDocument3 pagesIx. CSF AnalysisDee GeeNo ratings yet
- Analyzing Urinalysis ComponentsDocument7 pagesAnalyzing Urinalysis ComponentsDee GeeNo ratings yet
- II. Renal FunctionDocument3 pagesII. Renal FunctionDee GeeNo ratings yet
- QA MonitoringDocument3 pagesQA MonitoringDee Gee100% (1)
- Ix. CSF AnalysisDocument3 pagesIx. CSF AnalysisDee GeeNo ratings yet
- Analyzing Urinalysis ComponentsDocument7 pagesAnalyzing Urinalysis ComponentsDee GeeNo ratings yet
- QA MonitoringDocument3 pagesQA MonitoringDee Gee100% (1)
- Analyzing Urinalysis ComponentsDocument7 pagesAnalyzing Urinalysis ComponentsDee GeeNo ratings yet
- II. Renal FunctionDocument3 pagesII. Renal FunctionDee GeeNo ratings yet
- QA MonitoringDocument3 pagesQA MonitoringDee Gee100% (1)
- Analyzing Urinalysis ComponentsDocument7 pagesAnalyzing Urinalysis ComponentsDee GeeNo ratings yet
- Scripture Songs: 1 Corinthians, 1 John, 1 PeterDocument2 pagesScripture Songs: 1 Corinthians, 1 John, 1 PeterDee GeeNo ratings yet
- Ix. CSF AnalysisDocument3 pagesIx. CSF AnalysisDee GeeNo ratings yet
- Ix. CSF AnalysisDocument3 pagesIx. CSF AnalysisDee GeeNo ratings yet
- I. Safety in The Clinical LabDocument3 pagesI. Safety in The Clinical LabDee GeeNo ratings yet
- II. Renal FunctionDocument3 pagesII. Renal FunctionDee GeeNo ratings yet
- ABBDocument16 pagesABBDee GeeNo ratings yet
- GENDocument2 pagesGENDee GeeNo ratings yet
- 2HDocument6 pages2HDee GeeNo ratings yet
- Practice Test CCDocument3 pagesPractice Test CCDee GeeNo ratings yet
- QA MonitoringDocument3 pagesQA MonitoringDee Gee100% (1)
- 5 HDocument4 pages5 HDee GeeNo ratings yet
- God's Love LetterDocument1 pageGod's Love LetterDee GeeNo ratings yet
- Dna VirusesDocument3 pagesDna VirusesDee GeeNo ratings yet
- CC2 ChartDocument2 pagesCC2 ChartDee Gee100% (1)
- Haemoglobin Opa ThiesDocument4 pagesHaemoglobin Opa ThiesDee GeeNo ratings yet
- PH Le BotomyDocument4 pagesPH Le BotomyDee Gee100% (1)
- Hematology LaboratoryDocument3 pagesHematology LaboratoryDee GeeNo ratings yet
- RBC Studies MidtermDocument3 pagesRBC Studies MidtermDee GeeNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Special Pathology Viva Questions by AMS 46Document32 pagesSpecial Pathology Viva Questions by AMS 46Mohan Dass100% (1)
- Platelet Diluting Fluid ProcedureDocument1 pagePlatelet Diluting Fluid ProcedureJohnree A. EvangelistaNo ratings yet
- Schwartz Chapter 33 SpleenDocument20 pagesSchwartz Chapter 33 SpleenGay Solas Epalan100% (1)
- Anemia Nursing Care Management - A Study GuideDocument8 pagesAnemia Nursing Care Management - A Study GuideSachin Singh100% (1)
- Iso 10993 4 2017Document15 pagesIso 10993 4 2017Katerin MartínezNo ratings yet
- Medicine MCQDocument34 pagesMedicine MCQRuturaj JadejaNo ratings yet
- Chapter 3 - Blood Specimen CollectionDocument12 pagesChapter 3 - Blood Specimen CollectionMonica DomingoNo ratings yet
- Flash Notes SyndromesDocument8 pagesFlash Notes SyndromesschxzerrydawnNo ratings yet
- Acetic Acid As An Alternative Reagent in The Modified Knott TestDocument5 pagesAcetic Acid As An Alternative Reagent in The Modified Knott TestVicky Esteban MendozaNo ratings yet
- Int J Lab Hematology - 2021 - Kitchen - International Council For Standardization in Haematology ICSH Recommendations ForDocument12 pagesInt J Lab Hematology - 2021 - Kitchen - International Council For Standardization in Haematology ICSH Recommendations ForLaboratorium RS BELLANo ratings yet
- Clinical Approach For Diagnosis and Management of Anemia-1Document63 pagesClinical Approach For Diagnosis and Management of Anemia-1putri windianiNo ratings yet
- SEO-Optimized Title for Medical Exam DocumentDocument78 pagesSEO-Optimized Title for Medical Exam DocumentAdrian CaballesNo ratings yet
- Blood Analysis of Vaxed and Un Waxed PDFDocument35 pagesBlood Analysis of Vaxed and Un Waxed PDFTanvir SinghNo ratings yet
- Internal Medicine 1 Conrad FischerDocument35 pagesInternal Medicine 1 Conrad Fischerjaber fathiNo ratings yet
- Biomed 24 3 376Document7 pagesBiomed 24 3 376adrianaNo ratings yet
- Complication of Blood TransfusionDocument89 pagesComplication of Blood TransfusionyohannesNo ratings yet
- Spherocytosis AyurvedaDocument4 pagesSpherocytosis AyurvedaSameer HusainNo ratings yet
- Thalassemia Intermedia With Immune Hemolysis During Pregnancy A Report of Two Cases 2155 9864.1000185Document3 pagesThalassemia Intermedia With Immune Hemolysis During Pregnancy A Report of Two Cases 2155 9864.1000185Teky WidyariniNo ratings yet
- Hematology (Part-3) : B-ThelesemiaDocument4 pagesHematology (Part-3) : B-ThelesemiaPriyanka MonikaNo ratings yet
- Aabb Technical Manual 18th EdDocument901 pagesAabb Technical Manual 18th Ednathanmrrk pacayNo ratings yet
- AABB Technical Manual 20th EditionDocument841 pagesAABB Technical Manual 20th Editionparag f67% (3)
- MacConkey AgarDocument12 pagesMacConkey Agardessy asandraNo ratings yet
- Blood TransfusionDocument38 pagesBlood TransfusiontorkNo ratings yet
- Theophilus MicrobDocument4 pagesTheophilus MicrobRaymond NyarkoNo ratings yet
- Preparing Blood Samples for Blood Bank TestingDocument4 pagesPreparing Blood Samples for Blood Bank TestingKriziaNo ratings yet
- Normal FloraDocument30 pagesNormal FloraRaul DuranNo ratings yet
- Clinical Chemistry - Specimen Collection and Patient PreparationDocument6 pagesClinical Chemistry - Specimen Collection and Patient Preparationrosellae.No ratings yet
- HELLP SyndromeDocument29 pagesHELLP SyndromeJames Jayson LibertoNo ratings yet
- Guide to Hemoglobinopathies and ThalassemiasDocument10 pagesGuide to Hemoglobinopathies and ThalassemiashartNo ratings yet
- AnaemiaDocument43 pagesAnaemiaMy NameNo ratings yet