Professional Documents
Culture Documents
Direct Reduction-Are We Moving in The Right Direction?: by K. O. R. Gebhard
Uploaded by
NadyaZulfaniOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Direct Reduction-Are We Moving in The Right Direction?: by K. O. R. Gebhard
Uploaded by
NadyaZulfaniCopyright:
Available Formats
Direct reduction-are we moving in the
right direction?
by K. o. R. GEBHARD
INTRODUCTION
Numerous forecasts predict a rapid
growth
rate
for direct-reduction
processes. The figures quoted are so
impressive that many a top manager
must fear that he will fall behind in
technical development if he does not
base a substantial part of his future
steelmaking for mass production on
direct reduction.
In this context,
the question whether we are moving
in the right direction seems to be
somewhat out of place. But something that seems to be out of place
arouses curiosity, which is needed to
raise interest on a subject that is the
theme of numerous publications.
DIRECT
REDUCTION
OR
DIRECT
STEELMAKING?
All direct-reduction
processes are
based on the 'indirect reduction' of
iron ores with carbon monoxide or
hydrogen. This misnomer helps to
obscure the reasons why these processes, which are correctly
called
'direct steelmaking'
or sponge-iron
processes, were ever invented.
Until the middle of the sixteenth
century, all steel (i.e., iron) that
could be forged was made direct
from ore. Indirect steelmaking
via
blast furnaces, followed by a refining process, was slowly introduced to meet the ever-increasing
demand for steel, which could not be
manufactured
by the direct-steelmaking process. It was certainly a
detour to convert iron ore first into
an iron-carbon
alloy, which contained not only silica and manganese, but also phosphorus
and
sulphur. A second step, i.e., a costly
refining process, is required to convert the 'dirty pig' to clean steel. It
was more than fifty years ago that,
for various reasons,
metallurgists
took up the thread where it had
ravelled out centuries ago. One of
the main reasons was the excessive
energy consumption
in the indirect
process. The blast furnaces needed
1400 kg of coke and more per tonne
of hot metal. Steel refining in openhearth
furnaces
also required
a
considerable amount of energy. Very
forceful economic arguments invoked
the efforts to reintroduce an old art
into modern steelmaking.
In 1933,
more than 350 patents on direct reduction were known.
INDIRECT
STEELMAKING
TODAY
During
the last decades,
the
technology of indirect steelmaking
underwent drastic changes. The coke
consumption
is now about 500 kg
and less per tonne of hot metal. Hot
metal is refined to steel without
extraneous
fuel. Liquid steel can
now be made in the conventional
way at a lower heat consumption
than via sponge-iron production and
electric melting. A comparison made
by Mashlanka,
Knapp, and Kehll
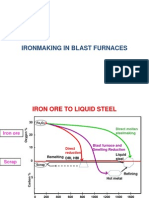
for the Mini Steel Works at Hamburg shows the following: 4,02 Gcal
is required per tonne of liquid steel
made by conventional methods, and
4,33 Gcal is required per tonne of
liquid steel made in the Mini Steel
plant and based on 80 per cent
sponge-iron and 20 per cent scrap.
This, of course, is not the full
story, because energy in coke is
more expensive
than
energy
in
natural gas. However, it is a good
illustration of the drastic changes in
iron and steel technology that once
upon a time gave birth to many
concepts in the field of sponge-iron
production.
Unfortunately,
some of the advances made in technology
have
been erased by rising coke costs.
In 1939, the cost of a tonne of coke
in Europe was DM 19 (about R5);
in 1971, DM 145 (about R36). In the
past, the blast furnace was not good
enough because it consumed
too
much coke. Today, its weak point is
that coke is still used.
What can be done about it? Is
sponge-iron production followed by
electric melting the only alternative
method for steel production
on a
large scale?
Let us imagine that sponge-iron
processes
are unknown.
Let us
further imagine that the top management of an integrated iron and steel
JOURNAL OF THE SOUTH AFRICAN INSTITUTE OF MINING AND METALLURGY
plant instructs
the Chief Metallurgist, as a first step, to reduce the
coke consumption in the blast furnaces and, as a final step, to eliminate, if possible, the use of coke
completely.
Let us also make the
not unlikely assumption
that top
management
expects results in the
not-too-distant
future.
It is hardly conceivable that the
Chief Metallurgist would sit down to
invent
a sponge-iron
process, to
nurse it from basic research in the
laboratory
to the pilot stage, to
arrive finally at the production
stage-which
certainly
would not
have been reached in the 'not-toodistant future'. Instead, we expect
our Metallurgist to look critically at
the various methods
for the injection of substitute
fuels into the
blast furnace. He will find that some
of the results obtained by the injection of fuels like natural gas and
oil are impressive, but he will also
realize that one cannot achieve a
drastic decrease in coke consumption.
After further studies, our Metallurgist will come to the conclusion
that the most promising injection gas
should contain
no hydrocarbons,
should have a low carbon dioxide
and water content, and should be
available
at high temperatures.
In his studies he will come across the
results obtained by Coheur2 on the
Liege low-shaft furnace. Coheur predicts a possible coke rate of 225 kg
per tonne of hot metal when a hot
reducing
gas is injected.
Sironi3
calculated
that coke rates of the
same order of magnitude
can be
expected if a reducing gas is correctly used.
We could take advantage of these
findings provided
a reducing gas
could be produced at an attractive
price in South Africa.
THE PRODUCTION
OF A REDUCING
GAS IN SOUTH
AFRICA
In countries in which natural gas,
oil, or naphtha
is available at a
reasonable price, a reducing gas can
FEBRUARY 1974 295
the Wiberg Process6. Briquetting
of the hot sponge iron should not
present difficulties. The advantages
of sponge-iron briquettes in storage,
handling, and charging are obvious.
Depending
on the degree of reduction that can be achieved economically and on the gangue content of the ore, sponge-iron briquettes can be used as feed stock for
electric furnaces or as a partially
reduced burden in blast furnaces.
be made by a reforming process,
which is considered to be the only
economic method for the production
of a reducing gas for iron ore reduction.
In South Mrica, a reducing gas
can be produced at an attractive
price from coal, which, because of its
ash content, has a rather limited
market value. These coals can be
gasified in the Koppers- Totzek Process4, which is based on the partial
oxidation
of powdered
coal with
oxygen. Up to now, all KoppersTotzek installations
are geared for
the production
of a gas for ammonia synthesis, and the hydrogen
content has to be as high as possible.
This is not needed for blastfurnace
injections,
but no difficulties will be encountered
in the
adaptation
of the Koppers-Totzek
Process for metallurgical purposes.
Preliminary
cost studies
have
shown that a reducing gas can be
produced at lower cost from South
African coal than is possible from
natural gas in Europe or from oil or
naphtha in South Africa.
to be determined.
The heat distribution in the furnace will be affected,
depending on the gas quantities injected, because less hot nitrogen is
ascending from the hearth, as a heat
carrier, to the shaft. This may require gas injections
on different
levels.
The conditions for low coke consumption are ideal when the degree
of indirect reduction
in the blast
furnace approaches
100 per cent,
i.e., when sponge iron is made in the
blast furnace.
It is hoped that design parameters
for a low-shaft furnace of medium
capacity can be deduced from experimental data obtained in a production blast furnace. Scaling down
should be more reliable than scaling
up, i.e., the evolvement
of design
data for an iron-making
unit for
Mini Steel plants could be a further
outcome of development work on a
larger furnace.
THE APPLICATION
OF THE
KOPPERS-TOTZEK
PROCESS
IN BLAST-FURNACE
OPERATION
In the Koppers- Totzek Process,
pure oxygen is used as a carrier gas
for powdered coal. Thus, complete
gasification of the coal is possible
over a short distance,
and it is
possible to attach a gasifying device
direct to the blast furnace.
The
powdered carbon entering the furnace will be converted
in front of
the burner to a hot gas consisting
of carbon monoxide and hydrogen,
the hydrogen content depending on
that of the coal. Because no steam is
added, the degree of oxidation of the
gas is low.
Because powdered
lime can be
added to the powdered coal, it is
possible to combine the advantages
of an acid bosh slag with good
desulphurization
in the hearth.
The numbers and positions of the
injection points, the gas volumes,
and the best temperature
have still
The results of experimental
work
on a production blast furnace will be
useful for the application
of the
Koppers- Totzek Process in the production of sponge iron. It will not
be possible to attach
a gasifier
direct to a shaft furnace for spongeiron production
because the gas
temperatures
are too high and the
gas has to be cleaned. The hot gas
leaving the gasifier can be desulphurized in a column of lime as
was practised
at the Norsk-Staal
sponge-iron plant at Bochum5.
In the sponge-iron processes using
shaft furnaces, part of the top gas
is used to reform natural gas. The
recirculation of the top gas improves
the heat economy of these processes
but complicates
layout and operation.
In a sponge-iron
process
based on gas from coal, part of the
top gas should be used as fuel for
the heating of fresh reducing gas,
and part should be burnt with air
in the upper section of the shaft to
preheat the burden as is done in
296
JOURNAL OF THE SOUTH AFRICAN INSTITUTE OF MINING AND METALLURGY
FEBRUARY 1974
THE APPLICATION
OF THE
KOPPERS-TOTZEK
PROCESS
IN SPONGE-IRON
PRODUCTION
CONCLUSION
Our imaginary Chief Metallurgist
would apparently be well advised to
concentrate his efforts on coal gasification and blast-furnace
injection.
So doing, he will have a good chance
of giving his top management,
in
the not-too-distant
future, an answer
to the pressing question on how to
make iron with little or no coke. He
has not to invent a new process or to
develop existing processes, but to
perfect a metallurgical tool, which so
far is the King-or
Queen-of
metallurgical
devices, i.e., the blast
furnace.
The direction in which we are
moving in respect of direct reduction is, of course, determined by
the position of the target. In broad
terms, the target is to by-pass the
blast furnace. As outlined, we should
set another
target,
namely,
the
making of sponge-iron in the shaft
of a blast furnace.
Sponge-iron production in a separate process (i.e., moving in the
fashionable direction) is one way of
saving coke. Perfecting blast-furnace
operation
(i.e., moving in another
direction) will, it is hoped, not require fifty years of hard development work to produce results that are
still not entirely satisfactory.
REFERENCES
1. International Symposium on Direct Reduction, Bucharest, September 1972.
2. COHEUR, P. C.R.M., no. 29. Dec. 1971.
pp. 3-6.
3. SIRONI, G. International Symposium on
Coal, Rome, March 1973.
4. GRATKOWSKI,V. Stahl u. Eisen, vo!. 80.
1960. pp. 397-407.
5. BULL-SIMONSON, I. Stahl u. Eisen,
vo!. 52. 1932. pp. 457-461.
6. STALHED, J. Stahl u. Eisen, vo!. 72.
1952. pp. 459-466.
You might also like
- ZX125 135 225us Elec eDocument1 pageZX125 135 225us Elec ejacklyn ade putra100% (3)
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNo ratings yet
- Axens CCR Reforming Octanizing Technology To Thailand Refinery-EnglishDocument1 pageAxens CCR Reforming Octanizing Technology To Thailand Refinery-EnglishmohanspathakNo ratings yet
- GGDB (Spec A-E) Genset Parts Manual (10-2009)Document72 pagesGGDB (Spec A-E) Genset Parts Manual (10-2009)Ron SchmittNo ratings yet
- The Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelRating: 5 out of 5 stars5/5 (4)
- 599ba9f5685ec58cc43993da9760b722Document65 pages599ba9f5685ec58cc43993da9760b722Cm SinghNo ratings yet
- Kho Swinbourne10 Mpm188 EAFDocument9 pagesKho Swinbourne10 Mpm188 EAFalilounahdisteNo ratings yet
- Benchmarking of Integrated Steel PlantsDocument38 pagesBenchmarking of Integrated Steel PlantsAnaruzzaman Sheikh100% (1)
- Automotive Interview and ReviewerDocument20 pagesAutomotive Interview and ReviewerMichael Radan90% (10)
- Intro To Organic PowerpointDocument49 pagesIntro To Organic PowerpointBarbra SinghNo ratings yet
- Blast Furnace IronmakingDocument83 pagesBlast Furnace IronmakingKumar Varun100% (1)
- Operation of 300MW CFBC BoilerDocument6 pagesOperation of 300MW CFBC BoilerSoodamany Ponnu PandianNo ratings yet
- Alstom - Integrated Solutions For Coal-Fired Power PlantsDocument3 pagesAlstom - Integrated Solutions For Coal-Fired Power PlantsAlmario SagunNo ratings yet
- Sponge IronDocument23 pagesSponge Ironhijzain0% (1)
- Deepwater Horizon Blowout Preventer Failure Analysis PDFDocument83 pagesDeepwater Horizon Blowout Preventer Failure Analysis PDFHosamMohamed50% (2)
- Iron Making TechnologyDocument53 pagesIron Making TechnologyAwas Ada UdinNo ratings yet
- The Basic Oxygen Steelmaking (BOS) ProcessDocument7 pagesThe Basic Oxygen Steelmaking (BOS) Processaecsuresh35No ratings yet
- Sponge Iron ManufacturingDocument14 pagesSponge Iron Manufacturingapi-2604165367% (3)
- GT13E2 General Data PDFDocument54 pagesGT13E2 General Data PDFThanapaet Rittirut100% (2)
- Catalogo Operacion y Manteniento GTA19Document5 pagesCatalogo Operacion y Manteniento GTA19Jorge Enrique Fuentes MarinNo ratings yet
- Present Indian Steel Making Practice and Its Scenario: Introduction: WHAT IS STEEL?Document10 pagesPresent Indian Steel Making Practice and Its Scenario: Introduction: WHAT IS STEEL?SarbajitManna100% (1)
- Sponge IronDocument11 pagesSponge IronVenkatadurgarao VendraNo ratings yet
- Submerged Arc TechnologyDocument11 pagesSubmerged Arc TechnologymerlonicolaNo ratings yet
- BF Reline ProjectDocument32 pagesBF Reline ProjectAnaruzzaman SheikhNo ratings yet
- Direct Reduction and Smelting ProcessesDocument40 pagesDirect Reduction and Smelting ProcessesAfza NurhakimNo ratings yet
- Coal Selection CriteriaDocument31 pagesCoal Selection CriteriaHardik Kumar MendparaNo ratings yet
- Manufacture of Metallurgical Coke and Recovery of Coal ChemicalsDocument166 pagesManufacture of Metallurgical Coke and Recovery of Coal ChemicalsMarco Milos Trentu100% (1)
- Coke Making TechnologyDocument166 pagesCoke Making TechnologyManab DuttaNo ratings yet
- Shaft Furnace Technology: For Scrap and Waste RoutesDocument6 pagesShaft Furnace Technology: For Scrap and Waste RoutesernandesrizzoNo ratings yet
- State of The Art and Future of The Blast FurnaceDocument16 pagesState of The Art and Future of The Blast Furnacesaibal_silNo ratings yet
- 1.2.2.1 CorexDocument3 pages1.2.2.1 CorexAnggiet HerdayantiNo ratings yet
- Proceso Finex y CorexDocument15 pagesProceso Finex y CorexAnthony AlvarezNo ratings yet
- Iron Making MM-15020 5 Sem B Tech Department of Metallurgy and Materials Engineering V.S.S.U.T, BurlaDocument83 pagesIron Making MM-15020 5 Sem B Tech Department of Metallurgy and Materials Engineering V.S.S.U.T, BurlaAshishNo ratings yet
- Cokeless CupolaDocument12 pagesCokeless CupolaGovind RaoNo ratings yet
- SRMB SteelDocument31 pagesSRMB SteelAmol Ujawane100% (1)
- Industrial Uses of Slag. The Use and Re-Use of Iron and Steelmaking SlagsDocument15 pagesIndustrial Uses of Slag. The Use and Re-Use of Iron and Steelmaking SlagsYinetdJerezNo ratings yet
- Blast Furnace Ironmaking Process Using Pre-Reduced Iron Ore: Nippon Steel Technical Report No. 94 July 2006Document6 pagesBlast Furnace Ironmaking Process Using Pre-Reduced Iron Ore: Nippon Steel Technical Report No. 94 July 2006Farhan AkhterNo ratings yet
- The HIsmelt Iron-Making Process AZOMDocument10 pagesThe HIsmelt Iron-Making Process AZOMalavi419No ratings yet
- A Novel Flash Ironmaking - US Department of EnergyDocument2 pagesA Novel Flash Ironmaking - US Department of EnergyprmthsNo ratings yet
- Chapter 5Document20 pagesChapter 5Oscar Espinosa BonillaNo ratings yet
- Energy: Ivan Najdenov, Karlo T. Rai C, Gordana KokezaDocument9 pagesEnergy: Ivan Najdenov, Karlo T. Rai C, Gordana Kokeza......No ratings yet
- Evraz Highveld SteelDocument6 pagesEvraz Highveld SteelCaio VaccariNo ratings yet
- Addition of Renewable Carbon To Liquid Steel: Plant Trials at Onesteel Sydney Steel MillDocument13 pagesAddition of Renewable Carbon To Liquid Steel: Plant Trials at Onesteel Sydney Steel MillRidwand KartikaNo ratings yet
- Study of Reduction Behaviour of Iron Ore Lumps A: BINAYAK MOHAPATRA (10504004) DHARANIDHAR PATRA (10504021)Document38 pagesStudy of Reduction Behaviour of Iron Ore Lumps A: BINAYAK MOHAPATRA (10504004) DHARANIDHAR PATRA (10504021)Anand BabuNo ratings yet
- The ALUREC ProcessDocument7 pagesThe ALUREC ProcesscarlosiqmNo ratings yet
- 3-0 BOF SteelmakingDocument7 pages3-0 BOF SteelmakingTamal Tanu RoyNo ratings yet
- Coal-Gas Residuals and Their Application.Document24 pagesCoal-Gas Residuals and Their Application.Sesan-David AyokanmiNo ratings yet
- Modern Blast Furnace Ironmaking TechnologyDocument9 pagesModern Blast Furnace Ironmaking TechnologyartNo ratings yet
- Martina Caldaroni Iron Carbide English Rev00Document29 pagesMartina Caldaroni Iron Carbide English Rev00Steve AguilarNo ratings yet
- Literature Review On Iron and Steel IndustryDocument6 pagesLiterature Review On Iron and Steel Industrysmvancvkg100% (1)
- Comparison of Cupola Furnace and BLDocument9 pagesComparison of Cupola Furnace and BLst5154706No ratings yet
- DC Arc Smelting - Conroast SAIMM 2004Document4 pagesDC Arc Smelting - Conroast SAIMM 2004witkerzNo ratings yet
- Continuous CastingDocument29 pagesContinuous Castingshailesh_tiwari_mechNo ratings yet
- Duplexing From Cupolas - DrV.P.guptaDocument16 pagesDuplexing From Cupolas - DrV.P.guptaVirendra GuptaNo ratings yet
- ITmk3 TechnologyDocument8 pagesITmk3 Technologyferozcan100% (1)
- Cupola Furnace Slag: Its Origin, Properties and UtilizationDocument14 pagesCupola Furnace Slag: Its Origin, Properties and Utilizationhemel hasanNo ratings yet
- Chapter 5 - SteelmakingDocument62 pagesChapter 5 - SteelmakingGRAHAM KUNDAI DENGEZANo ratings yet
- The Basic Oxygen SteelmakingDocument13 pagesThe Basic Oxygen SteelmakingEhsanulhaq786No ratings yet
- New Technology PDFDocument27 pagesNew Technology PDFbasavarajNo ratings yet
- Materials Development For Boilers and Steam Turbines Operating at 700 °CDocument19 pagesMaterials Development For Boilers and Steam Turbines Operating at 700 °CAnonymous lmCR3SkPrKNo ratings yet
- 2013-04 Iron and Steel CCS Study (Techno-Economics Integrated Steel Mill)Document642 pages2013-04 Iron and Steel CCS Study (Techno-Economics Integrated Steel Mill)imtinanNo ratings yet
- TSL 2009 349Document7 pagesTSL 2009 349Soo Sang ParkNo ratings yet
- What Is A Basic Oxygen FurnaceDocument2 pagesWhat Is A Basic Oxygen Furnacebiruk geseseNo ratings yet
- Venkat Spongeironrotarykilnmodel IMPC2010Document12 pagesVenkat Spongeironrotarykilnmodel IMPC2010PRASSAN SHAHNo ratings yet
- The HIsmelt Iron Making ProcessDocument6 pagesThe HIsmelt Iron Making ProcessamrohNo ratings yet
- Vizag Steel Plant ReportDocument54 pagesVizag Steel Plant ReportKalyan HalderNo ratings yet
- Innovation in Electric Arc Furnaces: Scientific Basis for SelectionFrom EverandInnovation in Electric Arc Furnaces: Scientific Basis for SelectionNo ratings yet
- Clean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions AbatementFrom EverandClean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions AbatementNo ratings yet
- Heavy Oil Upgrading - A Key Solution For Heavy Oil Upstream and Midstream Operations - IVANHOE ENERGY PDFDocument44 pagesHeavy Oil Upgrading - A Key Solution For Heavy Oil Upstream and Midstream Operations - IVANHOE ENERGY PDFGustavo Gonzalez ServaNo ratings yet
- Xrvs 1000 ServiceDocument10 pagesXrvs 1000 ServiceAbbas AhmedNo ratings yet
- DT60-DT70S (50 HZ)Document3 pagesDT60-DT70S (50 HZ)Md ShNo ratings yet
- Hand Out Kuliah Boiler Fuels and CombustionDocument53 pagesHand Out Kuliah Boiler Fuels and CombustionlirenavirenaNo ratings yet
- 4253462B-Select The Right Cal Bath Fluid WDocument5 pages4253462B-Select The Right Cal Bath Fluid WEyuNo ratings yet
- Harry Okolo Jr. - CV 10th Jan 2013Document5 pagesHarry Okolo Jr. - CV 10th Jan 2013Nuno Sa PunsoNo ratings yet
- Fire Code of The Philippines Practice Exam-MergedDocument17 pagesFire Code of The Philippines Practice Exam-MergedMayumi AngcaoNo ratings yet
- Modelling The Effects of Condensate Banking On High CGR ReservoirsDocument11 pagesModelling The Effects of Condensate Banking On High CGR ReservoirslikpataNo ratings yet
- Cara Kerja Mesin Diesel 4 TakDocument12 pagesCara Kerja Mesin Diesel 4 TakKhairulNo ratings yet
- 11-5417 FAQ Aspen HYSYS Petroleum RefiningDocument2 pages11-5417 FAQ Aspen HYSYS Petroleum RefiningfoamtrailerNo ratings yet
- 9718941Document6 pages9718941ramkrishnaNo ratings yet
- Predicting Hydrocarbon Recovery - AAPG WikiDocument3 pagesPredicting Hydrocarbon Recovery - AAPG WikiWassef MBNo ratings yet
- Properties and Reaction of Methyl BenzeneDocument21 pagesProperties and Reaction of Methyl Benzeneoasis_dessert100% (2)
- Minor Project LPG Gas SensorDocument18 pagesMinor Project LPG Gas SensorHimanshu kumarNo ratings yet
- Lesson 1 IntroductionDocument31 pagesLesson 1 IntroductionLIBRADA , CARLOSNo ratings yet
- Acknowledgment: Electricity Generation Form Speed BreakerDocument81 pagesAcknowledgment: Electricity Generation Form Speed BreakerMayank PatilNo ratings yet
- Cheap, But Challenging: The Disadvantage Is The High Investment For The ConstructionDocument2 pagesCheap, But Challenging: The Disadvantage Is The High Investment For The ConstructionMuhammad Jawad KhanNo ratings yet
- ExxonMobil EMTAM ProcessDocument2 pagesExxonMobil EMTAM ProcessAMANo ratings yet
- A Review - Weight Loss Studies On The Corrosion Behavior of Some Metals in Various MediaDocument8 pagesA Review - Weight Loss Studies On The Corrosion Behavior of Some Metals in Various MediaRonald GarciaNo ratings yet
- HS80 Owners ManualDocument37 pagesHS80 Owners ManualAndy TaborNo ratings yet
- Craftsman LawnmowerDocument30 pagesCraftsman LawnmowerBrendon Roberts100% (1)