Professional Documents
Culture Documents
Ovarian Dysgerminomas Pathology Overview of Ovarian Dysgerminomas
Uploaded by
Devi SyamOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ovarian Dysgerminomas Pathology Overview of Ovarian Dysgerminomas
Uploaded by
Devi SyamCopyright:
Available Formats
http://emedicine.medscape.
com/article/1951026-overview
Ovarian Dysgerminomas
Pathology Overview of Ovarian
Dysgerminomas
Author: Florette K Gray Hazard, MD; Chief Editor: Ramya Masand, MD more...
Updated: Oct 27, 2015
Overview of Ovarian Dysgerminomas
Differentiating Ovarian Dysgerminomas
Laboratory Markers
Gross and Microscopic Features
Immunohistochemistry
Molecular and Genetic Features
Prognosis of Ovarian Dysgerminomas
Show All
Multimedia Library
References
Overview of Ovarian Dysgerminomas
A dysgerminoma is a tumor of the ovary that is composed of primitive, undifferentiated germ
cells. Germ cell tumors arise from primordial germ cells of the ovary and the testis; however,
pathogenesis of the ovarian germ cell tumors is unknown. Of the ovarian lesions, 97% are
benign proliferations (ie, mature teratomas; the remaining 3% are malignant.[1]
Germ cell tumors may be distinguished by their line of differentiation. Primitive, unipotential
germ cells are the precursors to ovarian dysgerminomas and their testicular analogue, the
seminoma. However, pleuripotential germ cells diverge along several lines of differentiation:
trophoblasts, choriocarcinoma; embryonic cells, embryonal carcinoma; extraembryonic
components (endoderm, mesoderm, ectoderm), teratoma; presomite embryoid bodies,
polyembryoma; and yolk sac, yolk sac (endodermal sinus) tumor.
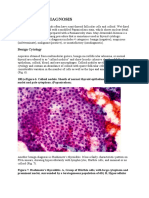
Dysgerminomas are the most common malignant germ cell tumor occurring in the ovary (see
the following image), and these lesions are found most commonly in adolescents and young
adults; in fact, approximately 60% of cases are diagnosed in patients younger than 20 years.[2]
http://emedicine.medscape.com/article/1951026-overview
Common signs and symptoms of ovarian dysgerminomas include abdominal/pelvic pain (5585%), abdominal mass (35%), fever (10-25%), vaginal bleeding (10%), and, occasionally,
ascites. Unlike other germ cell tumors, dysgerminomas often occur bilaterally (approximately
10-20% of cases).
and percentages of malignant germ cell tumors.
Extraovarian tumor spread of dysgerminomas often involves the retroperitoneal and pelvic
lymph nodes; these tumors are highly susceptible to radiotherapy.[1] In addition,
hematogenous spread may occur; common sites of involvement are the lungs, liver, and bone.
[2]
Go to Ovarian Cancer and Borderline Ovarian Cancer for complete information on these
topics.
Next
Differentiating Ovarian Dysgerminomas
The chief disorders in the differential diagnosis of dysgerminoma are lymphoma/leukemia,
yolk sac tumor, embryonal carcinoma, and Sertoli cell tumor. Large-cell lymphoma typically
occurs bilaterally; it is recognized on positive staining of markers for CD45 (LCA) but not
for OCT3/4, PLAP, or SALL4.[3]
Yolk sac tumor may exhibit a solid pattern, simulating dysgerminoma, but other classic
architectural patterns of yolk sac tumor (ie, reticular, microcystic, glandular, or SchillerDuvall bodies) are also invariably present.
Pure embryonal carcinoma is rare in the ovary. However, mixed germ cell tumors occur in
approximately 5% of cases. When present, embryonal cell carcinoma exhibits more nuclear
hyperchromasia and nuclear pleomorphism, amphophilic cytoplasm, high mitotic index, and
necrosis. Often, a glandular or papillary architecture is present. The cells of embryonal
carcinoma express CD30 and cytokeratin (strong, diffuse), whereas those of dysgerminoma
do not.
A study investigated nuclear protein in the testis (NUT) expression in ovarian germ cell
tumors (GCTs). The study found that most malignant ovarian GCTs express NUT protein,
albeit focally, and this should be considered when evaluating immunostaining in the
differential diagnosis of poorly differentiated malignancies, particularly NUT midline
carcinoma. The study further advised that since NUT protein appears to play a role in normal
germ cell maturation it may influence intestinal or hepatoid differentiation within malignant
GCTs.[4]
Sertoli cell tumors may be mistaken for dysgerminoma when tubules are indistinct and/or
solid, especially if they are poorly fixed. However, Sertoli cell tumors do not usually have a
background lymphocytic infiltrate; they express cytokeratin (strong, diffuse), calretinin, and
inhibin.
http://emedicine.medscape.com/article/1951026-overview
Previous
Next
Laboratory Markers
Dysgerminomas are associated with elevated serum levels of lactate dehydrogenase (LDH).
Although these tumors are thought to be hormonally inert, at least 1 case of precocious
puberty occurring in association with dysgerminoma has been reported.[5] The patient was a 6year-old girl whose precocious puberty was caused by elevations in the levels of betahuman
chorionic gonadotropin (beta-hCG), alpha-fetoprotein (AFP), and estradiol.
Additionally, elevated serum levels of neuron-specific enolase,[6] calcium,[7] inhibin,[8]
placental alkaline phosphatase (PLAP), and prolactin[9] have been reported. These serologic
elevations readily resolve following surgical excision; after the elevations resolve, the serum
levels may be used as tumor markers to monitor for recurrence. Because these markers are
more commonly associated with other germ cell tumors (ie, yolk sac, embryonal carcinoma,
choriocarcinoma), many scientists contend that secreting dysgerminomas are misdiagnosed as
pure lesions and that they actually represent mixed tumors containing other malignant germ
cell components.[2]
Go to Gynecologic Tumor Markers for complete information on this topic.
Previous
Next
Gross and Microscopic Features
Dysgerminomas are characterized by their solid nature and rapid growth. Grossly, these
tumors often measure more than 10 cm in maximum dimension at the time of diagnosis. The
classic histology of dysgerminomas features a proliferation of epithelioid cells admixed with
mature lymphocytes arranged in sheets or small clusters separated by thin, fibrous septae
resembling alveoli. The neoplastic cells are large and have moderate to high nucleus-tocytoplasm ratios. Other features are squared-off to round nuclei; vesicular chromatin;
prominent nucleoli; clear to eosinophilic cytoplasm rich in glycogen and lipid; and distinct
cell borders.
Multinucleated forms may be present. Mitotic activity may be significant and may vary
greatly, even within the same tumor; atypical mitoses may be seen. Noncaseating
granulomas, syncytiotrophoblastlike giant cells, and germinal center formation are not
uncommon. Additionally, foci of hemorrhage, necrosis, and small microcalcifications may
also be identified.
Examples of ovarian dysgerminoma histology are shown below.
Ovarian dysgerminoma histology (hematoxylin-eosin, 100)
http://emedicine.medscape.com/article/1951026-overview
Ovarian dysgerminoma histology.
The arrows indicate syncytiotrophoblastlike giant cells. A and B: Hematoxylin-eosin, 200.
C: hematoxylin-eosin, 400. D: hematoxylin-eosin, 600.
Although numerous architectural variants exist, the cytologic features remain constant. These
varying architectural patterns include but are not limited to sparse lymphocytes, trabeculae,
microcysts, and tubules, as well as extensive hyalinization. As yet, no prognostic significance
has been attributed to these architectural patterns.
Dysgerminomas demonstrate a characteristic tigroid background and loosely cohesive,
polygonal cells with round to oval nuclei, vesicular chromatin, and multiple (1-4) distinct
nucleoli on cytology preparations.
Previous
Next
Immunohistochemistry
Immunohistochemistry (IHC) plays an important role in characterizing germ cell tumors.
Although the wide range of architectural patterns may make establishing the pathologic
diagnosis difficult, the immunohistochemical profile is often informative.[1, 2]
The neoplastic cells of dysgerminomas express placental alkaline phosphatase (PLAP),
CD117 (c-kit), OCT 3/4, SALL4, and, variably, cytokeratin (see the images below). They do
not express epithelial membrane antigen (EMA), S100 protein, CD45 (LCA), or alphafetoprotein (AFP).
http://emedicine.medscape.com/article/1951026-overview
Dysgerminoma
immunohistochemistry (x200). CD117 = a proto-oncogen (c-kit) ; CKAE1/CAM5.2 =
cytokeratins; D2-40 = a monoclonal antibody; H&E = hematoxylin-eosin; OCT 3/4 = a
transcription factor; PLAP = placental alkaline phosphatase.
Syncytiotrophoblastlike giant cells are the source of beta-hCG production; this protein
expression is confirmed by immunohistochemistry. D2-40 membrane expression has been
established in testicular seminoma[10] but has not been extensively explored in dysgerminoma.
Previous
Next
Molecular and Genetic Features
Isochromosome 12 (i(12p)) is seen in dysgerminomas, as it is seen in seminomas of the testis.
A wide array of genetic syndromes has been associated with ovarian dysgerminomas;
however, many associations are loose, and establishing molecular/genetic relationships is
problematic. These syndromes include but are not limited to Frasier syndrome,[11] pseudoMeigs syndrome,[12] ataxia-telangiectasia,[13] and Apert syndrome.[14]
Swyer syndrome is an important disorder of intersex that has been shown to have a close
relationship with dysgerminoma, as well as its in-situ counterpart, gonadoblastoma.[15, 16]
Swyer syndrome (pure gonadal dysgenesis) is a disorder of sexual differentiation that is
characterized by mutations of the SRY gene responsible for male sex characteristics. This
gene is located on the Y chromosome (p11.31); mutations at this site result in phenotypic
females with mllerian external genitalia, underdeveloped (streak) internal gonads,
amenorrhea, and rudimentary development of breasts, pubic hair, and axillary hair. These
patients are at increased risk (approximately 20-50% by adulthood) for
dysgerminoma/gonadoblastoma in one or both ovaries.[16]
Gonadoblastomas are neoplastic proliferations found almost exclusively in patients with
gonadal dysgenesis (pure or mixed), male pseudohermaphroditism, and Turner syndrome
(45,XO and mosaics). More than 90% of patients with gonadoblastomas have a Y
chromosome.
Gonadoblastomas are histologically characterized by expanded nests containing Sertoli cells,
germ cells, and granulosa cells admixed with variable amounts of hyaline. These nests
morphologically resemble the Call-Exner bodies associated with granulosa cell tumors.
Leydig cells may also be found within the interstitium. Characteristically, these neoplastic
http://emedicine.medscape.com/article/1951026-overview
nests and/or fibrotic stroma between nests often contain large, irregular calcifications. Mitotic
activity, cytologic atypia, and necrosis are minimal or absent.
The following images depict examples of gonadoblastoma histology.
Dysgerminoma and
gonadoblastoma histology. A: Dysgerminoma with an adjacent zone of necrosis and large
calcifications (40). B: Gonadoblastoma with an adjacent dysgerminoma (40). C:
Gonadoblastoma with an adjacent dysgerminoma (40). D: Gonadoblastoma (200).
Gonadoblastoma
immunohistochemistry (200). CD117 = a proto-oncogen (c-kit), ; CKAE1/CAM5.2 =
cytokeratins; D2-40 = a monoclonal antibody; H&E = hematoxylin-eosin; OCT 3/4 = a
transcription factor; PLAP = placental alkaline phosphatase.
It is not uncommon to see regions of neoplastic overgrowth by dysgerminoma; however,
other malignant germ cell elements may be seen, such as yolk sac and choriocarcinoma.[1, 2]
Previous
Next
Prognosis of Ovarian Dysgerminomas
The prognosis and treatment of dysgerminomas are associated with their pathologic and
clinical stage. The overwhelming majority (approximately 75%) of these tumors are limited
http://emedicine.medscape.com/article/1951026-overview
to one or both ovaries (International Federation of Gynecologists and Obstetricians [FIGO]
stage 1) at the time of diagnosis.
Patients with stage 1A disease (ie, disease that is limited to 1 ovary) may be treated by
unilateral oophorectomy alone, especially when fertility is to be maintained. The relapse rate
ranges from 10% to 20%; the overall survival rate is 90-100%.[17] Patients who suffer relapses
may undergo chemotherapy; the survival rate for such patients is greater than 90%.
Radiotherapy and 3-4 cycles of adjuvant chemotherapy with cisplatinum,
etoposide/vinblastine, and bleomycin are often reserved for patients with at least stage 1B
disease (ie, disease that is limited to both ovaries) or for those patients who suffer
recurrences. For these patients, the results of therapy are similar to those of patients with
stage 1A disease.[18]
You might also like
- 250 Selected MCQs in Urology by Dr. Ahmed Adil-1Document56 pages250 Selected MCQs in Urology by Dr. Ahmed Adil-1siddavaram srideep67% (3)
- 2017 ESMO Essentials For Clinicians Gynaecological TumoursDocument100 pages2017 ESMO Essentials For Clinicians Gynaecological TumoursMaria RamosNo ratings yet
- Praktikum Reproduksi 2Document46 pagesPraktikum Reproduksi 2Andika Tatag100% (1)
- Cancer Stem Cells: Targeting the Roots of Cancer, Seeds of Metastasis, and Sources of Therapy ResistanceFrom EverandCancer Stem Cells: Targeting the Roots of Cancer, Seeds of Metastasis, and Sources of Therapy ResistanceHuiping LiuNo ratings yet
- Germ Cell Tumor Ovary PDFDocument70 pagesGerm Cell Tumor Ovary PDFmelysaNo ratings yet
- Ovarian Dysgerminomas PathologyDocument5 pagesOvarian Dysgerminomas PathologyUtiya Nur LailiNo ratings yet
- Ovarian Malignant Germ Cell Tumors PDFDocument26 pagesOvarian Malignant Germ Cell Tumors PDFFernanda RiveraNo ratings yet
- AlsnlasnfDocument13 pagesAlsnlasnfDina A. ŠabićNo ratings yet
- Flash Points in Special Pathology (Compiled) by Medical Study CenterDocument55 pagesFlash Points in Special Pathology (Compiled) by Medical Study Centerhhewadamiri143No ratings yet
- Yolk Sac Tumor EmedicineDocument5 pagesYolk Sac Tumor EmedicineUtiya Nur LailiNo ratings yet
- Female Genital System PathologyDocument6 pagesFemale Genital System PathologyHelenCandyNo ratings yet
- Breast Lesions Pic For BoardDocument179 pagesBreast Lesions Pic For BoardDr-Mohammad Ali-Fayiz Al TamimiNo ratings yet
- Practicalreviewof Ovariansexcord-Stromal Tumors: Krisztina Z. Hanley,, Marina B. MosunjacDocument34 pagesPracticalreviewof Ovariansexcord-Stromal Tumors: Krisztina Z. Hanley,, Marina B. MosunjacJulia LugonNo ratings yet
- Extragonadal Germ Cell Tumors: H.-J. SchmollDocument8 pagesExtragonadal Germ Cell Tumors: H.-J. SchmollAshutoshNo ratings yet
- Pathogenesis of Testicular Germ Cell Tumours: Leendert H. J. Looijenga and J. Wolter OosterhuisDocument11 pagesPathogenesis of Testicular Germ Cell Tumours: Leendert H. J. Looijenga and J. Wolter OosterhuisLatansa DinaNo ratings yet
- Nonepithelial Ovarian Cancers: ReviewDocument10 pagesNonepithelial Ovarian Cancers: ReviewSaeed HasanNo ratings yet
- Immunohistochemistry As A Diagnostic Aid in The Evaluation of Ovarian TumorsDocument30 pagesImmunohistochemistry As A Diagnostic Aid in The Evaluation of Ovarian TumorsFarah MutiaraNo ratings yet
- G Path-NeoplasiaDocument60 pagesG Path-Neoplasiachouchou124No ratings yet
- Neoplasma OvarialDocument9 pagesNeoplasma OvarialVicky TanNo ratings yet
- TESTICULAR CANCER BookletDocument33 pagesTESTICULAR CANCER BookletCheeBrendaNo ratings yet
- Germ Cell TumoursDocument32 pagesGerm Cell Tumoursapi-3705046No ratings yet
- Other TypesDocument17 pagesOther TypesJuan Maurencius CastleNo ratings yet
- Testicular Tumours 2020Document55 pagesTesticular Tumours 2020Ramesh ReddyNo ratings yet
- Stem CellsDocument16 pagesStem CellsdregopokeNo ratings yet
- Breast CancerDocument56 pagesBreast Cancersarguss14No ratings yet
- Definition of NeoplasiaDocument3 pagesDefinition of NeoplasiaMugiwara YzabelNo ratings yet
- Anatomy and Pathology of Testicular Tumors - UpToDateDocument26 pagesAnatomy and Pathology of Testicular Tumors - UpToDateBhargav YagnikNo ratings yet
- Introduction To OncologyDocument58 pagesIntroduction To OncologyserviceNo ratings yet
- It Does Exist Diagnosis and Management of Thyroid Carc 2023 Surgical PatholDocument12 pagesIt Does Exist Diagnosis and Management of Thyroid Carc 2023 Surgical PatholrubenmacaNo ratings yet
- NEOPLASIADocument15 pagesNEOPLASIADr-Mohammad Ali-Fayiz Al TamimiNo ratings yet
- Neoplasms of Testis NishDocument54 pagesNeoplasms of Testis NishRamesh ReddyNo ratings yet
- Imagenes Citologicas, Benignas, de MamaDocument19 pagesImagenes Citologicas, Benignas, de MamaPauloRosalesNo ratings yet
- Jurnal PDFDocument12 pagesJurnal PDFJanet UngNo ratings yet
- Cornejo 2019 Sex Cord Stromal Tumors of The TestDocument10 pagesCornejo 2019 Sex Cord Stromal Tumors of The TestfelipeNo ratings yet
- Pathology of Germ Cell Tumors of The TestisDocument14 pagesPathology of Germ Cell Tumors of The TestisAnonymous be1sWu6l6No ratings yet
- Multiple MyelomaDocument15 pagesMultiple MyelomaDinda YusditiraNo ratings yet
- Papillary CarcinomaDocument7 pagesPapillary Carcinomarazik89No ratings yet
- Non-Epithelial Ovarian Cancers: Risk ManagementDocument3 pagesNon-Epithelial Ovarian Cancers: Risk ManagementAngga PrabawaNo ratings yet
- 331-Book Chapter-3614-2-10-20210406Document20 pages331-Book Chapter-3614-2-10-20210406Yolla GitamayaNo ratings yet
- Cancer CellsDocument4 pagesCancer Cellsxdmhundz999No ratings yet
- Case Report Epithelial-Myoepithelial Carcinoma of The Breast With Rhabdoid FeaturesDocument4 pagesCase Report Epithelial-Myoepithelial Carcinoma of The Breast With Rhabdoid FeaturesGabriela Izabela BaltatescuNo ratings yet
- Citologie-Fna Tiroida DocumentDocument10 pagesCitologie-Fna Tiroida DocumentMocanu RalucaNo ratings yet
- Wilms Tumor NelsonDocument8 pagesWilms Tumor NelsonvegaNo ratings yet
- Molecular Genetics of Colorectal Cancer - UpToDateDocument35 pagesMolecular Genetics of Colorectal Cancer - UpToDateHartemes RosarioNo ratings yet
- Stem Cells in Breast Tumours - Are They Ready For The Clinicď Ą, 2012Document13 pagesStem Cells in Breast Tumours - Are They Ready For The Clinicď Ą, 2012Daniela GologanNo ratings yet
- Medsci 06 00031 PDFDocument113 pagesMedsci 06 00031 PDFCarolina VillalobosNo ratings yet
- Deteksi Kanker OvariDocument11 pagesDeteksi Kanker OvariFenny SestrianiNo ratings yet
- Neoplasia NotesDocument10 pagesNeoplasia NotesCharlene Fernández100% (4)
- PA 29 Testicular TumorsDocument46 pagesPA 29 Testicular TumorsFangNo ratings yet
- Cytologyofpleuralfluid PDFDocument33 pagesCytologyofpleuralfluid PDFAnonymous brvvLxoIluNo ratings yet
- Ovarian TumoursDocument41 pagesOvarian TumoursVlad RazvanNo ratings yet
- Topics: Nasopharyngeal CarcinomaDocument21 pagesTopics: Nasopharyngeal CarcinomaShruthi Y nursingNo ratings yet
- 4 Review of LiteratureDocument28 pages4 Review of LiteratureSarika RanaNo ratings yet
- Liao 2014Document13 pagesLiao 2014Jenivia LulileloNo ratings yet
- Stem CellsDocument9 pagesStem CellsMaria Del Mar RoblesNo ratings yet
- CAP Protocol-2016 Thyroid - HighlightedDocument8 pagesCAP Protocol-2016 Thyroid - Highlightedpath2016No ratings yet
- Rare Tumour - Sebaceous Carcinoma of The ScalpDocument2 pagesRare Tumour - Sebaceous Carcinoma of The ScalpInternational Organization of Scientific Research (IOSR)No ratings yet
- W de WenisimoDocument18 pagesW de WenisimoBJ CarminatorNo ratings yet
- Classi Fication of Endometrial CarcinomaDocument23 pagesClassi Fication of Endometrial CarcinomaKelly F RuizNo ratings yet
- 6 Tumors IntroductionDocument101 pages6 Tumors IntroductionReZky MusliminNo ratings yet
- Pathologic Quiz Case: Ovarian Mass in A 2-Year-Old Girl Presenting With Pleural EffusionsDocument4 pagesPathologic Quiz Case: Ovarian Mass in A 2-Year-Old Girl Presenting With Pleural EffusionsMateen ShukriNo ratings yet
- Unidad VDocument75 pagesUnidad VpablenqueNo ratings yet
- Sacrococcygealteratoma 170907115555Document19 pagesSacrococcygealteratoma 170907115555Rebuma MegersaNo ratings yet
- 9Document5 pages9Devi SyamNo ratings yet
- Rcog PpromDocument7 pagesRcog PpromDevi SyamNo ratings yet
- Jurnal Menopause KuDocument6 pagesJurnal Menopause KuDevi SyamNo ratings yet
- Jurnal PADocument11 pagesJurnal PADevi SyamNo ratings yet
- Protokol Kemoterapi BepDocument24 pagesProtokol Kemoterapi BepDevi SyamNo ratings yet
- JR 1 FerDocument16 pagesJR 1 FerDevi SyamNo ratings yet
- 1 s2.0 S0002937815003968 MainDocument9 pages1 s2.0 S0002937815003968 MainDevi SyamNo ratings yet
- Pineal Guidelines PDFDocument61 pagesPineal Guidelines PDFWilliam LeeNo ratings yet
- MR 28 Agustus-1Document7 pagesMR 28 Agustus-1BramaNo ratings yet
- Testicular TumorsDocument14 pagesTesticular TumorsJose SirittNo ratings yet
- Sobrevida Niños Cancer ImssDocument9 pagesSobrevida Niños Cancer ImssChristian Escalante DelgadoNo ratings yet
- 2017 Growing Teratoma SyndromeDocument5 pages2017 Growing Teratoma SyndromeOscar GarciaNo ratings yet
- Gyne 2.6 - Benign and Malignant Tumors of The Ovaries and Fallopian TubesDocument8 pagesGyne 2.6 - Benign and Malignant Tumors of The Ovaries and Fallopian TubesVon HippoNo ratings yet
- Testicular CancerDocument31 pagesTesticular CancerRezaGautamaNo ratings yet
- Ovarian Tumours PresentationDocument49 pagesOvarian Tumours PresentationChoden JamyangNo ratings yet
- Tumors of The Body + All Normal ValuesDocument16 pagesTumors of The Body + All Normal ValuesSifat DewanNo ratings yet
- Union Christian College School of Health and Sciences City of San Fernando La UnionDocument11 pagesUnion Christian College School of Health and Sciences City of San Fernando La UnionJobelle AcenaNo ratings yet
- Differential Diagnosis of The Adnexal Mass - UpToDateDocument38 pagesDifferential Diagnosis of The Adnexal Mass - UpToDateRamackNo ratings yet
- Testicular Tumor - Dr. FaizDocument54 pagesTesticular Tumor - Dr. FaizFemale calmNo ratings yet
- Testicular CancerDocument21 pagesTesticular CancerRachel Julianne MoralesNo ratings yet
- 8.male Genitourinary CancersDocument7 pages8.male Genitourinary CancersWoo Rin ParkNo ratings yet
- Testicular Tumors in Undescended Testes in Children Below 5 y of AgeDocument4 pagesTesticular Tumors in Undescended Testes in Children Below 5 y of AgeDhruv MahajanNo ratings yet
- KNTE Patent ApplicationDocument32 pagesKNTE Patent ApplicationCharles GrossNo ratings yet
- GYNE 5.01 - Neoplastic Disease of The Ovary - Dr. TinioDocument7 pagesGYNE 5.01 - Neoplastic Disease of The Ovary - Dr. TinioMonique BorresNo ratings yet
- Testicular Cancer BrochureDocument2 pagesTesticular Cancer BrochuretburkleNo ratings yet
- Chapter 21 Male ReproDocument34 pagesChapter 21 Male ReproIsfahan MasulotNo ratings yet
- K19 - Pathology of OvaryDocument26 pagesK19 - Pathology of OvaryMuhammad AbdillahNo ratings yet
- Jurnal Kanker TestisDocument9 pagesJurnal Kanker TestisDiatni FibriNo ratings yet
- Turner Syndrome: Update The Paradigm of Diagnosis, Clinical Care and Consequences of Y Cell LinesDocument10 pagesTurner Syndrome: Update The Paradigm of Diagnosis, Clinical Care and Consequences of Y Cell LinesBastiaanNo ratings yet
- Ultrasound Differentiation of Benign Versus Malignant Adnexal Masses - UpToDateDocument31 pagesUltrasound Differentiation of Benign Versus Malignant Adnexal Masses - UpToDateRamackNo ratings yet
- Sdxxxxy MRPDocument10 pagesSdxxxxy MRPnon_zenseNo ratings yet
- Ovarian TumoursDocument41 pagesOvarian TumoursVlad RazvanNo ratings yet
- ESGO-SIOPE Guidelines For The Management of Adolescents and Young Adults With Non-Epithelial Ovarian CancersDocument9 pagesESGO-SIOPE Guidelines For The Management of Adolescents and Young Adults With Non-Epithelial Ovarian CancersAna100% (1)
- Urology Case ReportsDocument3 pagesUrology Case ReportsEdo Bagus TantyoNo ratings yet