Professional Documents
Culture Documents
DOC0744835 - Rev11 - Field Service Work Instruction
Uploaded by
Anonymous WBrY38uHOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
DOC0744835 - Rev11 - Field Service Work Instruction
Uploaded by
Anonymous WBrY38uHCopyright:
Available Formats
Rendered PDF File Page 1 of 43 DOC0744835, Rev:11
Electronic Signature Information
Name DOC0744835
Revision 11
Type Controlled Document
Title Field Service Work Instruction
Originator 502304713_prashanth_c_h
Release Date 09/14/2015 11:27:14 AM
Obsolete Date
Name Reason For Change File Size (Bytes)
Field Service Work Instruction.doc Field Service Work Instruction 2196992
Route Signer Function Status Comments Completion Date
R-6358548 305002478_thirumalai__sudhakar Approve APPROVED 11 Sep 2015 11:56:23 GMT
R-6358548 305007933_geetha__s s Approve Approved 11 Sep 2015 09:09:03 GMT
Periodic Review
There are no signatures or routes related to this business object.
Obsolesence Approval
There are no signatures or routes related to this business object.
* Printed versions are For Reference Only *
+ Indicates a task was reassigned from an original assignee
Released
Rendered PDF File Page 2 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Table of Contents
Purpose.....2
Scope.....2
Reference..2
Definitions....2
Organization & Responsibility....5
Training ....7
Infrastructure.14
Document and Data Control .14
Installation Process ....17
Quality Goals and Objectives.......17
Service Process ..18
Service Contract Review ...23
Control of Parts 27
ESD procedures ......30
Record retention ..31
Tools & Calibration equipment ...33
Measurement and Analysis ......35
India Region Regulatory Requirements.....36
Appendix .......38
Service Process Flow References......39
Ownership, Revision History and Effective Date ...41
Page 1 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 3 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Purpose
This procedure explains how the Field Service organization of Wipro GE Healthcare
Private Limited meets the requirements of the GEHC QMS in conjunction with the Site
Quality Plan DOC0695721.
Scope
This document applies to all Field Service personnel in India Region Services. The scope
of this procedure is to define the structure of the Quality System in the Field Service
organization and to specify how roles and responsibilities are assigned.
Reference
External Reference
a. ISO 9001: 2008
b. ISO 13485: 2003
Internal reference
a. Global Quality Manual DOC0041547
b. Site Quality Plan DOC0695721
c. Global Quality Procedures in BOK14210
d. Guideline on India CHU Defects Feedback DOC1410781.
e. PM Work instruction for India Region DOC1705924
f. Customer Refusal letter for Preventive Maintenance DOC1743086
g. Customer Refusal letter for GE approved part replacement DOC1752178
h. All manuals available in Common documentation Library at URL.
http://olcweb.olc.med.ge.com:8020/servlet/ClientServletProp?REQ=Enter%20D
ocumentation%20Library
Definitions
Term Definitions
Customer complaint Written, electronic or oral communication that alleges
deficiencies related to the identity, quality, durability, reliability,
safety or performance of a medical device that has been placed
on the market.
Labeling Written, printed or graphic matter affixed to a medical device
or any of its container or wrappers or accompanying medical
device related to identification, technical description, & use of
the medical device but excluding the shipping documents.
Page 2 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 4 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Term Definitions
Medical Device Any instrument, apparatus, implement, machine, appliance,
implant, in-vitro reagent or calibrator, software materials or
other similar related article, intended by the manufacturer to
be used alone or combination for human beings for one or
more of the specific purpose of Diagnosis, prevention,
Monitoring, treatment alleviation of disease or Injury
Investigation, replacement, modification or support of the
anatomy or of the physiological process
Supporting or sustaining life
Control of conception
Disinfection of devices
Providing information for medical purpose by means of in vitro
examination of specimens derived from the human body and
which does not achieve its primary intended action in or on the
human body by pharmacological, immunological or metabolic
means, but which may assist in its function by such means.
Imaging Equipment Equipment used to diagnose by a way of viewing various images
obtained from the body.
Life Support System Equipment used to sustain life & whose failure to perform its
primary function is expected to result in imminent death in the
absence of immediate intervention.
Corrective repair Service given to a customer when he has a concern issue in
intended usage of the medial device.
Preventive The field engineer performs this proactively as per the
maintenance recommendation of the manufacturer to ensure the machine
function as per the intended usage.
FMI This is a service performed to correct or prevent the issue,
which has been identified by the design team.
Installation This is an activity to fix the medical device at customer site for
the intended use.
De-installation This is an activity to remove the medical device from customer
site.
Calibration Checking or adjusting a tool or equipment by comparison with a
standard.
Install Date The day when customer is able to take his first case on the
equipment for the intended purpose.
Warranty End date The day when machine completes its warranty obligation as per
the manufacturer.
Failure Date The day when the equipment is not able to perform its
intended use.
Page 3 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 5 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Term Definitions
Failure Date The day when the equipment is not able to perform its
intended use.
Call center Pool of people who log the customer reported problem, creates
(Customer Technical a dispatch reference & deputes a service personnel for
Center CTC ) attending to the equipment
Remote Service Person who remotely accesses the system for service
(Leader) Personnel requirements and proactively monitors customer equipment
/Online engineer
Field Engineer / Person who maintains / performs corrective repair at the
Service Engineer customer site. He could be direct employee of GE or a ITP
engineer
Technical Service Interface between service team & Engineering/ Manufacturing.
Manager/ Regional
Service Engineer
Zonal Technical Interface between Zonal service team & technical service
support manager / manager.
Engineer
Service Sales Person who sells the service contract, parts, equipment &
accessories during the course of maintenance of the
equipment.
Site Leader Service Engineer with responsibility for equipment installation
at a site with end to end management
Zonal Service Person who is responsible to adherence of the service process
manager in their zone.
Area Service Person who is responsible to adherence the service process in
manager their Area.
Site down tracker Tracker for part orders & Customer issue escalation process.
(SDT)
Support central (SC) Tracker for part orders, Installation progress, and Customer
issue escalation process.
GE learning Web based training system.
On Job Training Demonstration of activities by a trained engineer to a trainee
and observation of the trainees activities prior to qualifying the
trainee for independent operation.
ISD Integrated service desktop An application used by Remote
service leaders to support Customer call, FE calls. It is also used
by RSL for connecting to customer equipment and for file
transfers
InSite ExC Connectivity application used for connecting product with the
remote engineer
Page 4 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 6 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Term Definitions
ITP Independent Third party. Third party Personnel who provided
service as per the agreed terms & conditions.
SME Subject matter expert is a Personnel who has experience for
more than 3 years in GEHC or Taken TTT / ERT Training course
on the Subject from GEHC.
Trainer SME with experience of the teaching skills through training in
GEHC.
HO (Head office) Team of people who supports the field service team for its
function from Bangalore.
Organization & Responsibility
Field Service is divided into Service Zones, managed by Zonal Service Managers (ZSMs)
who report directly to the Vice President and General Manager of India service. Each
zone has a specified management structure, identified by geographic service area that is
consistent in delivering service activities within the quality system.
The specific organizational structure will be described in an organization chart. Local
Customer Teams (LCTs) are organized throughout the field to focus on key business
goals in support of our customers. LCTs are generally organized around geographic
areas within each Service zone.
The organization chart is available at DOC0705852.
The list of LCTs is available at DOC0745960.
Responsibilities, Authority and Delegation
All responsibilities and authority of applicable process to the Field Service organization
have been defined within the global procedures; see SQP DOC0695721 for applicable
procedures and work instructions.
Indias Service Management Quality Representative has been appointed as per
document DOC0652185.
Job Description for various positions in organization chart will be maintained in
myworkshop under LIB00089 by Human Resource team.
Human Resource team will be able to change the qualification criteria as per the local
requirement in India region.
Hiring of Employee
Page 5 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 7 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Employees are hired into their positions by meeting the position competency
requirements documented in the COS (Career Opportunity System) or Non-exempt Job
Announcement. This is typically a combination of education, previous experience, and
training. Competency evaluations are an integral part of the hiring process and only
those deemed competent to perform the required activities are placed into position.
There may be situations where an employee is hired without a formal posting process.
In those situations, it is the responsibility of the hiring manager/leader to assure that
the employee is capable of meeting the position requirements as documented in the
Goals and Objectives or position guide and is competent to perform the required
activities.
If a position is newly developed, the hiring manager will create, review and approve the
position competency requirements. Human Resources will maintain the position
competency requirements or job description in LIB00089.
Independent Third Party
Independent Third process has been established as per DOC0762117
Outsourced Process
All out sourced vendor supporting service process need to be approved by sourcing
process as defined in SQP DOC0695721.
Some of the outsourced process use by service is listed below.
Warehousing
Disinfection/Fumigation
Installation Support activities
RF Caging
Painting
Transportation
Packaging
Performance of these vendors needs to be reviewed by the process owners and if they
are any Quality concerns it has to be raised with the Quality team.
Quality team will review the concern and if required will take corrective or preventive
action.
Page 6 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 8 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Goals & objectives
Employees need to discuss with their manger on the Goals & Objectives at the beginning
of every year.
Once the Goals & Objectives are established it needs to be captured in the Goals &
objectives Tool in performance appraisal tool.
Guiding Principle for setting up goal for each employee will be established & sent by
Human resource Manager on yearly basis.
Performance Review
Employees are appraised and evaluated as to their job performance on an annual basis
to assure they continue to meet their job requirements, Goals & Objectives for that year
and maintain their job competency. This evaluation may be accomplished using the
EMS, Performance Appraisal forms, or other appropriate forms.
Training
Education Manager is responsible for the co-ordination of training related activities with
all relevant roles within the Service organization.
New Employee Training
Upon being placed into a position, the employee will receive all applicable Trainings.
All employees new to GE shall be given a period of training in accordance with a
program prepared by their manager/leader. The program shall include, but not limited
to company rules, safety and quality policies, EHS training for the position, GEHC
products, GE values, Session C process and other policies.
Records of such training will be documented. Upon completion of the employee
training, a training record shall be created and maintained.
Training Plan
Training Plan Quality
As per the Job Description, Quality team will assign required quality training for each
new employee as per master training plan DOC0702556 within 30 days of joining.
Training Plan EHS
As per the Job Description, relevant EHS training will be assigned to new employees as
per the EHS Training Matrix within 30 days of joining.
Page 7 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 9 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Training Plan - Technical
New Employees will be assigned to Hand Holding process through On job Training. New
Employee immediate Supervisor will assign the mentor. Mentor will be field engineer/
senior field engineer/Technical support specialist who are fully trained on that product.
Mentor will support new employees to learn and get On Job Trainings (OJT). New
employees will be assessed for either single or Multi-Modality roles. Hand holding
process will go for 6 months to 1 year depending on the new employee knowledge,
Experience and number of IBs.
LCT manager will request Training manager for classroom training for new employees.
Training manager consolidates technical training requirements from different LCTs and
arrange for class room training depending on schedule availability.
The training plan for Dealer Service engineer will be created by Authorized dealer
representative along with their Area Service Managers of the respective LCT in
consultation with Dealer Manager.
Technical Training Process
Hand Holding Process
After training plan is made, new Employees will be assigned to Hand Holding process
through OJT (On job Training). New Employee immediate Supervisor will be the Coach.
Coach will identify a senior field engineer and assign the new employees to the senior
field engineer. Senior field engineer will support the new employees to learn and get On
Job Trainings (OJT). New employees will be assessed for either single or Multi-Modality
roles. For employee trained on Hand holding process, OJT certificate will be issued.
On Job Training (OJT)
OJT training needs to be given by experienced employees to the personnel who are new
to the job or task.
On completion competency need to be demonstrated and achieved.
The OJT certificate shall include the name of the instructor; a brief description of the job
carried out on the competencies delivered e.g. tube change. Evidence/records shall be
maintained by the training admin/Training manager.
In certain cases it can be documented through opening a service dispatch with
appropriate code and document the same.
Typically service manuals are used for such training. In case of any additional material
developed locally by the trainer it should be controlled document.
Page 8 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 10 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
On Completion of such training he will be qualified to perform the task, which has been
trained for.
The trainer signing off On Job Training Record should be a full service qualified engineer
either with the class room training certificate or with an approved Full service form.
Demonstrate EHS policies and safe work practices in the service environment. Have read
and understood all modality specific Safety Risk Assessment (SRA) & LOTO procedures.
Mandatory requirement of all basic EHS & modality specific procedures as defined in the
competency model should be completed.
In case the FE has to work in radiation environment he/she need to have valid TLD
badge and should complete Radiation Safety 575 course before attending a call.
FE will not work in High Risk Operations identified independently until he/she completes
all required modality & EHS trainings, but he/she can accompany a full service engineer
or act as a secondary engineer.
Note: On the Job Training record DOC1133454 & Full Service Form DOC1064097.
Field Based Technical Training
Field based training is conducted by the local resource within India region as per the
requirement and LCT managers request.
All such trainings schedules need to be created in GE learning by the training Admin, Full
service credit should be given after completion of training.
The following field based resources are utilized, but not limited to, in meeting specific
training needs: Modality leaders, Support Engineers or other qualified employees
Vendor provided training programs and Consultants
In case of Dealer Service engineer attending the training certificate will be issued & the
dealer will maintain it.
Typically service manuals are used for such training in case of any additional material
developed locally by the trainer it should be controlled document.
GEHC Learning Solutions (Health Care Institute)
Once the training need is identified and agreed upon, the Service Engineer will send
their requests to the Training manager/training administrator.
Training admin check for various courses available using the Global training website or
training schedule given by the training center & enroll for the course in GE learning.
GEHC Learning Solutions utilizes the request list to adjust class sizes or create new
sessions.
Learning Solutions notifies field management of schedule changes, new course
offerings, prerequisites and students who fail to report for training. All documents will
be prepared and maintained by learning solutions.
Other global training centers are located at Japan, China and Europe.
Also time-to-time the manufacturing center will conduct trainings on various products.
Page 9 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 11 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
They will communicate schedules for such trainings to Training manager/Training
administrator so that he can assign the required engineers to these locations.
Qualification of engineers will be as per the process established at their respective
locations. Once qualified at this training center they will be treated has Full service
qualified.
Class Room Training
As per LCT managers request and available slots Training manager will arrange class
room training. In class room training, technical managers will train the employees and
exam will be conducted. Qualified employees will get Class room certificate.
To attend class room training, employee should be trained on basics.
Virtual or Synchronous trainings
Trainer will conduct training through Internet virtually. Field engineer can attend these
courses from their location.
Trainer shall record the attendance sheet and maintained with training administrator.
On completion of training they will be qualified for skills they have been trained.
Grand Father Course
Employee who has experience on the subject for more than 3years in GEHC can elect for
the grandfather course.
This will be conducted through a challenge exam on the subject.
If employee passes this exam he can be considered as full Service qualified employee.
In case of not being qualified classroom course is recommended.
Employee needs to take approval from respective Technical support manager for
electing such course.
Training Competence
Technical training are classified as below,
Full Service
Demonstrates Basic Service competencies.
Able to install the system using appropriate tools, test equipment, perform Calibration
and safety procedures.
Page 10 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 12 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Operate the system at the executive software level to verify proper operation and
duplicate customer complaints.
Able to perform all periodic maintenance and calibrations, including electrical,
mechanical and other adjustments to keep system performing within specified limits.
Isolate faults and system errors using standard and advanced service documentation,
tools and diagnostics.
Remove and replace parts to repair systems at the Field Replaceable Unit level, using
functional checks and performance tests to ensure proper operation.
Demonstrate safe work practices in the Service environment: Including LOTO, proper
use of PPE, HazMat, modality specific Radiation/RF/Cryogen safety, driving safety and
ergonomics awareness as applicable.
In addition to the safety requirements defined in the Basic Service Form, the Field
engineer should be trained on all HRO operations and should have demonstrated to
TSM/ZTSM SM.
Basic Service
Able to take first call, escalating as required.
Demonstrate EHS policies and safe work practices in the service environment. Have read
and understood all modality specific Safety Risk Assessment (SRA) & LOTO procedures.
Mandatory requirement of all basic EHS & modality specific procedures as defined in the
competency model should be completed.
In case the FE has to work in radiation environment he/she need to have valid TLD
badge and should complete Radiation Safety 575 course before attending a call.
FE will not work in High Risk Operations identified independently until he/she completes
all required modality & EHS trainings, but he/she can accompany a full service engineer
or act as a secondary engineer.
Comply with regulatory requirements like AERB, PNDT.
Follow proper RFS procedures, including accepting a dispatch, splitting a dispatch and
debriefing an RFS using service database / recording tools.
Seek support to solve difficult problems by Escalation.
Report product, documentation and Service related defects through service database /
recording tools.
Use FEMC and standard procedures to order parts and tools necessary to repair and
maintain customer equipment.
Use Service Technology tools to aid in customer service and fault isolation.
Demonstrate a working knowledge of modality physics and theory as it applies to
modality that is being worked on.
Identify the basic subsystems required and factors affecting overall system operation.
Operate the system at the Applications level to verify proper system operation.
Can accompany Service Engineers with Full Service qualifications onsite for servicing
activities.
Page 11 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 13 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Job Specific Training
Each employees manager/leader is responsible for reviewing training
needs/recommendations with the employee. These reviews will take place when an
employee changes positions and at a minimum, on an annual basis, typically during the
annual performance review. The format for documenting training needs is described
later in this section. It is understood that some training needs/recommendations are for
the growth of the employee and are not necessarily required to maintain position
qualifications or competency.
In the case of an employee returning to a job for which they have been previously
trained, the manager/leader must evaluate the employees competency, identify any
training needs, and develop a plan as necessary. If it is determined that no training is
required, no action is necessary. Identified training needs/recommendations should be
completed during the next fiscal year unless otherwise stated.
Completion of training may not be credited until effectiveness of training has been
completed. Once that is done, the training record will be updated. Some non-product
or soft skill training may be exempted by the organization from their requirement as
those courses are for employee personnel growth and are not required to maintain
position qualifications and competencies.
Training Documentation
Identified training needs will be completed within the fiscal year unless otherwise
specified. Once the training has been completed the appropriate training record will be
updated. In some cases, no additional training will be required to maintain employee
competency.
The format for identifying training needs may vary as several methods are employed.
Training needs shall be identified and agreed upon by both employee and
manager/leader as needed throughout the fiscal year. The following are examples of
documented training needs plans.
GE Learning: A planned course in GE Learning will become a record upon completion of
the course and no longer will show on the Learning Plan list.
Goals and Objectives
EMS Improvement/Development Needs section
Other forms if applicable
The format for Training Records may vary as several methods are employed and for
some employees, a combination may be utilized. The following are examples of training
records.
Page 12 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 14 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
EMS Major Programs & Training section.
GE Learning System
Other forms if applicable
Functional managers ensure that all assigned personnel are aware of the relevance and
importance of their activities and how they contribute to the achievement of the quality
objectives.
This can be achieved through Zone/LCT/meetings, monitoring of quality objectives and
metrics, and documentation in Gs and Os.
Training material used for local training should be a controlled document & it needs to
be stored in BOK45261 in MyWorkshop.
Trainer Qualification
A subject matter expert in the subject with the TTT qualification can conduct training
example (Technical Support manger of the product).
On certain products Trainers may be trained through Train the Trainer program or early
readiness-training program conducted by GEHC. Once they go through such program
they will be qualified for training on such subject.
Training administrator will maintain all such records as per the training documentation
guidelines given above.
Qualification Criteria
Full Service
FE has completed the course through Class/Lab.
OR
FE has received collateral credit for Full service course.
OR
Has passed the challenge exam after receiving Basic Qualification through
Grandfather Course. Challenge exams will be through GE Learning.
OR
FE has achieved Full Service definition requirements either through experience /OJTs
and evidence available in Full Service Form.
Note: Full Service Form DOC1064097.
Basic Service
Page 13 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 15 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
After assessing the skills as per the Basic Skill definition given above reporting manger
can qualify the person to perform basic service.
FE has achieved Basic Service definition requirements either through experience
/OJTs/On Job Training Record and evidence available in Basic Form.
Note: On the Job Training record DOC1133454 & Basic Service Form DOC1064055
Environmental Health and Safety
Field Services follows the EHS framework established by GEHC.
Infrastructure
The appropriate management staff has the responsibility for determining & providing
the infrastructure needed to achieve conformity to product requirements.
Buildings, Workspace and Associated Utilities
The Field Service Organization leases some local offices any field service employees
work remotely. Local Customer Team offices are covered under appropriate service
maintenance agreements with the lesser for items such as heating, air conditioning,
cleaning, etc.
Process Equipment
Service Engineers are provided with a support infrastructure that provides them with
the necessary support to maintain process hardware and software. Examples of support
include GEHC Help Desk/Client Services, GEHC Global Systems Group, GEHC Telecom,
etc.
Document and Data Control
All materials used for servicing the equipment shall be able to be verified as appropriate
for the equipment being serviced.
Good Documentation Practice
Good documentation practices as detailed below must be followed for documentation
of original data and for all quality documents/records (in written or electronic form).
Installation records, PM Record and Service records are the commonly used records by
Service.
Page 14 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 16 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Original Data recorded as quality data, for example, the test result that you observe
visual checks done on the products, calibration results, etc. which can be handwritten,
printed or stored electronically is maintained as a quality record
When recording original data adhere to the following requirements:
Use indelible ink and make sure that the writing is clear and legible.
Record information directly onto approved and current data collection forms.
Sign and date entries on the day and at the time that the entry is made.
Complete all parts of the form.
Record only pertinent information
Enter data even if it is repetitive (Dont use ditto marks or as above.)
Mistakes happen. Be sure to follow these rules when correcting data:
Correct data errors and omissions as soon as the error or omission is identified.
Do not use correction fluid on any quality record.
Correct errors in such a way that the original entry is not altered, overwritten, or
erased.
Good Documentation Management
Good document management and control ensures compliance with quality
requirements by making sure you have the right information:
Only the current and approved revision of the document is used and procedures
are consistently performed. Ensure that you have the latest revision of Field
Service work instruction downloaded from MyWorkshop.
Obsolete documents are removed from unintended use.
Employees who discover inconsistencies between the procedures and current practice
or who have identified any necessary updates should escalate these changes to the
Quality Manager.
Verifying Materials:
Here is the link that you could use to access Field Service work instruction
DOC0744835.This is the work instruction that guides and details all the Service
processes that you need to carry out in Wipro-GE.
Here is the link where you can access QMS work instruction.
http://myworkshop.health.ge.com/ematrix/common/emxNavigator.jsp?timeZoneOffset
=-5.5
Page 15 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 17 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Here is the link where you can access Service manuals, CDL (Common Documentation
Library).
http://apps.gehealthcare.com/servlet/ClientServlet?REQ=RNEW&MODALITY=XR
Info center link for manual for devices, LSS & DO.
http://dom03.em.health.ge.com/B/Marketing/fi3017d.nsf/Pages/Home.
All documents, CD-ROMs, tapes, instructions, etc. utilized to perform a job function
must be verified by the user as the latest or appropriate revision for the job being
performed. This includes GEHC created documents as well as documents of external
origin.
Methods of verification for service manuals include but are not limited to: electronic
product document management (MyWorkShop), contacting the originator, OEM, using
intranet URLs, etc.
If the user cant verify, he/she will escalate through the proper channels.
The service materials that are shipped with the system are recognized as appropriate for
the equipment at the time of equipment delivery.
It is recommended that site materials be marked with system ID.
Disposition of Materials:
Employee Reassignment - If an individual is reassigned to a new modality or job
function, the employee's manager will make an assessment as to whether any assigned
materials the employee may possess needs to be collected. The manager may also pull
the consignment by site information for any materials co-located at site locations.
Termination The terminated employee's manager is responsible for collecting all
assigned material such as FE consignment, tools, badge, Service report pad, service keys
etc. that the employee may possess.
The manager or designee will ensure that the employee is removed from the
appropriate TAB Distribution Lists. The manager will contact the appropriate individuals
(e.g. - modality specialists) to evaluate the materials for appropriateness.
The manager will ensure materials are in appropriate revisions before transferring
materials to newly assign or replacement employee. The manager may assign this task
to a modality specialist or a ZTSM.
The manager ensures that the new/replacement individual is added to the appropriate
Tabs.
Page 16 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 18 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Any obsolete materials that are retained must be suitably identified as being obsolete,
along with name of the owner. The owner must also be able to explain the rationale for
retaining such documentation.
All other materials used for the purpose of performing a job function, if not found to be
appropriate; will be disposed of in appropriate and timely manner.
Installation Process
Installation processes are defined within Installation global procedure GEHC_GQP_12.05
DOC0373357 and India Service installation WI DOC0748218
Dealer Installations for High end equipments (eg: CT)
Site preparation activities co-owned by ITP and Project Manager. AERB drawing
release done by Project team.
Project manager drives site readiness activities.
Shipment trigger is done by the project manager upon successful site readiness.
Shipment delivery and installation is carried out by the ITP.
QA testing is done by the ITP /Supported by GE FE/Third party QA vendor as per
arrangement with in the dealer location.
ITP submits IR report for back office processing and to activate post installation
setups.
Deinstallation Process
Deinstallation of equipment may be required if a buy back happens or a customer needs
a relocation.
The following are the requirements for Deinstallation process:
A dispatch needs to be opened for Deinstallation activity.
Post Deinstallation as per applicable manuals the service report and
deinstallation report needs to be made.
eGIB updates to indicate deinstallation of parts to be completed.
Quality Goals and Objectives
Annual communication from the Service Head will be sent out for the Goals and
Objectives to Region Service Functions.
Page 17 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 19 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Service Process
Customer Property
Adequate precaution should be taken while working on customer equipments at
customer site.
While working on customer equipment at our premises necessary communication need
to be given to customer on the activity performed on the equipment. Care is exercised
to identify, protect and safeguard customer properly.
In case of any Data loss or damage to the equipment happens while working at either
location necessary communication to be made to the customer through the service
report or letter.
Customer Data Base
CRM information system contains equipment information necessary to create a service
request and to dispatch a qualified Service Engineer.
Service Engineers are assigned to individual equipment in CRM by the Call center with
the help of the Area Service Manager based on the training, experience and competency
of the Service Engineer. These assignments identify the Service Engineer as primary,
secondary, etc. Every Install Base must have at least a primary Service Engineer assigned
to the equipment.
In some cases, a secondary or tertiary engineer is also assigned to the equipment to
provide additional resources. In the event if the primary engineer is not available, the
secondary engineer will be assigned to attend the customer for service.
Weekend and off hours coverage is also a part of the call center protocols.
Customer calls for service are handled 24 hours in a day & 7 days in a week, and are
dispatched based on the customers contract coverage.
Call Handler calls the service engineer or the remote engineer and assigns the dispatch
as per the protocol given to them.
Dispatch Type
Emergency (EMG)/ Breakdown Maintenance (BM)
This type of dispatch is opened for customer calls where they ask for corrective repair of
the equipment where they are not able to use the intended purpose.
Planned Dispatch
This type of dispatch is opened for customer calls where they ask for corrective repair of
the equipment in case they are able to use the machine partially.
Page 18 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 20 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Preventive Maintenance Dispatch
This type of dispatch is opened for preventive maintenance of the equipment.
FMI Dispatch
This type of dispatch is opened for execution of any corrective action / preventive action
on the equipment recommended by the manufacturer.
Billing Dispatch
This type of dispatch is opened where customer does not have any contractual
obligation and they will be billed for such service.
Customer Courtesy call
This type of dispatch is opened in case of visiting the customer without any obligations
as mentioned above.
Training Dispatch
Service engineer to record the training obtained On The Job during the customer visit
with the qualified personnel opens this type of dispatch.
Dispatch Opening
Customers call the call center or Field engineer to request service on their equipment.
Call center nos will be communicated to customer through system id stickers, service
report and etc.
A service dispatch is created and directed to an RSL or ROLE or to a Service Engineer.
Systems equipped with the iLinq feature, customers can automatically create a service
dispatch which goes to the RSL for resolution.
If any customer concerns are received through letter or email the person receiving the
customer letter needs to asses whether it is a service complaint & if applicable open a
dispatch through call center for tracking the issue.
Note: Call center facility is exempted for neighboring country where they dont have this
facility. Customer calls the field engineer and the engineer will open the dispatch
instead of call center.
RSL Response to Service Request
Upon requesting service via CRM or iLinq, the customers service request will be
directed to the RSL where applicable.
Page 19 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 21 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
A Call Handler attempts to transfer the customer live to the RSL. Where a live transfer is
not possible or iLinq is utilized, the service request is transferred to the RSL queue
electronically for support from the first available RSL.
When speaking with a Call Handler the customer can opt to have an FE paged directly in
place of receiving remote support from the RSL.
The RSL remotely connects to the customers system, review error logs, run diagnostics,
and use other service tools to attempt to resolve the problem. If a customer call was
resolved by the RSL, they will debrief the service call.
If the RSL was unable to resolve the problem, RSL will split the call. CTC associates
monitor the queue, assigns the split RFS to Primary FE. Alternatively RSL also informs
CTC to assign the split RFS to FE. The call handler will call the primary Service Engineer &
assign a call.
Service engineer contacts the customer as described in the Service Engineers Response
to Dispatch section.
Service Engineers /Field Engineer Response to Emergency / Break fix Dispatch
The Call Handler provides the Service Engineer responding to the dispatch with the
customer name, system ID, equipment type, equipment status, contract coverage
details and the hospital contact. Service Engineer can also obtain this information from
CRM.
The Service Engineer contacts the customer and the symptoms are discussed. An
attempt is made to either resolve the customers problem over the phone or to
schedule a site visit.
The Service Engineer is responsible for updating the equipment status code to reflect
the operational condition of the equipment.
If the service call is an application issue, the customer is put in contact with the
applications specialist or the RSL at the Call Center and the Service Engineer then closes
the dispatch using the appropriate codes.
If the Service Engineer is required to visit the site, he/she troubleshoots the equipment
including analyzing error logs, running diagnostics, and/or performing various
operational and functional adjustments.
If replacement parts are needed, the parts are ordered through SDT/Support Central
and are installed upon receipt.
Parts/Consumable replacement: It is important to use GE recommended parts
with machines for optimal performance and best results. . Use of
parts/consumables not approved by GE is not recommended.
Always use only GE approved Parts/Consumable where there is a requirement
for parts/ consumables replacement on GE equipment
When there is a situations where customers request GE FE to replace non-GE
Parts/ consumable on GE products, Customer letter (DOC1752178) should be
Page 20 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 22 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
given to such customers who intend or have a tendency to use third party
unapproved parts/consumables with our GE equipments. GE is not responsible
for any safety or reliability issues arising out of using unapproved parts
If the Service Engineer needs technical support, they can contact for support at zone or
HO.
If it becomes necessary for the Service Engineer to transfer the call to another engineer,
the first engineer gives the second an update on site status. The call is split and the
second engineer receives a new dispatch with links to the original dispatch.
While transferring the call or splitting the call there is a need to understand the work
status of the equipment, in case if the equipment is fully down where customer is not
able to use the intended purpose then it should be transferred has emergency status
only.
In the service record the assigned field engineers should capture who all attended the
call.
In case of equipment working partially and the customer is able to use the machine for
intended purpose then they can change the call status to planned call.
Each Service Engineer accepting a call is responsible for updating their original dispatch
with any change in equipment status or service call status.
In case of CRM based system it is responsibility of the service engineer to send the
service report to the administrators to update the system
Service engineer need to maintain the service reports signed by the customer in the
customer file.
In case of any Potential Safety issue escalated by customer or noticed by any of the GE
representative during their customer visit it need to be captured in the relevant section
of the service dispatch in CRM.
In case of CRM system same information need to be updated to the GEHC service
administrator for capturing the same while dispatch closure.
Note: After expiry of contract offering or warranty, which includes part supply & labor
to be provided on billing basis unless approved by the Zonal Service manager or Area
service manager or Zonal Service Sales manager & Technical Operations Manager,
Finance manager at Head office.
Service report requirements
Refer to Guideline on India CHU Defects Feedback DOC1410781for more details on
CHU 35 requirements.
iCenter Tool for Customers
Page 21 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 23 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Some customers have the iCenter feature, which allows them to access information and
data regarding their GE equipment that is installed at their site.
This data includes equipment service history, system uptime, PM results, contract
summary, etc.
Planned Maintenance (PM) Service
The frequency of planned maintenance (PM) inspections is specified in the individual
product manuals or on the GE Maintenance Service Agreements.
Service Dispatch needs to be opened through CRM by call center or Service Admin or
field engineer before attending the PM.
PM Service is scheduled at a mutually agreeable time between the customer and the
local GE service team. Modality specific material, known as PM manuals, PM checklists
or PM schedules provides guidelines on how to perform the activities. These
documents are provided by Engineering and are typically a part of the appropriate
service manuals. These documents provide a high level overview to more specific
procedures that are documented in the service manuals. When required by the service
manuals instructions, recording of data or results of the functional tests will be done by
the service engineer.
During the PMS appropriate tools need to be used & results need to be documented as
per the recommendation of the Service manual.
After completion of the planned maintenance, a copy of the service report need be
given to the customer & signature need to be taken.
The service report for CRM based system needs to be routed to the service
administrator for updating the system.
The official record of PM completion is the dispatch, which is maintained on the GE CRM
system.
If a customer refuses a PM, the customer signing the PM dispatch must document it.
This copy may be maintained locally or will be forwarded to the service administrator. If
a customer postpones or reschedules a PM for a later date, this is not considered a
refusal to perform the PM. The PM will remain open until it is officially completed.
Any problems noted during a PM that cannot be corrected during the PM will be
documented and a fresh dispatch opened.
The system will be put back into service only after the service engineer has verified the
problem identified during the PM will have no effect on patient or operator safety or no
impact on performance specifications.
Field Modification Instruction
Field Modification Instruction (FMI) - FMIs are categorized as per procedure
DOC0070412
Page 22 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 24 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
All FMIs are to be implemented only by GEHC service or authorized GEHC representative
unless otherwise indicated on the FMI document.
The units are identified and electronically tracked by model numbers and serial numbers
/ system ID.
In case of devices all FMIs need to be updated in the CLIFS by the FMI administrator.
In case for recalls (safety and mandatory FMIs), each affected system as identified in the
IB database is required to have the FMI installed.
Optional FMIs are performed per the service engineer or customer's request.
When the FMI is performed, the CRM dispatch debriefs automatically updates the FMI
tracking system.
The FMI kits are shipped directly to the FMI address as specified by the service engineer
or nearest service branch of the customer.
The FMI charge code, model number, customer name, system ID, service class, FMI
number and description need to be filled in the dispatch.
Once the kit is received, the service engineer schedules the modification with the
customer.
Service engineer must fill in the completion code and serial number in order to debrief
the dispatch correctly.
The FMI administrators track the FMI completion status on weekly basis & send the
update to the field.
If the customer refuses the FMI, then the service engineer/FMI coordinator need make
at least 3 attempts with interval of 30 days for completion of FMI. If still customer
refuses to accept or sign, the service engineer may sign and note on the FMI
certification report that the customer verbally refused the FMI. Customer delays or
postponements on installation dates are not typically viewed as refusals.
Service Contract Review
Responsibilities
The Service Sales team is responsible for establishing, releasing and maintaining
contract pricing guidelines (Service Price book) and definition of customer offerings.
The Risk Management on contracts terms & conditions will be evaluated by the
Legal/Compliance, Operations team; they will issue a standard contract terms &
conditions. If there is any deviation-required from the Standard terms & condition the
service sales team should take approval from Legal/ compliance & Operations Manager
of the modality before entering into contract.
The Zonal/Area Service Sales Managers are responsible for ensuring that the customer
requirements can be met, proprietary considerations are satisfied and should be made
aware of all contract activity in their service area (i.e. from LCT, and modality meetings)
before presentation to the customer.
Page 23 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 25 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Customer Operations is responsible for the maintenance and retention of all Service
Contracts.
Contract Review
New and renewal service agreements are negotiated, configured, reviewed and
approved by the appropriate GE representative. GE representative could be a service
sales personnel or Field engineer or service dealer.
Each contract need to be reviewed by Service Sales team & if they notice any terms &
conditions varying from standard published, which may need new clauses to be added
to the contract terms as per the customer requirement then, appropriate approvals
need to be taken from Legal/Operation manager/Service sales Business manager before
submitting the proposal.
Contract terms need to be reviewed before sign off.
The Service Sales team from head office will float the standard contract prices, which
need to be offered for various products.
If any deviation in this price then appropriate approval need to be taken from the
Pricing Manager/Business manager/Finance manager in head office as per the
established process.
In case the equipment is not listed in the Service Price book, the equipment
configuration should be reviewed and a technical evaluation should be made. Factors
used to develop system pricing are, but not limited to, age of the equipment, level of
service available (including parts, technical documentation, and technical experience
availability), past hourly-billed service history, and configuration of the contract of a
similar system. For current production equipment, Service sales at head office will be
contacted to review the pricing and add to the Service Price book.
Before putting equipment under contract, an inspection of the equipment should be
considered to identify items that should be repaired or excluded from the contract.
All repairs to be made before the contract is signed or excluded from the contract &
should be documented and signed by a GEHC representative and the customer.
The preparer of the Service Contract DOC0745921 will review the contract for
completeness before submitting to the GE Representative. It is acceptable for the
preparer to be the GE Representative. The preparers name will be noted on the
contract.
Preparer need to check with the respective Operations team to understand the
serviceability of the machine for the contract period being negotiated.
Terms and conditions, legal requirements, Planned Maintenance offered and different
service coverage offerings are negotiated between the customer and the appropriate GE
Representative.
The GE Representative will then review the contract with the customer, obtain the
customers acceptance and their signature, and sign on behalf of GEHC.
Page 24 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 26 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
If the Service Contract includes only standard terms, Payment terms and meets the
Service Contract process guidelines, no additional contract review is required beyond
the signatures of the customer and a representative from GEHC.
Contracts outside the Normal Guidelines
Any contracts that are outside the normal guidelines stated in the standard terms &
condition or the Service Price book, the person signing the contract as the GE
representative must review & take necessary approvals.
Factors to consider during this internal review include but are not limited to: Quality of
the business decision, ability to deliver the service specified, and overall effect on
customer satisfaction.
In the event that the customer requests a change to the "Terms and Conditions" section
of the contract, escalation to the Legal/Service operations team should occur.
If there is agreement to accept a contract outside the normal guidelines or one with
non-standard terms and conditions, two original contracts are sent to the customer for
signature and then returned to GE Healthcare.
After review and customer signature, the appropriate GE representative per the
guidelines will sign the contract.
Any written communications between legal counsel and the contract negotiator will be
added to the customer file.
During the term of the contract if the contract is renegotiated, the review process must
be repeated. This includes extensions, contract renewals, and adding or deleting of
equipment on contracts.
Customer Contract Renewals
Telemarketing team / Service sales team in Head Office & Area Service Sales
Manager monitors contracts that are up for renewal in that month using data available
in CRM
system.
Contract renewals follow the same contract review process as the original contracts
unless extended by an addendum.
While renewing the contract the person needs to check the Offering Type, Account
Receivable, EOPL status & any Price escalation as per the contract guidelines, in case of
deviation in pricing approval need to be taken from Pricing manger & finance manager
& for Terms & conditions Business manager/Operations manager/Legal need to
approve.
Page 25 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 27 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Proactive approach needs to be followed for contract renewal, Effort should be made to
renew contract prior to expiry. Also recommended practice is to take contract for more
than a year
Note: After expiry of contract offering or warranty, which includes part supply & labor
to be provided on billing basis unless approved by the Zonal Service manager or Area
service manager, Zonal Service Sales manager & Technical Operations Manager, Finance
manager at Head office.
Government Contracts
Government Service terms & conditions reviewed and approved by the legal Team.
GE should do preparation, pricing, contract review, approval and record retention.
Any deviation needs to go through the Service sales Business manager or person
authorized by him need to approve.
In government contracts, if the terms & conditions are accepted as part of tender, all
further contract documents can be signed by Area Service Manager.
Similarly in case of non-government contracts, if the terms & conditions are accepted as
part of Sales PO, all further contract documents can be signed by Area Service Manager.
Billable Service
Billable service will be performed in response to a written / verbal request from a
customer and invoiced as per Service Price Book published by the service sales team.
Recommended practice for such service is to submit a Quotation to the customer as per
the price book & once the purchase order issued by the customer we will proceed with
the service.
Any billable service request will be reviewed by Area Service / Sales Manager and
depending on the review results non-contract customer will be assigned.
In case of labor only billable service, FE should fill in all the information on the call after
completion of the job and keep the dispatch in open status, the same dispatch should
be send for billing at back office against the right customer and back office will close the
dispatch.
In case of parts billing, the sales team will get the purchase order and the back office
team will open a dispatch and bill the part to the customer. The Field engineer will open
additional dispatch for covering the labor services and capture all the issue status in the
dispatch and this dispatch will not be billed to the customer.
In case FE closes the dispatch with the comments and labor hours covered in both the
cases, a separate dispatch will be open by back office for billing the customer and no
billing will happen against dispatch which has been closed by FE.
Contract for Accessory supplies.
Page 26 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 28 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Service support for all vendor supplied Non-listed equipments (Non-GE
accessories/equipments) will be given directly by the vendor.
The type of Service support can be of two types
Labor Contract: This covers Preventive maintenance and labor support for breakdowns.
Comprehensive Contract: This covers Preventive maintenance, Breakdown support and
also part support. But excludes consumables.
The type of contract that Service will enter in to would depend on the Customer and
Business needs.
IB/Customers not in any contract would be supported through Hourly Bill Service from
the vendor for any breakdown.
If GE maintains any of the Vendor supplied items the required manuals and
documentation will be maintained as documents of external Origin.
The activity is limited to cleaning and regular checks .In case of a breakdown the
accessory would be replaced.
The contract with the customer to mention exclusions on those accessories which GE
will not maintain or support customer on maintenance/Breakdowns.
In case of accessories that are not serviceable and can only be replaced, GE may or may
not have a Service agreement.
In case of standard items/off the shelf items like computer hardware and software too
GE may or may not have a Service agreement.
Control of Parts
Field Service will store & distribute all replacement service material used during
equipment service as per the warehouse work instruction DOC0821695.
Preservation of Product
Equipment will be delivered to the site via appropriate distribution processes of the
specific modality. The modality maintains responsibility of the equipment until delivery
is made at customer site. Once on site, India region Service will assure the conformity of
the equipment throughout the installation process until handed over to the customer.
Preservation of replacement parts
Page 27 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 29 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
While storing the replacement service material used for equipment, GE Field Engineer
need to follow the guidelines given below.
Warehousing
Need to maintain & monitor the environmental requirement as per the storage
requirement of each package.
Need to have location marked for Good/Non Conformance parts.
Need to Store the Good part in GOOD location & Defective material need to be stored in
the area identified for Non-conforming material and should not be mixed with other
material.
In case of parts, which have Shelf life expiry date need to be monitored. It is
recommended to practice first in first out.
Check expiry date of shelf-Life parts before delivering such part to the customer.
Need to have pest control measures implemented in case sterile products are stored.
Packaging Requirements
All material (i.e., replacement parts and finished goods) must be transported in their
original container whenever possible. No parts should be transported without adequate
packaging. Need to take care of ESD requirements where applicable.
Service Parts Orders
Service parts orders include Parts, tools, and proprietary items. Order instructions are
included in Support Central/SDT:
Tools and Test Equipment
Tools are to be kept in the protective case recommended by the manufacturer and
secured to prevent damage during transportation.
Control of Service Parts
When it has been determined that a replacement part is needed during a service call,
the FE will order the necessary parts using SDT too or Support central and specifying the
delivery address.
Part receipt
Page 28 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 30 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Once the material is received and prior to use in the equipment the field engineer will
inspect and confirm that the part is good by one of the following means
1. Green sticker in case of GPRS supplied parts unless the shipment document
explicitly states that the part is defective it is considered a good part.
2. Part identified as good by either writing on the box/ Tag/Label that indicates that
this is a good part.
Unpack & do a visual inspection of the material for damage & whether part supplied is
matching the requirement as per the order placed. In case of part which has shelf life
check for the expiry date.
In case of part, which has been damaged during shipment, Field engineer needs to
escalate the same to logistics team & logistics will place an order for another part.
If the part is deemed as conforming, i.e. good, service engineer will repair the
equipment using the part supplied.
In the event the Service Engineer determines the replacement part did not resolve the
equipment problem, the original part should be removed from the equipment and
repacked using the original packing.
The field engineer will affix a Green Label (part # 5163644) to the container identifying it
as good part to return to distribution center / point.
If the part is deemed as nonconforming part i.e. needs to be returned back has defective
or recycling,
Service engineer will place the part in the original packing,
If non-conformance due to part supplied is not matching as per the order or damaged
while transportation need to use the Defective Red Label (part # 5162860) & capture
the right part no in the defective sticker after declaring the same in FEMC. The defective
part removed from the customer equipment that has Due in on FEMC should be
labeled with Red sticker
In case there is no due in the part needs to have a purple label. These parts will be
sent for disposition as per GEHC EHS guidelines.
In case GE supplied labels are not available with the part identify as Good or
Defective either by writing on the box, identifying by location, putting on labels with
the word Good or Defective as applicable.
Page 29 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 31 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Any labels like "Good to stock, Defective" or "Recycle need to be filled with complete
information like part numbers, SSO ID/Name ,Remarks etc as applicable on the label .
Note: All parts/materials supplied by GEHC, (unless customer wants it for insurance
purpose or purchased has non-returnable material) needs to be disposed /scrapped
through GEHC only.
Training / Loaner Equipment / Sales Demo Equipment
Field training equipment - Equipment used for training of Service Engineers to
supplement Service Technical Training.
Loaner Equipment - Service equipment given to customer while his equipment is being
repaired or awaiting parts. While getting the customer equipment for repairing it should
be sterilized/decontaminated/patient data removed before repairing.
For further information, refer DOC0874313 - GEHC_GQP_12.08.001 Handling of
Demonstration, Evaluation, and Loaner Units
All such equipment needs to be identified and traceable in the applicable system.
Sales Demo-Equipment used by sales for equipment demonstrations at customer sites is
Sales responsibility to ensure equipment is maintained in good working order.
In the event there are deviations from this procedure, approval from Operations
Manager must be obtained.
ESD procedures
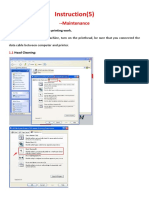
ESD wrist strap testing using Multimeter
Carry out ESD wrist strap test with multimeter every time a Wristband is used as
per instruction in the next slide.
Record on the Service report the resistance value observed and confirms it is
within acceptable range.
Record on the Service report.
All personnel handling the PCBs and any other static sensitive parts should follow
adequate ESD precautions such as wrist strap.
Personnel using such ESD devices shall ensure Wrist strap need to be replaced every
two years or check with the multimeter for continuity periodically if the continuity test
fails then replace the strap.
Page 30 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 32 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Record retention
Quality Records
Quality records are maintained to demonstrate achievement of the required quality of
Field Service are detailed here.
This is a listing of records, owners, retention period and how identified.
Record Owner Identified by System Retention period
Document 5 years from the date of record
Quality Audit QARA Myworkshop
Number. completion
Management Document 5 years from the date of record
QARA Myworkshop
Reviews Number. completion
Corrective 5 years from the date of record
QARA CAPA Number Trackwise
actions completion
Page 31 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 33 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
The minimum retention time is the
greatest of the following:
Design and expected life of the
Service Operation
Dispatch number CRM /Hardcopy Device
dispatches Manager
The stop of production and
distribution + 5 years from the
last shipment date
The minimum retention time is the
greatest of the following:
Design and expected life of the
Operation
CSO logs Log Number CRM device
Manager
The stop of production and
distribution + 5 years from the
last shipment date
The minimum retention time is the
greatest of the following:
Design and expected life of the
Operation
GIB Records System ID CRM device
Manager
The stop of production and
distribution + 5 years from the
last shipment date
The minimum retention time is the
greatest of the following:
Design and expected life of the
Installation Operation
System ID CRM / Hardcopy device
Report Manager
The stop of production and
distribution + 5 years from the
last shipment date
Service Sales The stop of production and distribution
Service contract Contract number CRM /Hardcopy.
Manager + 5 years from the last shipment date
The stop of production and distribution
Education Employee
Training Mylearning + 5 years from the last shipment
Manager and HR number or Name
Date
Additional records may be called out within the individual sections in this manual. All
Hardcopies where electronic record storage is available should be stored for a minimum
of two years. All hard copy record to be stored in manner to ensure they are safe and
not retarded. However if legal, compliance, regulatory requirements exists they should
be stored as long as required for other activities.
Service Dispatches
Service Dispatches are documents created on the GE CRM for service on customers
equipment.
Service Dispatches document parts and labor for planned maintenance, emergency
service, installation, warranty, and FMIs.
The owner of these records is Information Systems.
Service report hard copy DOC0737562 or DOC0745902 signed by the customer against
each dispatch need to be stored at customer file & the content need to be entered in
the information system like CRM against each dispatch.
Page 32 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 34 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
It is recommended to store such document in customer file at least for 2 years.
Training
Training covers both training records and training plans.
Training includes technical, non-technical and OJT.
All records will be stored as per the documentation requirement procedure.
Tool Calibration Records
The tools coordinator of each area accounts for tools and test equipment within the
Zone. The tool admin maintains all calibration records.
Service Contracts
Original service contract documents (between GE and a customer) are kept for 2 years.
This data is loaded into the information system for tracking purposes.
Install report
The Notice of Install Complete is the document that indicates the installation is
complete and ready for 1st patient use. When completed, it starts the Transfer Process
to collect final payment and start the warranty process.
Tools & Calibration equipment
Procedures
Service Engineer communicates tools & calibration needs to designated service
Coordinator in their location.
Area Service Manager in each location is responsible to assure that all Service Engineers
have access to the basic tools and measurement equipment to perform their work.
The Service Engineer will NOT utilize any personnel or uncontrolled test and
measurement equipment on any customer equipment.
Time to time Technical Service Manager will evaluate the Tools required for maintaining
the equipments.
Test and Measuring Equipment Tracking
CRM is tracking system for tools, test and measurement equipment and other assets
that doesnt require calibration.
All test and measurement equipment requiring calibration will be documented.
Items in CRM will contain enough information to adequately describe and identify the
item.
Page 33 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 35 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Test and Measuring Equipment Identification
Equipment is tracked through its Model No & serial no.
Test and measuring equipment is distributed by HO and shipped to the respective
branches. Unique identification number is assigned at HO.
Each branch is responsible to ensure the calibrated tools are used.
Calibration status will be displayed on equipment as per Calibration Procedure
DOC0373363.
Service providers must meet the same criteria as company owned equipments.
Master list of equipments will be maintained at HO.
Receiving Tools and Test Equipment
Upon receipt of test equipment the receiver must perform the following as guidelines of
a visual inspection:
When a new tool is purchased.
When a new tool is purchased,
1. Verify calibrations certificate for validity and check for the calibrations label with
due date if not available the same.
2. Apply asset tag label.
Verify calibrations certificate for validity and check for the calibration label with due
date if not available affix the same.
Check to ensure that the calibration sticker, if applicable, is attached, dated correctly
and current.
Check to ensure that tamper seals, if applicable, are attached and not damaged.
Check to ensure that the equipment is not physically damaged
Check to ensure that the equipment operates in the appropriate manner.
If service or operator documentation from the manufacturer is received with the test
equipment, the recommended function check, when applicable, should be completed.
If the unit is not deemed to be operationally ready, then the tool should be returned to
the calibration supplier.
Test and Measuring Equipment Calibrations/Repairs
The User of the test equipment is responsible for the day-to-day integrity of the
calibration / repair.
Items sent out for calibration or repair must be sent only to approved vendors, which
includes, but is not limited to, the original manufacturer of the equipment.
When the calibrated/repaired tool has been returned from the vendor, the coordinator
will update records.
The hard copy of the calibration record should be stored in a place where it can be
retrieved easily.
Page 34 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 36 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Should any item requiring calibration go past its calibration due date, the Area Manger
will ensure that the FE, and designated tool coordinator are aware of the non-compliant
equipment.
When the tool is returned from the calibration vendor, the service engineer will follow
the process documented in the Receiving Tools and Test Equipment section above.
Tools past due for calibration will not be used to service customer equipment.
Quality Records
Calibration Certificates for all In Service calibrated items will be stored at the head
office or branch.
Measurement and Analysis
Technical Operation Manager can decide the parameters and frequency of
measurement needs to be monitored for quality purpose.
Back office will publish the data to the field team on various parameters as specified by
Technical Operation Manager.
Field team should review and take necessary actions, if required escalation process can
be followed to get the issue addressed.
Some of the parameters monitored currently are specified below
PODA
FMI report
PM Adherence report
15 min and 12-hour response time report
All these reports should have at a minimum Branch Location, FE name, Dispatch no,
more fields can be added as per the reporting requirement.
Customer Feedback report will be owned by the lean leader and he will analyze the data
and take necessary action with the help of various process owners.
Above report shall be reviewed during the management review.
If required corrective or preventive measures should be taken to address any issues.
Document of external Origin
Documents from external origin like User manuals, Service manuals from accessory
suppliers to be maintained at the branches/Head office.
Page 35 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 37 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Individual users are responsible for ensuring that only the current and effective revision
of the document is used for operation.
India Region Regulatory Requirements
Local Regulations
AERB
Type Approval / No Objection Certificate
Prior to import / installation of X-ray equipment, the manufacturer shall obtain a
valid NOC Type Approval Certificate from the competent authority for
indigenously made equipment.
If the equipment is of foreign make, the importing/vending agency shall obtain a
No Objection Certificate (NOC) from the competent authority, prior to importing
the First equipment.
Type Approval shall be issued only if the equipment satisfies the safety
specifications and the standards in force, as demonstrated by actual type testing
of the equipment.
Only type-approved / NOC obtained equipment shall be imported and used in
India.
Structural Shielding requirements for X-ray emitting product installation to be
met as per requirements mentioned at www.aerb.gov.in
Registration of X-ray emitting Equipment Responsibility by Customer
All the X-ray equipment shall be registered with the competent authority by the
person/hospital who owns the equipment.
Send the registration form duly filled with Quality Assurance Report to AERB to
obtain license. There is no fee for registration.
QA test needs to be conducted once in two years.
Diagnostic X-Ray AERB Guidelines link:
http://www.aerb.gov.in/T/XRay/forms/Guidelines_users.pdf
Nuclear Medicine AERB Guidelines link:
http://www.aerb.gov.in/T/forms/regforms/nuclear_medicine/docs/Guidelines_NM.pdf
For more details refer to Layout Approval requirements for DI products and AERB
requirements DOC1190838.
Page 36 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 38 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
PNDT Registration -India
User should register their Ultrasound equipment with PNDT authorities and
follow their requirements. It is required that dealer shall not sell, distribute,
supply, rent, allow or authorize the use of ultrasound equipment at any location
that does not have the PNDT certificate.
PNDT & affidavit must be collected prior invoicing any Ultrasound system from
dealer stock & sale.
Bangladesh Bangladesh Atomic Energy commission (BAEC) regulates all
radiation emitting devices. Any such devices should get import permit from BAEC
before importing the unit. The permit number sticker needs to be put on the
product during installation. Responsibility Importer.
Sri Lanka CDDA regulates all medical devices. Any medical devices including
softwares, accessories & spares should have valid license / No Objection letter
(NOL) before importing the device to Sri Lanka. In addition, Atomic Energy Authority
(AEA) regulates the radiation emitting device. No Objection Certificate should be
obtained from AEA for importing the first unit. Obtaining NOC is the responsibility
of the manufacturer/dealer.
Site plan approval should be obtained from AEA before starting the installation,
which is the responsibility of the customer. Dealer should ensure the site meets the
AEA dimensional requirements. If any deviation found, dealer should inform to get
authoritys consent.
Dealer should inform AEA before and after installation of the radiating emitting
devices. FEs working on the installation activity of the radiating emitting device
should have valid license and radiation monitoring devices obtained from AEA,
which needs to be renewed every one month.
NOTE: Any further details or clarifications on regulations get in touch with India Wipro-
GE regulatory representative.
Page 37 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 39 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Appendix
Acronym Description
ASM Area Service Manager
ASSM Area Service Sales Manager
CAPA Corrective Action Preventive Action
Acronym Description
CARES Computer Assisted Remote Engineering Service
CCPL Customer Cares Program Leader
CSO Customer satisfaction opportunity
CT Computerized Tomography
ECG Electro Cardio Graphic
EHS Environment Health & Safety
EMS Employee Management System
FE/SE Field Engineer/Service Engineer
FEMC Field engineer Mobile Computing
FMI Field Modification instruction
G&O Goals & Objectives
GEHC GE Healthcare
GIB Global Install Base
IB Install Base
LCT Local Customer Team
SDT Site Down Tracker
SE Service Engineer
SSM Service Sales Manager
TAB Technical Bulletin
URL Uniform Resource Locator
WGE Wipro GE Healthcare
ZRSE Zonal Support Engineer
TSM/ZTSM Technical Support manager/Zonal Technical Support manager
ZSSM Zonal Service Sales manager
ITP Independent Third Party
RSE Region Support Engineer
RSL Remote Support Leader/Engineer
SC Support central
MIPS Manual Inventory Processing system
MRI Magnetic Resonance Imaging
OEM Original Equipment Manufacturer
OJT On the JOB Training
OTR Order to Remittance team
Page 38 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 40 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 REV 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
PET Positron emission tomography
PM Preventive maintenance
QARA Quality Assurance & Regulatory Affairs
EOPL End of Product Life
HO Head office
CRM Customer Relationship Management
Service Process Flow References:
Refer DOC1593361 in MWS for Process flow.
Refer DOC1593787 in MWS for Process SOPs.
Page 39 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 41 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 Rev 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Ownership, Revision History, and Effective Date
Ownership
Quality Manager/ Technical Operations/Business/Installations Manager.
Authorization
See MyWorkshop for approval signatures.
Revision History and Effective Date
Next
Rev Effective
Date Reason for change /Change Control No Author Review
No Date
Date
1 21/Dec/2010 Initial Release T.Sudhakar 01/Jan/2010 Jan-2011
2 14/Apr/2010 Added GOALS & objective Sub section. T.Sudhakar 01/May/201 Jan-2011
Added Independent Third party Sub section. 0
Added Trainer Qualification Sub section.
Rearranged the Section layout
Modified the content in following sections and sub sections
Contract review
New employee training
On the JOB training
Control of parts section.
Acronym
Service process
Organization structure
Demo & loaner equipment
3 01/Aug/2010 Modified content in the training session, Control of Parts record T. Sudhakar 15/Aug/201 14/Aug/2011
retention session and Service process session 0
Added Definition for SME, Trainer
Added more Acronym
Moved ITP requirement in another work instruction
Corrected the process map
4 15/Nov/2010 Modified content in training process for giving more clarity and T. Sudhakar 15/Nov/201 14/Nov/2011
added qualification criteria. 0
Added Outsourced process, Measurement and analysis and
customer property section to give more clarity.
Modified Content in Control of parts section to give more clarity
on labeling.
Added reference to warehousing process in Control of parts
session by removing the warehouse definitions.
5 15-Mar-2011 Added ownership Name, ESD Procedure, Added documents of T.Sudhakar 15-Mar- 16-Mar-2012
external origin, Added Calibrations Notes. 2011
Page 40 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 42 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 Rev 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
Next
Rev Effective
Date Reason for change /Change Control No Author Review
No Date
Date
6 17-June2011 Requirements for accessory supplies for contracts/PM added T.Sudhakar 15-July-2011 15-Jul-2012
.Training section added requirement to assign training plan 30
days from joining date and after EMS cycle if required.
Education Manager high level responsibility detail added.
7 17-May-2012 Complete rewrite of Field Service Work Instruction Procedure Hari 17-May- 16-May-2013
Removed Instructor Led Training in Hand Holding Process in 2012
Training Section
Added the Document number for On the Job Training Record
certificate.
Updated Qualification Criteria section for Full Service and Basic
Service
In Document and Data Control section, added GDP and added
links for CDL & QMS Work station
Updated Quality Goals and objective
Updated PAT Section with patient impact requirements
Updated Government Contract section
Updated Billable Service Section
Updated details to the Control of Parts Section on Part receipt
and also added warehousing section.
Quality Record retention section updated as per global procedure
DOC0371392- GEHC_GQP_02.02 Quality Document Retention
Procedure.
Section added for India region regulatory requirements.
Updated details on Area Service Manager can approve contracts.
Added the On the Job Training Record Document number in
OJT Section
Updated Sales demo section with the Global reference document
number.
8 09-Jul-2013 Added Guideline on India CHU Defects Feedback DOC1410781 to Hari 30-Jul-2013 30-Jul-2015
Internal Reference section.
Updated Full Service, Basic Service and On Job training (OJT)
sections will EHS requirements.
Added Dealer installations for High end equipment and
Deinstallation Process to Installation Process Section
Added Service report requirements to Service Process Section.
Removed PAT +PI section and provided reference to Guideline on
India CHU Defects Feedback DOC1410781.
Updated India Region Regulatory Requirements section with
latest details.
9 27-AUG-2014 All flow charts are removed and MWS reference has given to Prashanth 11 SEP 2014 11 SEP 2015
Service Process Flow and SOPs. HC
MFG PRO/CARES/SIEBEL words are replaced with CRM (Customer
Relationship Management).
10 8-APR-2015 Provided more clarity on Training Plan. Prashanth 24 APR 2015 23 APR 2016
Training assignments not required. HC
11 11 Sep 2015 Use only GE approved Parts/Consumable is added under section Prashanth 11 Sep 2015 21 Sep 2015
Service Engineers /Field Engineer Response to Emergency / Break HC
fix Dispatch (Page no 20 & 21)
Reference for PM WI and customer refusal letter for PM are
Page 41 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
Rendered PDF File Page 43 of 43 DOC0744835, Rev:11
GE Healthcare Quality Work Instruction
08.142_ INDIA REGION Field Service Work Instruction DOC0744835 Rev 11
Parent Document: DOC0373359 GEHC_GQP_ 12.08 Product Servicing Procedure
added in reference section (Page# 2)
This update will be communicated to field through Service
communication and Training is not required.
Page 42 of 42
Released
* Printed versions are for Reference Only *
Before using this document, consult MyWorkshop for the latest revision.
You might also like
- Final - Schedule M IIIDocument23 pagesFinal - Schedule M IIIdnalokeshNo ratings yet
- Pharmaceutical Industry Documents: 90 Pharmaceutical Quality Assurance Interview Questions & AnswersFrom EverandPharmaceutical Industry Documents: 90 Pharmaceutical Quality Assurance Interview Questions & AnswersNo ratings yet
- A Review On Qualification of Auto Clave, RMG, FBD, Cone Blender, Tablet Compression Machine.Document11 pagesA Review On Qualification of Auto Clave, RMG, FBD, Cone Blender, Tablet Compression Machine.DANIBATANo ratings yet
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- 002 PSC TS - Maintenance and CalibrationDocument16 pages002 PSC TS - Maintenance and Calibrationmuhammad nomanNo ratings yet
- Mission-Critical and Safety-Critical Systems Handbook: Design and Development for Embedded ApplicationsFrom EverandMission-Critical and Safety-Critical Systems Handbook: Design and Development for Embedded ApplicationsRating: 5 out of 5 stars5/5 (1)
- Artigo 1fwM9K 2014Document10 pagesArtigo 1fwM9K 2014Robert DanielsNo ratings yet
- Software Extension to the PMBOK® Guide Fifth EditionFrom EverandSoftware Extension to the PMBOK® Guide Fifth EditionRating: 5 out of 5 stars5/5 (1)
- Drystar5302 - Manual - 2008 - 09 - 22 6.0Document433 pagesDrystar5302 - Manual - 2008 - 09 - 22 6.0jolujan6258100% (2)
- ZFP Op Man - EN PDFDocument142 pagesZFP Op Man - EN PDFMohamed Abd El-Fattah GalalNo ratings yet
- Quality Manual, IsO 13485 and MDR, Free TemplateDocument13 pagesQuality Manual, IsO 13485 and MDR, Free TemplateNADJAH YOUSFINo ratings yet
- TIS0002908.001 en-US-PV-351-SOP-CRI-PTZ CameraDocument24 pagesTIS0002908.001 en-US-PV-351-SOP-CRI-PTZ CameraPedro DebiaNo ratings yet
- Site Master FileDocument59 pagesSite Master FileMohammed Zubair100% (3)
- Global Journal of Advanced Engineering Technologies and Sciences Risk Management and Sterilization Process in Medical Device IndustryDocument13 pagesGlobal Journal of Advanced Engineering Technologies and Sciences Risk Management and Sterilization Process in Medical Device Industryhuyen.vtk98No ratings yet
- General Principles of Software Validation Final Guidance For Industry and FDA StaffDocument47 pagesGeneral Principles of Software Validation Final Guidance For Industry and FDA StaffTanusri GhoshNo ratings yet
- Continual Improvement Using Jishu Hozen Pillar of Total Productive Maintenance in Manufacturing OrganizationDocument7 pagesContinual Improvement Using Jishu Hozen Pillar of Total Productive Maintenance in Manufacturing OrganizationPE QANo ratings yet
- 29 GRPMDDocument16 pages29 GRPMDMohd Shuib Abd RahmanNo ratings yet
- 789 G5500 Rev. 9.2 Quality Manual Final1.UnlockedDocument23 pages789 G5500 Rev. 9.2 Quality Manual Final1.Unlockeddrmohamed120No ratings yet
- AGFA Drystar 4500 Film Printer Service Manual - Revision 2Document662 pagesAGFA Drystar 4500 Film Printer Service Manual - Revision 2Brandon Briggs83% (6)
- Reference Guide: Quality Control FunctionDocument40 pagesReference Guide: Quality Control FunctionMED PRONo ratings yet
- PIP-Guidelines For Spare Parts ManagementDocument16 pagesPIP-Guidelines For Spare Parts ManagementrizaNo ratings yet
- Procedure For Service and Maintenance - AOCDocument3 pagesProcedure For Service and Maintenance - AOCMohamed EzzatNo ratings yet
- Validation of Sterile Water For Injection in PharmDocument12 pagesValidation of Sterile Water For Injection in PharmSehrish KanwalNo ratings yet
- JDS-G223 Training AidDocument52 pagesJDS-G223 Training Aidhmp90No ratings yet
- Ag Devicediagnostics PDFDocument22 pagesAg Devicediagnostics PDFAnonymous cXjAZTNo ratings yet
- Feedback FormDocument4 pagesFeedback FormKhushboo WarhadeNo ratings yet
- The Preparation of Validation Master Plan: Manual: 035Document6 pagesThe Preparation of Validation Master Plan: Manual: 035Rambabu komati - QA71% (7)
- Complaint Handling SOPDocument10 pagesComplaint Handling SOPPrashant Khare100% (3)
- Complaint Handling SOPDocument10 pagesComplaint Handling SOPkkvbn100% (1)
- Pws 010189Document17 pagesPws 010189Ishit DhadaNo ratings yet
- QSP Format Product SafetyDocument4 pagesQSP Format Product Safetydhir.ankurNo ratings yet
- DGU-403 DGU-405: Instruction ManualDocument30 pagesDGU-403 DGU-405: Instruction ManualCrystal LinNo ratings yet
- Procedimientos TecnicosDocument10 pagesProcedimientos TecnicosDomingo esteban perez ceballoNo ratings yet
- Dissolution AutosamplerDocument12 pagesDissolution Autosampleravinash peddintiNo ratings yet
- Complaint Handling SOPDocument9 pagesComplaint Handling SOPVidhya GGNo ratings yet
- Article Ejbps Volume 2 June Issue 3 1433741132Document25 pagesArticle Ejbps Volume 2 June Issue 3 1433741132Nitin KashyapNo ratings yet
- AQQQQDocument18 pagesAQQQQDhruvi KansaraNo ratings yet
- Workcentre 5335 SeriesDocument1,513 pagesWorkcentre 5335 SeriesРома Кузнецов100% (2)
- BS-230 Operation Manual V8.0 enDocument373 pagesBS-230 Operation Manual V8.0 enTheoWiranadiNo ratings yet
- Manual de Servicio Xerox WC5325Document1,513 pagesManual de Servicio Xerox WC5325Antonio HerreraNo ratings yet
- Cleaning and ReuseDocument22 pagesCleaning and ReuseMarcBenetPozoNo ratings yet
- Doc-00027-02 Usp Dietary Ingredient Verification Program Manual For Participants - Docxjan202024070533Document73 pagesDoc-00027-02 Usp Dietary Ingredient Verification Program Manual For Participants - Docxjan202024070533Angel FloresNo ratings yet
- BioScan BSR Series Operations ManualDocument68 pagesBioScan BSR Series Operations Manualquijote1381No ratings yet
- Xerox XD100-102-104 Service ManualDocument331 pagesXerox XD100-102-104 Service ManualNorton YuNo ratings yet
- Computer System Validation in The Perspective of T PDFDocument7 pagesComputer System Validation in The Perspective of T PDFFkNo ratings yet
- Computer System Validation in The Perspective of TDocument7 pagesComputer System Validation in The Perspective of Tttugce29No ratings yet
- User Requirement Spec - ContohDocument9 pagesUser Requirement Spec - ContohDenySidiqMulyonoChtNo ratings yet
- Workcentre 7220/7225 Service Documentation: November 9, 2012 Ecat VersionDocument1,017 pagesWorkcentre 7220/7225 Service Documentation: November 9, 2012 Ecat VersionDoicho AndonovNo ratings yet
- Icppr322c R1Document13 pagesIcppr322c R1habteyes abateNo ratings yet
- User Requirement Specifications Medical DevicesDocument16 pagesUser Requirement Specifications Medical DevicesIndra VaitkevičiūtėNo ratings yet
- Computer Validation StandardDocument13 pagesComputer Validation Standardahmed AwadNo ratings yet
- 31 Conformity Assessment For Medical DeviceDocument28 pages31 Conformity Assessment For Medical DeviceMohammed HammedNo ratings yet
- Fresenius 5008 Hemodialysis System - Service Manual PDFDocument168 pagesFresenius 5008 Hemodialysis System - Service Manual PDFTrung Nguyen100% (1)
- GHTF Sg3 n18 2010 Qms Guidance On Corrective Preventative Action 101104Document26 pagesGHTF Sg3 n18 2010 Qms Guidance On Corrective Preventative Action 101104grovuNo ratings yet
- PB-1042431 - D M22 Service ManualDocument636 pagesPB-1042431 - D M22 Service ManualAnang Sunandar100% (3)
- Xerox WC 7855FDocument1,500 pagesXerox WC 7855Fvladimir242667% (12)
- Testing and Commissioning Manual PDFDocument29 pagesTesting and Commissioning Manual PDFmrizalygani99100% (1)
- 16 Notification For Clinical Research or Performance EvaluationDocument57 pages16 Notification For Clinical Research or Performance EvaluationrevathiNo ratings yet
- Module 4Document3 pagesModule 4sri manthNo ratings yet
- PreDCR Do and DontDocument31 pagesPreDCR Do and Dontvenkata satyaNo ratings yet
- Add Info B-64485EN 01Document104 pagesAdd Info B-64485EN 01clausNo ratings yet
- Job Description FOR SAPDocument3 pagesJob Description FOR SAPp6tzk7No ratings yet
- Online Cosmetics: A Project ReportDocument82 pagesOnline Cosmetics: A Project Reportpraveen goddatiNo ratings yet
- How To Set Up A Call Centre From Scratch - The Checklist PDFDocument12 pagesHow To Set Up A Call Centre From Scratch - The Checklist PDFOmar BenkiraneNo ratings yet
- PySpark Data Frame Questions PDFDocument57 pagesPySpark Data Frame Questions PDFVarun Pathak100% (1)
- Trik Nih-Versi AssoyDocument6 pagesTrik Nih-Versi AssoyMulya Nurmansyah ArdisasmitaNo ratings yet
- Utilizing The Linux Userfaultfd System Call in A Compaction PhaseDocument9 pagesUtilizing The Linux Userfaultfd System Call in A Compaction PhaseAhmad huzai FahriNo ratings yet
- Python Introduction New 2021-22 - OddDocument120 pagesPython Introduction New 2021-22 - OddRahul A 1si19ei049No ratings yet
- CS - IT Gate Books ListDocument2 pagesCS - IT Gate Books ListSatyam GuptaNo ratings yet
- Desktop PublishingDocument11 pagesDesktop PublishingMANISHA S GANGURDENo ratings yet
- Non-Matching Scheme For Financial Assistance For DigitizationDocument7 pagesNon-Matching Scheme For Financial Assistance For DigitizationsarafepramodNo ratings yet
- Evidence (Links) and Copy of Publications PDFDocument48 pagesEvidence (Links) and Copy of Publications PDFJunaid AkramNo ratings yet
- The Adventures in Harmony CourseDocument183 pagesThe Adventures in Harmony CourseSeth Sulman100% (10)
- Introduction To SuperMap IDesktopDocument36 pagesIntroduction To SuperMap IDesktopArif W. SunarsoNo ratings yet
- Read This First: Setting DefaultDocument42 pagesRead This First: Setting DefaultmathoneNo ratings yet
- Aerospace Systems Engineering A Modern ApproachDocument137 pagesAerospace Systems Engineering A Modern ApproachSree NivasNo ratings yet
- Presentation9 - Query Processing and Query Optimization in DBMSDocument36 pagesPresentation9 - Query Processing and Query Optimization in DBMSsatyam singhNo ratings yet
- ItpDocument2 pagesItphazim gomer PangodNo ratings yet
- C DemoDocument197 pagesC DemoZeeshan ShaikhNo ratings yet
- Running PHP On Embedded DevicesDocument46 pagesRunning PHP On Embedded Deviceskaplumb_aga100% (4)
- Mlinaric Mario FPZ 2016 Diplo SveucDocument76 pagesMlinaric Mario FPZ 2016 Diplo SveucAdrian Marian RosuNo ratings yet
- Penetratuioin ToolsDocument9 pagesPenetratuioin ToolsMian Talha RiazNo ratings yet
- Bizagi How To Create A Timeline For Digital TransformationDocument10 pagesBizagi How To Create A Timeline For Digital TransformationJosé María Roberto CidNo ratings yet
- Ansible Introduction For RHEL8-CentOS8Document134 pagesAnsible Introduction For RHEL8-CentOS8Prashant DhuriNo ratings yet
- 5-Instructions of Machine Maintenance A3 A3 Uv l1800Document3 pages5-Instructions of Machine Maintenance A3 A3 Uv l1800Madjid El ayariNo ratings yet
- JP - 2 - 3clase AbstractaDocument61 pagesJP - 2 - 3clase AbstractaFelicia Candelario GuzmánNo ratings yet
- UserManual Coding VCM v1.4.Document51 pagesUserManual Coding VCM v1.4.ImSW1100% (2)
- Itil Cobit Mapping TemplateDocument6 pagesItil Cobit Mapping Templategobits100% (3)
- InternshipDocument9 pagesInternshipDipesh TripathiNo ratings yet