Professional Documents
Culture Documents
EE 432/532 Silicon Dioxide - 1
Uploaded by
Nguyễn Viết Huy0 ratings0% found this document useful (0 votes)
83 views7 pagesMEMS
Original Title
Silicon Dioxide
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentMEMS
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
83 views7 pagesEE 432/532 Silicon Dioxide - 1
Uploaded by
Nguyễn Viết HuyMEMS
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 7
Silicon dioxide, SiO2
Sand (silica) one of the most common minerals in the earth.
Main component in common glass
mixed with sodium carbonate and calcium oxide (lime) to
make soda-lime glass for window panes, bottles, drinking
glasses, etc.
Main component in optical fibers
Crystalline SiO2 is quartz
fancy drink ware and artwork
crystal oscillators using the piezoelectricity)
http://en.wikipedia.org/wiki/Crystal_oscillator
Laboratory equipment
Food additive (?!)
Microelectronics
thin electrical insulator (MOSFET gate)
diffusion barrier
EE 432/532 silicon dioxide 1
SiO2 structure
Tetrahedral arrangement with one silicon bonded to four oxygen atoms.
Most oxygen atoms will be bonded
to two silicon atoms, so that two
tetrahedra are joined at a corner.
(bridging atoms)
The orientation can be random,
leading to an amorphous structure.
Some oxygen atoms will be bonded
to only one silicon atom (non-
bridging atoms). The relative
amounts of bridging to non-bridging
determines the quality of the
oxide.
If all oxygen atoms are bridging, then
a regular crystal structure results
quartz.
EE 432/532 silicon dioxide 2
SiO2 properties
In microelectronics, we use thin layers of pure SiO2. The layers are
amorphous (fused silica)
Density: 2.0 - 2.3 gm/cm3
Dielectric constant at low frequencies: r = 3.9 (remember this!)
refractive index at optical wavelengths: n 1.5
Breakdown field: > 107 V/cm (1 V across 1 nm)
The interface with silicon always results in electronic trap levels and
some negative interface charge. Typical interface defect density 1011
cm2. This is not a high density of defects at an interface. It can be
made even lower by annealing in hydrogen.
The combination of the relatively good electrical properties of silicon,
the excellent insulating properties of SiO2, and the low-defect interface
between them is the key ingredient of modern integrated circuit
electronics.
EE 432/532 silicon dioxide 3
Oxidation of silicon
There are several ways to form a layer of SiO2 on the surface of silicon.
The two pre-dominate methods are:
Thermal oxidation of silicon - react silicon from the wafer with
oxygen to create oxide.
Deposition of a thin film by chemical vapor deposition.
(Well discuss this in detail later.)
Thermal oxidation is a simple process - introduce an oxidizing
atmosphere to the surface of the silicon with sufficient temperature to
make the oxidation rate practical. There are two commonly used
variants:
Pure oxygen: Si + O2 SiO2 (dry oxidation)
Water vapor: Si + 2H2O SiO2 + 2H2 (wet oxidation)
Typical oxide thicknesses range from a few nanometers to about 1
micron. The details of the process and a mathematical model are
presented in the next lecture.
EE 432/532 silicon dioxide 4
Consumption of the silicon substrate
In the reaction forming SiO2, silicon atoms at the surface of the wafer
must be converted to make the oxide. For a given volume of SiO2 that
is formed, a corresponding volume of the silicon substrate is lost.
In crystalline silicon, each silicon atom corresponds to a volume of
2x1023 cm3 (= 0.02 nm3). In SiO2, each silicon atom corresponds to a
volume of about 4.4x1023 cm3, depending on the density of the oxide,
or about 2.2 times more than the volume in the silicon.
However, as the SiO2 is forming, it cannot expand in all directions
equally it is constrained in the plane of the wafer. So all of the
volume difference is taken up by expansion in the vertical direction.
EE 432/532 silicon dioxide 5
Thinking about this in reverse, for a given thickness of oxide, tox, the
fraction of the thickness that corresponds to consumed silicon is 1/2.2
or 0.455. So, growing the oxide, we convert 0.455tox of the silicon
thickness to oxide, and the grown oxide extends 0.545tox above the
original surface of the silicon.
original 55%
tox
silicon surface
45%
EE 432/532 silicon dioxide 6
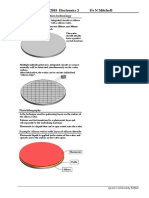
Steps at the surfaces and interfaces
Then grow a second oxide
Grow an oxide layer. Then layer. The oxide in the
pattern and etch away the exposed region grows faster
exposed region. than in the other area.
Steps are created at both the
oxide surface and the Si/SiO2
interface.
EE 432/532 silicon dioxide 7
You might also like
- Silicon Dioxide, SioDocument7 pagesSilicon Dioxide, SioAinnur SyabihaNo ratings yet
- Lec 3 Thermal OxidationDocument21 pagesLec 3 Thermal OxidationVikram MeenaNo ratings yet
- Silicon and Silicates AssignmentDocument6 pagesSilicon and Silicates AssignmentUsman GhaniNo ratings yet
- Silicon and SilicatesDocument6 pagesSilicon and SilicatesUsman GhaniNo ratings yet
- Utility of Thermal OxidationDocument7 pagesUtility of Thermal OxidationRishi JhaNo ratings yet
- Si Wafer Growth and Preparation ProcessDocument87 pagesSi Wafer Growth and Preparation ProcessSriramNo ratings yet
- Course Title: VLSI Technology Course No.: EEE 489Document25 pagesCourse Title: VLSI Technology Course No.: EEE 489Rubel RiadNo ratings yet
- EBB 323 Semiconductor Fabrication Technology: OxidationDocument57 pagesEBB 323 Semiconductor Fabrication Technology: Oxidationdildar123No ratings yet
- Silicon, Silicates and Their TypesDocument6 pagesSilicon, Silicates and Their TypesUsman GhaniNo ratings yet
- Silicon and silicates assignmentDocument6 pagesSilicon and silicates assignmentUsman GhaniNo ratings yet
- Chapter 6 Thermal OxDocument22 pagesChapter 6 Thermal OxheNo ratings yet
- Lecture 1 (Corrosion)Document24 pagesLecture 1 (Corrosion)Ansh DhankharNo ratings yet
- 03 - Elec5804 - f2023 - CMOS ProcessingDocument116 pages03 - Elec5804 - f2023 - CMOS Processingclarice.wenNo ratings yet
- Silicon DioxideDocument14 pagesSilicon DioxidesuganthiNo ratings yet
- OxidationDocument39 pagesOxidationSHAIK MUSTHAFANo ratings yet
- Basic Corrosion (Oxidation & Aqueous) : Prepared Nasrizal Mohd Rashdi LecturerDocument29 pagesBasic Corrosion (Oxidation & Aqueous) : Prepared Nasrizal Mohd Rashdi LecturerAlhadidstoner NabilNo ratings yet
- Basic Corrosion (Oxidation & Aqueous)Document29 pagesBasic Corrosion (Oxidation & Aqueous)Alhadidstoner NabilNo ratings yet
- 03 Fabrication TechnologyDocument9 pages03 Fabrication TechnologyiramNo ratings yet
- Oxidation Process in IC FabricationDocument11 pagesOxidation Process in IC Fabricationhermas67No ratings yet
- Unit II - Corrosion and Its ControlDocument17 pagesUnit II - Corrosion and Its ControlC BNo ratings yet
- 2-EECE 6041 OxidationDocument10 pages2-EECE 6041 OxidationAnh LuuNo ratings yet
- VLSI Design (WEEK 3 & 4)Document29 pagesVLSI Design (WEEK 3 & 4)Software EngineerNo ratings yet
- Isolation: Chapter 3 CMOS Processing TechnologyDocument1 pageIsolation: Chapter 3 CMOS Processing TechnologyCarlos SaavedraNo ratings yet
- Atomic Structure of SiO2-Si InterfaceDocument11 pagesAtomic Structure of SiO2-Si Interfacewer809No ratings yet
- MODULEDocument82 pagesMODULENithin GopalNo ratings yet
- Thermal Oxidation Process in IC TechnologyDocument39 pagesThermal Oxidation Process in IC TechnologyCyrille MagdiNo ratings yet
- Part I ADocument206 pagesPart I AsenthilkumaranvNo ratings yet
- Group 14: By: Shafiq Rasila Mag Nova N HasDocument34 pagesGroup 14: By: Shafiq Rasila Mag Nova N HasShafiq HamzahNo ratings yet
- MOSFET cross section schematicDocument19 pagesMOSFET cross section schematicBarış YaradanakulNo ratings yet
- Integrated Circuit (IC) FabricationDocument39 pagesIntegrated Circuit (IC) FabricationSHAIK MUSTHAFANo ratings yet
- Module 3 Corrosion KKDocument65 pagesModule 3 Corrosion KKAastha MandaliaNo ratings yet
- Silicio UllmanDocument29 pagesSilicio UllmanPaula OlazabalNo ratings yet
- Oxidation 2Document35 pagesOxidation 2mNo ratings yet
- 제15주차 Chapter11 웨이퍼 접합기술Document6 pages제15주차 Chapter11 웨이퍼 접합기술K SiriusNo ratings yet
- OxidationDocument21 pagesOxidationMalavika R NairNo ratings yet
- Unit - IDocument69 pagesUnit - IsaravananNo ratings yet
- Chapter 5 Fabrication of Microelectronic DevicesDocument35 pagesChapter 5 Fabrication of Microelectronic Devicesmuhamadsaidi60% (5)
- A Major ProjectDocument14 pagesA Major ProjectVarun ChauhanNo ratings yet
- 1-3-13fab TechniquesDocument4 pages1-3-13fab Techniquessandeep_surya432No ratings yet
- Unit No.6: Corrosion ScienceDocument30 pagesUnit No.6: Corrosion SciencesaritaNo ratings yet
- Passivation (Chemistry)Document8 pagesPassivation (Chemistry)Carlos BustamanteNo ratings yet
- Silicon Would Not Be TheDocument10 pagesSilicon Would Not Be TheZhafrandy Eka SenidaNo ratings yet
- CH-310 Lectuer # 29Document23 pagesCH-310 Lectuer # 29Saddiqa MansoorNo ratings yet
- Oxidation Behaviour of Silicon Carbide - A Review PDFDocument11 pagesOxidation Behaviour of Silicon Carbide - A Review PDFcleitononline4599No ratings yet
- Why Do Metals Rust? An Easy Read Chemistry Book for Kids | Children's Chemistry BooksFrom EverandWhy Do Metals Rust? An Easy Read Chemistry Book for Kids | Children's Chemistry BooksNo ratings yet
- Corrosion: EngineeringDocument149 pagesCorrosion: EngineeringarielNo ratings yet
- Chemistry Investigatory Project: Topic: To Study The Effect of Metal Coupling On Rusting of IronDocument12 pagesChemistry Investigatory Project: Topic: To Study The Effect of Metal Coupling On Rusting of IronSumit Chakrabarti100% (1)
- Traditional Ceramics: A Guide to Properties and ApplicationsDocument82 pagesTraditional Ceramics: A Guide to Properties and ApplicationsJB HIFINo ratings yet
- AISSCE-2022 Chemistry Project on Effect of Metal CouplingDocument14 pagesAISSCE-2022 Chemistry Project on Effect of Metal CouplingPUSHKAR PANDEYNo ratings yet
- Oxidation - Fabrication of Oxide LayersDocument5 pagesOxidation - Fabrication of Oxide Layersfidel.certucheNo ratings yet
- T2 VLSI FabricationDocument24 pagesT2 VLSI FabricationM. Bilal NoorNo ratings yet
- Properties of Matter: Oxidation, Rusting and CorrosionDocument13 pagesProperties of Matter: Oxidation, Rusting and CorrosionSelwah Hj AkipNo ratings yet
- Feynman's Prediction of Storing Encyclopedia on Pin HeadDocument7 pagesFeynman's Prediction of Storing Encyclopedia on Pin HeadGopinath ChakrabortyNo ratings yet
- Semiconductor FabricationDocument20 pagesSemiconductor FabricationMahabub HossainNo ratings yet
- Oxidation - VLSI TechnologyDocument15 pagesOxidation - VLSI TechnologyDeepak Khushalani0% (1)
- Tilli 2020Document15 pagesTilli 2020Athiyan RNo ratings yet
- 6 ElectrochemistryDocument83 pages6 ElectrochemistryILEENVIRUSNo ratings yet
- CorrosionDocument30 pagesCorrosionNikhilNo ratings yet
- Extractive Metallurgy 2: Metallurgical Reaction ProcessesFrom EverandExtractive Metallurgy 2: Metallurgical Reaction ProcessesRating: 5 out of 5 stars5/5 (1)
- Solutions Manual to accompany Engineering Materials ScienceFrom EverandSolutions Manual to accompany Engineering Materials ScienceRating: 4 out of 5 stars4/5 (1)
- Calibration The Magnetic SensorDocument45 pagesCalibration The Magnetic SensorNguyễn Viết HuyNo ratings yet
- Linear Algebra, Geodesy, and GPS by Gilbert Strang, K. Borre PDFDocument324 pagesLinear Algebra, Geodesy, and GPS by Gilbert Strang, K. Borre PDFNguyễn Viết Huy100% (3)
- An Introduction To Inertial NavigationDocument37 pagesAn Introduction To Inertial NavigationZee ZouNo ratings yet
- Tracking & Kalman Filtering Made Easy by Eli Brookner PDFDocument492 pagesTracking & Kalman Filtering Made Easy by Eli Brookner PDFNguyễn Viết HuyNo ratings yet
- UV Chapt in Handbook of Cleaning PDFDocument42 pagesUV Chapt in Handbook of Cleaning PDFNguyễn Viết HuyNo ratings yet
- Tracking & Kalman Filtering Made Easy by Eli Brookner PDFDocument492 pagesTracking & Kalman Filtering Made Easy by Eli Brookner PDFNguyễn Viết HuyNo ratings yet
- Project Avoidance MEMS Dielectric ChargeDocument17 pagesProject Avoidance MEMS Dielectric ChargeNguyễn Viết HuyNo ratings yet
- SensorDocument25 pagesSensorNguyễn Viết HuyNo ratings yet
- Figure 1: Basic Design of Fluidized-Bed ReactorDocument3 pagesFigure 1: Basic Design of Fluidized-Bed ReactorElany Whishaw0% (1)
- Vertical Drains May Not Work Alone Due To Thick Uniform Soft ClayDocument77 pagesVertical Drains May Not Work Alone Due To Thick Uniform Soft ClayChitharanjan VishnukripalNo ratings yet
- Heat Fusion of Ice ReportDocument8 pagesHeat Fusion of Ice Reporthasifah abdazizNo ratings yet
- 3466Document8 pages3466sk m hassanNo ratings yet
- Polyalk FixoprimeDocument2 pagesPolyalk FixoprimeAjay Kumar AgrawalNo ratings yet
- AOCS Recommended Practice Ca 12-55 Phosphorus PDFDocument2 pagesAOCS Recommended Practice Ca 12-55 Phosphorus PDFMaximino Alvarez100% (1)
- Types of Packing Materials for Distillation ColumnsDocument4 pagesTypes of Packing Materials for Distillation ColumnsAngelica Joyce BenitoNo ratings yet
- Styrene Acrylic FTIRDocument9 pagesStyrene Acrylic FTIRDesi Rahma PrihandiniNo ratings yet
- X20 CR Mo 13 KGDocument2 pagesX20 CR Mo 13 KGBonthala BadriNo ratings yet
- Fruit Juice ClarificationDocument26 pagesFruit Juice ClarificationRoshan JainNo ratings yet
- Assessment PN1096617Document14 pagesAssessment PN1096617Amr TarekNo ratings yet
- J Est 2018 11 027Document14 pagesJ Est 2018 11 027Hiraya HaeldrichNo ratings yet
- Analysis and Design of Steel I-Girder Bridge Using CSI-Bridge SoftwareDocument300 pagesAnalysis and Design of Steel I-Girder Bridge Using CSI-Bridge SoftwareChinmay TejaswiNo ratings yet
- Chapter No-02-GT2Document5 pagesChapter No-02-GT2VikasPatilVickyNo ratings yet
- X17CrNi16 2Document2 pagesX17CrNi16 2madodandembeNo ratings yet
- Mechanical SealDocument64 pagesMechanical SealKhoh Kai ShengNo ratings yet
- Equilibrium Constants and Le Chatelier's PrincipleDocument20 pagesEquilibrium Constants and Le Chatelier's Principlekrishna100% (1)
- Brosur Globond Alumunium Composite PanelDocument8 pagesBrosur Globond Alumunium Composite PanelDede SubhanNo ratings yet
- Chapter 10 Solution Lecture Solution Ecture NotesDocument37 pagesChapter 10 Solution Lecture Solution Ecture NotesLiew KahJiannNo ratings yet
- Adilabad ROB SCHDocument17 pagesAdilabad ROB SCHKota RavichandNo ratings yet
- JH Sir Ionic DPP 3Document4 pagesJH Sir Ionic DPP 3Tavishi SinghNo ratings yet
- Chlorine Dioxide PDFDocument4 pagesChlorine Dioxide PDFSudhakar Rao100% (1)
- Designer Brick Brochure WEBDocument28 pagesDesigner Brick Brochure WEBdirtapatroNo ratings yet
- Filter Paper TestDocument14 pagesFilter Paper TestSallam MohammedNo ratings yet
- HYDRAZINEDocument19 pagesHYDRAZINEDamla Taykoz100% (2)
- Lec 6 Module 1Document18 pagesLec 6 Module 1vedant chavanNo ratings yet
- FIB TheoryDocument38 pagesFIB TheorySabri TraderNo ratings yet
- In-Situ Rock StressesDocument16 pagesIn-Situ Rock StressesAslam KhanNo ratings yet
- VERITAS PHARMACEUTICALS LIMITED MICROCRYSTALLINE CELLULOSE SPECIFICATIONDocument1 pageVERITAS PHARMACEUTICALS LIMITED MICROCRYSTALLINE CELLULOSE SPECIFICATIONShagorShagorNo ratings yet
- Calculation Sheet For Spit Anchors: TAPCON XTREM HFL Min. Anchorage 10x120/65-35Document6 pagesCalculation Sheet For Spit Anchors: TAPCON XTREM HFL Min. Anchorage 10x120/65-35abdallah badrNo ratings yet