Professional Documents
Culture Documents
tmp669F TMP
Uploaded by
FrontiersOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
tmp669F TMP
Uploaded by
FrontiersCopyright:
Available Formats
Indian Journal of Biotechnology
Vol. I, July 2002, pp 298-300
Short Communications
Agrobacterium-mediated Transformation of strategy for improvement (Schroeder et ai, 1991).
Lucerne (Medicago sativa Linn.): Optimizing Similarly, introduction of genes controlling lignin
(reduce digestibility) and tannin production (which
Biological and Physical Parameters
acts as the anti-bloat factor), would improve quality
Suresh Kumar l *, Vishnu Bhat, B V Bhat and M G Gupta (Baucher et ai, 1999). Lucerne has a number of
inherent problems for its improvement through
Biotecll.,ulogy Section, Divi sion of Crop Improvement, Indian
Grassland and Fodder Research Institute, Jhansi 284 003 , Ind ia conventional breeding methods, biotechnological
tools provide tremendous scope for its genetic
Received 14 Seplelllber 2001; accepted II February 2002
improvement. Methods for transformation of higher
An optimized protocol for the transformation of Indian plants employing Agrobacterium are well-established;
cultivars of lucerne (Medicago sativa Linn.) using produce stable transgenic plants (Baucher et ai,
Agrobacterium tlllnejaciefls has been developed. It involved a 1999); do not require complicated protocols or
selectable marker gene encoding hygromycin sophisticated equipments; result in higher
phosphotransferase (hpt II) which confers resistance to the
antibiotic hygromycin, together with a reporter gene (uid A) transformation efficiency as predictable pattern of
encoding P-D glucuronidase (GUS); cotyledonary and foreign DNA integration (Chan et ai, 1993). Using
hypocotyl explants from ill vitro grown 5 days -old seedlings this technique, optimization of biological and physical
raised from mature seeds of genotype L-2 and IL-75 as target parameters for transformation of Indian cultivars of
tissues, and co-cultivation of explants with A. tumejaciells lucerne is described in thi s paper.
strain LBA-4404 harbouring plasmid pCAMBIA-1301.
Cotyledonary explant was found to be more responsive for Mature seeds of lucerne genotypes (L-2 & IL-75)
transformation. Bacterial density of 5xl09 cells/ml and co-
cultivation for three days were found to be most effective for
obtained from germplasm collection available at
stable GUS expression in 91 % of the hygromycin-resistant Indian Grassland and Fodder Research Institute,
calli. (IGFRI) Jhansi, were surface-sterilized in 0.1%
mercuric chloride solution with a drop of Tween-20
Keywords: lucerne, Agrobacterium, genetic transformation, for 5 min and washed 4-5 times with sterile di stilled
forage quality improvement
water. They were germinated and grown on half
Lucerne or alfalfa (Medicago sativa Linn .; family- strength MS agar medium (Murashige & Skoog,
Fabaceae; Papilionaceae) is one of the most 1962) under 16 hrs photo-period at 252C.
important leguminous forage crops of India. Widely Cotyledon and hypocotyl segments (0.5 to 1.0 cm)
known as the "queen of the forage", it is cultivated from 5 days-old axenically grown seedl ings were used
throughout the world in diverse environments (Bolton as explants for transformation. Several wounds were
et aI, 1972; Hanson et aI, 1988). It contains protein, made on the coty ledon surface, whi le thin cylindrical
15-20; calcium 1.5 and phosphorus, 0.2%, and also is hypocotyl segments were having wounds on the cut
a rich source of vitamin A, B and D for animal ends only. A. tumefaciens (LBA-4404 bearing
feeding (Bickoff et aI, 1972; McCoy & Walker, plasmid construct pCAMBIA-130 I), reporter genes
1984). The deficiency in sulphur contain ing amino for GUS (uid A gene interrupted by catalase intron)
acids and saponins, which reduce its nutritional value, and hygromycin resistance (hpt II), both of them
make it a candidate crop for forage quality driven by CaMV 35S promoters with nos terminators
improvement (McCoy & Walker, 1984; Bickoff et aI, (Fig. 1), was grown in Yeast Extract Mannitol (YEM)
1972). Introduction of gene for ovalbumin (which has medium supplemented with Kanamycin (100 mg/l)
balanced amino acids profile including 6.5 % cysteine and Rifampicin (10 mg/l) at 282 C for 2-3 days.
and methionine) in lucerne cultivars would be a right The bacterial pellet was obtained by centrifugation
(5000 rpm, 5 min at room temperature) and
*Author for correspondence: resuspended in YEM medium (density, 5x I0 7-5x lO lo
Tel: 5824787/2 14; Fax: +91 11 -5823984
E-Mail : sureshkumar33@ red iffma il.com
cells/ml). The explants were immersed in it for 15
Ipresent Addre~s: NRC on Plant Biolechnology, IARI, min, excess bacterial suspension was blot dried and
New Delhi 11001 2 explants transferred onto MS ca llusing medium
SHORT COMMUNICATIONS 299
pCAMBIA1301 T-DNA Hilfd rn (2455)
pUC II MC:> NM I (S230)
5607 bo
XIM 1(210)
uoR I (2404) Nco 1 (3217) /'M/I (S2S3)
border (L) Xho 1(1374) 81/ XI (:1161) 8,/11 (32:14) Bit Ell (n66)
~~~~~~~I!!!!!"'-M~....~~~, .
Lla alphl I at.,... Inlron
GUS Firat Exon
I T-border (R)
N S.POL YA
iabdtne tag
:fss promot.r Gus Second EKon
Fig.l-T-ONA region of the plasmid pCAMBIA-130 I used for Agrobaclerilllll-m ediated tran sformation of luce rne.
(CLM), supplemented with 9.0 ).AM 2,4-0, 5.0 ).AM
NAA and 0.8 ).AM BAP, with or without 50 ~lM
acetosyringone (3' ,5'-dimethoxy-4'-hydroxy-aceto-
phenone; Aldrich Chemical Co, USA) . Co-cultivation
on this medium was continued at 282 C for 2-5
days in dark. The explants were rinsed thoroughl y
with 250 mg/l Cefotaxime so lution (Melford Lab Ltd,
UK) , to get rid of bacteri al cells from the surface of
the exp lant, blot dried and tran sferred on CLM
medium contaln1l1g hy gromycin 50 mg/l and
Cefotaxime 250 mg/l (ClmHC). Embryogenic calli
induced from the explants were subcultured at two
weeks interval o n freshly prepared ClmHC medium.
The uid A gene expression was determined by a
Fig. 2-S~able GUS gene ex pression in transformed hy gromycin -
histochemical assay (Jefferson, 1987) in randomly
resis tant calli , 4 weeks after co-c ulti vation (T). Un stain ed
selected ca lli , 4-5 weeks after co-cultivation. GUS ch imeric reg io n on th e calli lost the ge ne express ion. 0
assay staini ng so luti on was prepared by dissolving 5 exp ress io n in control (C).
mg X-gluc (5-bromo, 4-chloro, 3-indolyl-~-D
glucuronide; Sigma Chem Co, USA) , 359 mg sod ium their genotypic behaviour. GUS gene expression was
dihydrogen ph osphate dihydrate and 0.01 ml of observed in calli from 82 cotyledonary and 48
Tween-20 in 20 ml of double distilled water (pH 7.0), hypocotyl explants, out of 90 from each in a set of
filter sterilized an d stored at 4C. GUS gene experiment with IL-75 genotype. The difference
express ion was observed with Nikon-SMZ-2T might be due to more wounded surface on cotyledon
binocular stereozoom microscope as blue spots. (wounding was easier) than that on hypocotyl and
Transformation freq uency is the number of GUS wounded cotyledons offered comparatively more sites
ex pressing hygromycin-resistant calli divided by the for infection by Agrobacterium. Wounded leaves of
number of calli assayed and ex pressed as percentage. M. varia plants gave four times higher transformation
The experimental data (replicated three times), were frequency than petioles (Chabaud et al, 1988).
analyzed using analysis of variance and significant Although the effects of plasmid-vector/
differences among treatment mean s at 5 per cent level Agrobacterium strain on tran sformation efficiency
of significance were calculated using Duncan's were not examined by the authors, such effects of
multiple range test (IRRIST AT modul e). genotype, bacteri al strain and vector combination
Cotyledonary and hypocotyl explants of the test have been observed by others (Byrne et al, 1987;
genotypes, L-2 and IL-75 , were co-cultivated with A. Desgagnes et al, 1995 ; Du et al, 1994). GUS gene
tlll1lefaciens strain LBA -4404, and hygromycin 50 expression was observed in 91 % randomly selected
mg/l for proliferation of transformed cells. GUS assay calli proliferating on selection medium (ClmHC) in
was performed in callus after 4-5 weeks co- the form of blue spots (Fig. 2).
cultivation. While GUS gene expression was not Significant difference in transformation efficiency
detected in control (Fig. 2), genotype IL-75 responded was also observed with different concentration of
better for both in vitro culture and transformation . bacterial cells used for co-cultivation. The optimal
Significant differences observed in transformation transformation efficiency of 91.3 % ((J = 3.05) was
efficiency of the L-2 and IL-75 might be purely due to obtained with 5xl09 cells/ml (Table 1). Bacterial
300 INDIAN J BIOTECHNOL, JULY 2002
Table I-Erfect of bacterial cell density on transform ation facilities, reviewers for constructive suggestions and
effi ciency CAMBIA, Australia for providing the plasmid
Explant/cells/ml Transformation efficiency (%) construct.
Cotyledon s 66.6C 91.3"
References
Baucher M el ai, 1999. Down-regulation of cinnamyl alcohol
dehydrogenase in transgenic alfalfa (Medicago sativa L.) and
Hypocotyl 53.6"
the effect on li gnin composition and di gestibility . Plant Mol
Co-culti va tion was continued for three days. Figures are the mean Bioi, 39, 437-447.
of three replications show ing percentage of calli with GUS Bickoff E M et ai, 1972. Chemical composit io n of herbage. ill
positi ve response. In a row, means follow ed by a different letter Alfalfa Science and Technology, edi ted by C H Hanson.
are significantly different at 5 per cent level of significance. ASA Mad ison, Wi sconsin , USA Pp 247-282 .
Bolton J et ai, 1972. World di stribution and hi storical
Table 2-Erfect of duration of co-cultivati on on transformation developments. in Alfalfa Science and Technology, Agro n
efficiency Monogr - 14, ed ited by C H Hanson. CSSA . Mad ison,
Ex plant/Co- Transformation efficiency (%) Wisconsin, USA . Pp 1-34.
cultivation 2 days 3 days 4 days 5 days Byrne M C el ai, 1987. Strain and culti var specifi ci ty in the
Agrobacleriulll-soybean interaction. Plant Cell TisslIe Organ
Cotyledons 91.3" 41.6 C Cult, 8,3-15 .
Chabaud M et ai, 1988. Parameters affec tin g the frequ ency of
Hypocotyl 53.6" 31.3 c
kanamycin resistant alfalfa obtained by Agrobacterilllll
Optimal density (5 x 109 cells/ml) was used for co-cultivation. lumeJaciens medi ated tra nsformation. Plallt Cell Rep, 7,5 12-
Figures are the mean of three replications showing percentage of 516.
calli with GUS positive response. In a row, means followed by a Chan M T et ai, 1993. Agrobacteriulll-mediated production of
diffe rent letter are significantly diffe rent at 5 per cent level of transgenic ri ce plants expressi ng a chimeri c a -amy lase
signi fi cance. prom o t e r/~ -g lucuronid ase gcne. Plan t Mol Bioi, 22, 491 -506 .
Desgagnes R et ai, 1995. Genetic tra nsformation of commercial
concentrati on is an important parameter for efficient breeding lines of alfalfa (Medicago sativa ). Plallt Cell Tissue
Organ Cult, 42,129- 140.
transformation (Chabaud et ai, 1988). Further, co- Du S et ai, 1994. Effect of plant ge notype on the tran sformation of
cultivation of explants for three days recorded culti vated alfalfa (Medicago sativa) by Agrobacteriu/II
significantly hi gher (optimal) transform ation tllllleJaciells. PICllII Cell Rep, 13,330-334 .
efficiency (Table 2) . When acetosy ringone was added Hanson A A et ai, 1988. Alfalfa and alfalfa impro veme nt. Agron
Monogr - 29, ASA , ASSA and SSSA, Madison , Wisconsin ,
in the co-cultivation medium to induce bacterial vir
USA. Pp 38-72.
genes, no significant difference in transformati on Horsch R B et ai, 1985. A sim ple and ge neral method for
efficiency was observed. transferrin g genes into plants. Science, 227, 1229- 123 1.
It is hoped that optimized parameters for Je fferson R A, 1987. Assaying chimeric genes in pl ants: the ge nes
Agrobacteriul11-mediated transform ation of Indi an fu sion system. Plallt Mo l Bioi Rep, 5, 387-405.
McCoy T & Walker K, 1984. Alfalfa. ill Handbook of Plant Cell
cultivars of lucerne will be useful for the development Culture. Vol 3. Crop species, ed ited by P V Ammirato, 0 A
of transgenic plants harbouring genes of interest, Evans, W R Sharp & Y Yamada. Mac millan Publishing
particularly for forage quality improve ment. Company , New York . Pp 171 - 192.
Muras hige T & Skoog F, 1962. A revised medium for rapid
grow th and bioassays with tobacco ti ssue cultures. Physiol
Acknowledgement
Plant, 15,473-497.
The authors thank Dr R N Choubey, Head of the Schroeder H E et ai, 199 1. Expression of a chicken ovalbumin
Crop Improvement Division, and Dr P S Pathak , ge ne in three lucerne cultivars. Aust J Plallt Physiol, 18,495-
Director, IGFRI, Jhansi for providing necessary 505.
You might also like
- tmpF178 TMPDocument15 pagestmpF178 TMPFrontiersNo ratings yet
- tmpE3C0 TMPDocument17 pagestmpE3C0 TMPFrontiersNo ratings yet
- Tmp1a96 TMPDocument80 pagesTmp1a96 TMPFrontiersNo ratings yet
- Tmpa077 TMPDocument15 pagesTmpa077 TMPFrontiersNo ratings yet
- tmp998 TMPDocument9 pagestmp998 TMPFrontiersNo ratings yet
- tmp3656 TMPDocument14 pagestmp3656 TMPFrontiersNo ratings yet
- tmp27C1 TMPDocument5 pagestmp27C1 TMPFrontiersNo ratings yet
- tmp96F2 TMPDocument4 pagestmp96F2 TMPFrontiersNo ratings yet
- tmpA7D0 TMPDocument9 pagestmpA7D0 TMPFrontiersNo ratings yet
- tmp97C8 TMPDocument9 pagestmp97C8 TMPFrontiersNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Speaking Topics For Final ReviewDocument3 pagesSpeaking Topics For Final ReviewNhư ÝNo ratings yet
- This Is No Way To Treat An Aorta.: Edwards EZ Glide Aortic CannulaDocument5 pagesThis Is No Way To Treat An Aorta.: Edwards EZ Glide Aortic CannulaAhmadNo ratings yet
- UBKV Ranking Proforma With Annexures 2018 PDFDocument53 pagesUBKV Ranking Proforma With Annexures 2018 PDFSubinay Saha RoyNo ratings yet
- HDFC Life Insurance (HDFCLIFE) : 2. P/E 58 3. Book Value (RS) 23.57Document5 pagesHDFC Life Insurance (HDFCLIFE) : 2. P/E 58 3. Book Value (RS) 23.57Srini VasanNo ratings yet
- Pure Vegeterian: Kousika (CaterersDocument2 pagesPure Vegeterian: Kousika (CaterersShylender NagaNo ratings yet
- Ethnomedicinal Plants For Indigestion in Uthiramerur Taluk Kancheepuram District Tamilnadu IndiaDocument8 pagesEthnomedicinal Plants For Indigestion in Uthiramerur Taluk Kancheepuram District Tamilnadu IndiaGladys DjeugaNo ratings yet
- Chemrite SP 200Document3 pagesChemrite SP 200ghazanfarNo ratings yet
- Bartos P. J., Glassfibre Reinforced Concrete - Principles, Production, Properties and Applications, 2017Document209 pagesBartos P. J., Glassfibre Reinforced Concrete - Principles, Production, Properties and Applications, 2017Esmerald100% (3)
- WEEK 7-8: Health 9 Module 4Document8 pagesWEEK 7-8: Health 9 Module 4Heidee BasasNo ratings yet
- Lesson Plan On Tuberculosis (Health Talk)Document8 pagesLesson Plan On Tuberculosis (Health Talk)Priyanka Jangra100% (2)
- Phoenix Contact DATA SHEETDocument16 pagesPhoenix Contact DATA SHEETShivaniNo ratings yet
- HEAS 1000 Health Assessment: Reflection of PracticeDocument4 pagesHEAS 1000 Health Assessment: Reflection of PracticePreet ChahalNo ratings yet
- Method of Toxicity Test 1Document59 pagesMethod of Toxicity Test 1Widya AnggrainiNo ratings yet
- Top 6 Beginner Work Out MistakesDocument4 pagesTop 6 Beginner Work Out MistakesMARYAM GULNo ratings yet
- Cariprazine PDFDocument162 pagesCariprazine PDFige zaharaNo ratings yet
- Policy Schedule Personal Accident Insurance Policy: (Plan 5)Document2 pagesPolicy Schedule Personal Accident Insurance Policy: (Plan 5)Rana BiswasNo ratings yet
- URICA TestDocument3 pagesURICA TestCristy PagalanNo ratings yet
- Tryout Consent Form - 2014 - Sign and ReturnDocument2 pagesTryout Consent Form - 2014 - Sign and ReturnSanjeevan BaraNo ratings yet
- Notes, MetalsDocument7 pagesNotes, MetalsindaiNo ratings yet
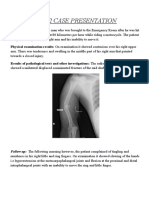
- PBL 2 Case PresentationDocument12 pagesPBL 2 Case PresentationRamish IrfanNo ratings yet
- Additional Activity 3 InsciDocument3 pagesAdditional Activity 3 InsciZophia Bianca BaguioNo ratings yet
- Impact of Job Design On Employee Engagement: A Theoretical and Literature ReviewDocument6 pagesImpact of Job Design On Employee Engagement: A Theoretical and Literature ReviewAnonymous CwJeBCAXpNo ratings yet
- Written Assignment Unit 4 Health ScienceDocument6 pagesWritten Assignment Unit 4 Health SciencesafsdaNo ratings yet
- Cataloge ICARDocument66 pagesCataloge ICARAgoess Oetomo100% (1)
- Foundation Engineering. 02 Soil CompressibilityDocument63 pagesFoundation Engineering. 02 Soil Compressibilitysammy lopezNo ratings yet
- Chemistry DemosDocument170 pagesChemistry DemosStacey BensonNo ratings yet
- Proposal Semister ProjectDocument7 pagesProposal Semister ProjectMuket AgmasNo ratings yet
- The Coca-Cola Company - Wikipedia, The Free EncyclopediaDocument11 pagesThe Coca-Cola Company - Wikipedia, The Free EncyclopediaAbhishek ThakurNo ratings yet
- NCP Ineffective Breathing ActualDocument3 pagesNCP Ineffective Breathing ActualArian May Marcos100% (1)
- RDG UNIT 2 Skimming Class A 2021Document17 pagesRDG UNIT 2 Skimming Class A 2021Yuly Rumondang Wulan SiallaganNo ratings yet