Professional Documents
Culture Documents
Hydrogen Plant
Uploaded by
duongchitrung0 ratings0% found this document useful (0 votes)
28 views2 pageshydrogen plant
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documenthydrogen plant
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
28 views2 pagesHydrogen Plant
Uploaded by
duongchitrunghydrogen plant
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Steam Reforming for Hydrogen.
resulting in less costly and more efficient plants, in part because of
The Process and the Mechanism better materials for reformer tubes, better control and understanding
of carbon limits, and better catalysts and process concepts with high
Jens R.. Rostrup-Nielsen and Jens Sehested feedstock flexibility. This progress has been accompanied by a better
Haldor Topse A/S understanding of the reaction mechanism.
Lyngby, Denmark
Results
Efficiency and costs. With no steam export, theoretical
Introduction energy consumption of 300 BTU/scf H2 (11,8 MJ/Nm3) on LHV
Steam reforming has been a well-established process in (lower heating value) basis (using liquid water as feed). The
industry for more than 70 years and it will play an important role in industrial value2 for natural gas based plants is about 320 BTU/scf H2
future applications related to a new hydrogen economy1,2. There is (12.6 MJ/Nm3H2) corresponding to 94% of the theoretical efficiency.
also a fast growing need for more hydrogen production capacity in At locations with high natural gas prizes, the energy efficiency
refineries, as the hydrogen balance is negative which means that becomes critical. For a natural gas price of 4 USD MM BTU, the
more hydrogen has to be produced at the refinery or being imported. feedstock and utility costs makes about 65% of total operating costs.
In spite of efforts to produce hydrogen by schemes involving solar High temperature and high steam to carbon ratio favor high
energy, wind energy and biofuels, fossil fuels remain the most conversion of the endothermic steam reforming reactions. However,
feasible feedstock for hydrogen generation in the near term2. modern hydrogen plants are normally designed for low steam-to-
The choice of hydrogen technology is dictated by the cost and carbon ratios (1.8-2.5 mole/C-atom). A low steam-to-carbon ratio
availability of feedstock, and by the scale of operation. reduces the mass flow through the plant and thus the size and costs of
Other parameters than efficiency play a role for small units equipment2. Furthermore, low steam-to-carbon ratios result in a more
such as simplicity, compactness and (for automotive units) short energy-efficient plant and thus a lower operating cost. In principle, a
start-up time. Air blown catalytic partial oxidation (CPO) fulfils these low steam-to-carbon ratio increases the amount of unconverted
requirements in particular for fuel cell applications where it is methane from the reformer, but is compensated for by increasing the
normally acceptable that the hydrogen stream contains nitrogen3. reformer outlet temperature, typically to ca. 1700oF.
However, for commercial scale production of pure hydrogen, steam
reforming remains the most economic and efficient technology for a - High heat flux
wide range of hydrocarbon feedstocks. - Low steam to carbon ratio and high
exit temperature
- Prereformer and high inlet temperature
Figure 2. Parameters resulting in lower reformer costs
The thermal efficiency of the tubular reformer and waste heat
recovery section approaches 95%. The heat transferred to the process
is about 50% of the heat input to the reformer and the remainder is
recovered from the flue gas. This heat is used for steam production
and for preheating of the reformer feed, combustion air, etc. The
same is true for the heat contained in the hot product gas exiting the
reformer. Very often, there is little need for export steam and todays
design aims at minimum steam production.
It is possible to increase the amount of heat transferred to the
process gas in the reformer from about 50% to about 80% of the
supplied heat when using a convective heat exchange reformer5, in
which the flue gas as well as the hot product gas are cooled by heat
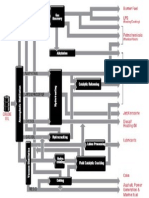
Figure 1. Hydrogen plant. Simplified scheme. exchange with the process gas flowing through the catalyst bed. This
results in a more compact piece of equipment. In all types of heat-
exchange reformers, however, the heat exchange is by convection,
A typical lay-out of a hydrogen plant is shown in Figure 1. which generally leads to lower heat fluxes than in reformers with
The reforming process is followed by water gas shift at 410-625 F to radiant heat transfer. However, the compact heat exhange reformer is
ensure high conversion of carbon monoxide and clean-up of the well suited for small skid-mounted) hydrogen plants. For large scale
hydrogen product in a PSA-unit. reforming, tubular reforming remains the most economic solution.
In many situations when natural gas is not available, higher There have been strong efforts to minimize the costs of the
hydrocarbons become the preferred feedstock for the reforming tubular reformer. A smaller size is achieved by improving the heat
process. Many refineries can benefit from flexibility in feedstock, transfer and hence reducing the number of tubes6. Tubular reformers
taking advantage of the surplus of various hydrocarbon streams in today are designed for operation at average heat fluxes exceeding
the refinery. Steam reforming of liquid hydrocarbons is also 37000 BTU/sqft/h) (100,000 kcal/m2/h) almost two times higher than
considered for hydrogen generation for fuel cells4 with diesel and jet what was industrial practice 20 years ago. Such reformers are built
fuel considered as logistic fuels. today for capacities up to 270 MM SCFD H2 (300,000 Nm3/h).This
The steam reforming process may appear straightforward can be achieved by using a side wall fired furnace with better control
from an overall consideration as the product composition is of the tube wall temperatures.
determined by simple thermodynamics, but in reality it is a complex Prereformer. Steam reforming generally involves the risk of
coupling of catalysis, heat transfer and mechanical design. In recent carbon formation1. Whisker carbon may be formed on the catalyst
years, there has been progress in steam reforming technology and at high temperatures, ethylene from the pyrolysis of higher
Fuel Chemistry Division Preprints 2003, 48(1), 218
hydrocarbons may lead to pyrolytic coke, which may encapsulate the
catalyst pellets.
These constraints are eliminated when a prereformer7 is
installed before the tubular reformer. All higher hydrocarbons are
converted in the prereformer in the temperature range of 750-1000 F.
After a prereformer, it is possible to preheat to temperatures around
1200 F. The prereformer offers great feedstock flexibility ranging
from natural gas and refinery off-gas to liquid fuels and it also serves
as an effective sulfur guard for down-stream catalysts.
Catalyst. The typical steam reforming catalyst contains
nickel1. The catalyst properties are dictated by the severe operating
conditions in the reformer with high temperatures an steam partial
pressures. Sintering is an important cause of deactivation of nickel-
containing steam reforming catalysts8. The most important
parameters are the temperature and the atmosphere in contact with
the catalyst. The catalyst support can affect the sintering in various
ways by loss of surface area. The sintering ceases when the nickel Figure 3. Potential energy curves for steam reforming of methane
particle size exceeds a given size. This maximum size increases with reaction on Ni(111) and Ni(211) surfaces9
temperature8.
The catalyst activity is rarely a limiting factor. The catalyst Conclusions
volume (space velocity) is fixed from the tubular reformer design. The Fundamental studies of the steam reforming reactions have
equilibrium conversion at high reforming temperatures is achieved at led to a more consistent understanding of the mechanism of the main
very high space velocities (above 106 vol CH4/vol cat/h) when reactions and the competing reactions leading to carbon formation.
extrapolating the intrinsic rates1. In practice, however, the utilization of This forms a more solid basis for development of better catalysts and
the activity (as expressed through the effectiveness factor) is smaller for their optimum use in advanced reforming technologies. It remains
than 10% because of transport restrictions. It can be shown by a challenge to take advantage of the huge surplus of catalyst activity
computer simulations that the catalyst is not the limiting factor for the in present reformer designs and there is still room for developing
design of a tubular reformer. An increase of the heat flux and the load catalysts being better in withstanding the risk for carbon formation
at a given exit temperature by a factor of two results in an increase in and sulfur poisoning.
methane leakage by only 10%7.
References
Discussion
The mechanism of steam reforming. Recent studies of the 1. Rostrup-Nielsen, J.R.; Nrskov, J.K; Sehested, J., Adv.Catal. 2002,
fundamentals of the steam reforming reactions have lead to a more 47 (2002), 65 (in print).
consistent understanding of the mechanism of the main reactions and 2. Rostrup-Nielsen, J.R.; Rostrup-Nielsen, T. Cattech, 2002, 6 (4)
the competing reactions for carbon formation1,9. The dissociation of 150 (2002).
3. Rostrup-Nielsen, J.R.; Aasberg-Petersen, K. in: Fuel Cell
methane on nickel surfaces has been investigated extensively, and
Handbook (John Wiley & Co.) (in press).
several details of the reaction pathway are known from fundamental 4. Rostrup-Nielsen, J.R. Phys. Chem. Chem. Phys,. 2001, 3, 283.
studies, in-situ high resolution electron microscopy and theoretical 5. Dybkjr, I.; Winter Madsen, S.E.L. Int. J. Hydrocarb. Eng. 3 (1)
calculations. 56 (1997/1998).
In-situ high resolution electron microscopy has provided new 6. Rostrup-Nielsen, T. Hydrocarb.Eng,, .2002, 7 (8) 51.
information on sintering mechanisms and for the importance of steps 7. Aasberg-Petersen, K.; Bak Hansen, J.-H.; Christensen, T.S.;
in nucleation of whisker carbon. DFT-calculations have quantified Dybkjr, I.; Seier Christensen, P.S.; Stub Nielsen, C., Winter
the energetics of methane activation and shown that activation Madsen, S.E.L.; Rostrup-Nielsen, J.R. Appl.Catal. A. 2001, 221,
379.
barriers are smaller on surface steps where also carbon is the most
8. Sehested, J.; Carlson, A.: Janssens, T.V.W.; Hansen, P.L.; Datye,
stable surface species. A.K. J. Catal,. 2001, 197, 200.
Fig. 3 shows the full potential energy diagram of the steam 9. Bengaard, H.S., Nrskov, J.K.; Sehested, J.; Clausen, B.S.;
reforming reaction. The figure shows the energies of the Nielsen, L.P.; Molenbroek, A.M.; Rostrup-Nielsen, J.R., J. Catal.
intermediates on the surface and activation barriers separating the 2002, 209, 365.
intermediates along the reaction path. Two different nickel surfaces
were considered. The Ni(111) surface represents the stable dense
packed surface, whereas the Ni(211) surface contains steps.
The steps are much more reactive than the close packed
surface. All intermediates are also more strongly bound at the steps
than on the terraces. For example, adsorbed atomic carbon is much
more stable at the steps than at the terraces1. Consequently, the steps
should be better nucleation sites for graphite than the terraces. The
availability of step sites is therefore important both for a high
reaction rate and for graphite formation. This raises the question of
where promoters (such as potassium) are located on the surface
during the catalytic reaction. Again, these were found to be
considerably more stable at a step than on a terrace1,9.
Fuel Chemistry Division Preprints 2003, 48(1), 219
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Property Tables and Charts (Si Units)Document50 pagesProperty Tables and Charts (Si Units)Q_TNo ratings yet
- Hess's LawDocument10 pagesHess's LawduongchitrungNo ratings yet
- DAC Simulator D610Document1 pageDAC Simulator D610duongchitrungNo ratings yet
- Trung TestDocument1 pageTrung TestduongchitrungNo ratings yet
- Cat CrackingDocument1 pageCat CrackingduongchitrungNo ratings yet
- Upload SCRBDocument1 pageUpload SCRBduongchitrungNo ratings yet
- Present Azy ADocument1 pagePresent Azy AduongchitrungNo ratings yet
- Do ThiDocument1 pageDo ThiduongchitrungNo ratings yet
- NH3 ProductionDocument1 pageNH3 ProductionduongchitrungNo ratings yet
- Cat ReformingDocument1 pageCat ReformingduongchitrungNo ratings yet
- Brochure Producedwater Sorbwater For WEBDocument12 pagesBrochure Producedwater Sorbwater For WEBManvendra SinghNo ratings yet
- Amine Gas SweeteningDocument2 pagesAmine Gas SweeteningCameron Kashani0% (1)
- PVT SurfaceDocument1 pagePVT SurfaceduongchitrungNo ratings yet
- Pollution Acid Plants: NitricDocument7 pagesPollution Acid Plants: NitricduongchitrungNo ratings yet
- Refinery SchemeDocument1 pageRefinery SchemeduongchitrungNo ratings yet
- Steam ReformingDocument1 pageSteam ReformingduongchitrungNo ratings yet
- DCDocument34 pagesDCduongchitrungNo ratings yet
- Steam Cracking 2Document1 pageSteam Cracking 2duongchitrungNo ratings yet
- Steam ReformingDocument1 pageSteam ReformingduongchitrungNo ratings yet
- PVT SurfaceDocument1 pagePVT SurfaceduongchitrungNo ratings yet
- Lam Kho Khi Bang ZeoliteDocument1 pageLam Kho Khi Bang ZeoliteduongchitrungNo ratings yet
- Steam CrackingDocument1 pageSteam CrackingduongchitrungNo ratings yet
- Restaurant ViewDocument1 pageRestaurant ViewduongchitrungNo ratings yet
- Ammonium Carbamte P, T - BASFDocument4 pagesAmmonium Carbamte P, T - BASFVinh Do ThanhNo ratings yet
- RSC Nutrients and FertilisersDocument2 pagesRSC Nutrients and FertilisersduongchitrungNo ratings yet
- PVT SurfaceDocument1 pagePVT SurfaceduongchitrungNo ratings yet
- AP Dung Thi Nghiem Ao o Hong KongDocument6 pagesAP Dung Thi Nghiem Ao o Hong KongduongchitrungNo ratings yet
- Nitric AcidDocument31 pagesNitric AcidBon Bon100% (1)
- Pollution Acid Plants: NitricDocument7 pagesPollution Acid Plants: NitricduongchitrungNo ratings yet
- Lange (2007)Document10 pagesLange (2007)Ally BNo ratings yet
- Biomass CombustionDocument71 pagesBiomass Combustionchaiya sonwong100% (1)
- 1 s2.0 S0141391014000913 MainDocument9 pages1 s2.0 S0141391014000913 Maintsing takNo ratings yet
- Thermal Pretreatment of Lignocellulosic Biomass PDFDocument6 pagesThermal Pretreatment of Lignocellulosic Biomass PDFW00WNo ratings yet
- Nikos Kostas Master ThesisDocument99 pagesNikos Kostas Master ThesisVILLAINZ83No ratings yet
- Waste Management - Wikipedia PDFDocument64 pagesWaste Management - Wikipedia PDFSanjib SarkarNo ratings yet
- Chapter 2. Wood Carbonisation and The Products It YieldsDocument10 pagesChapter 2. Wood Carbonisation and The Products It YieldsKalidhas YogarajanNo ratings yet
- Direct-Thermochemical-Liquefaction Commercialization OverviewDocument28 pagesDirect-Thermochemical-Liquefaction Commercialization OverviewJairoVidalNo ratings yet
- Moving Bed GasificationDocument19 pagesMoving Bed GasificationObriantendekai MarushoNo ratings yet
- Goma XantanaDocument11 pagesGoma XantanaDayanneNo ratings yet
- Thermal Conversion of Plastic-Containing Waste-A ReviewDocument77 pagesThermal Conversion of Plastic-Containing Waste-A ReviewVishal BhagwatNo ratings yet
- F&P Paul AndersonDocument41 pagesF&P Paul AndersonMax Calun Rosellon PalmieryNo ratings yet
- Potential Thermochemical Conversion of Bioenergy From Acacia Species in Brunei Darussalam: A ReviewDocument18 pagesPotential Thermochemical Conversion of Bioenergy From Acacia Species in Brunei Darussalam: A ReviewFernando AmoresNo ratings yet
- Model Calculation of Heat Balance of Wood PyrolysiDocument10 pagesModel Calculation of Heat Balance of Wood PyrolysiqwerNo ratings yet
- A Review of Biomass Hydrothermal Processing PDFDocument15 pagesA Review of Biomass Hydrothermal Processing PDFVignesh NvNo ratings yet
- RRLDocument4 pagesRRLRejed VillanuevaNo ratings yet
- The Potential of Waste Tire and Waste Plastics Pyrolysis in Southern Savonia Region PDFDocument94 pagesThe Potential of Waste Tire and Waste Plastics Pyrolysis in Southern Savonia Region PDFWilmer Max Espinoza ChancaNo ratings yet
- Project Work of Eph On Waste DisposalDocument10 pagesProject Work of Eph On Waste DisposalSuzon Mndr100% (1)
- Reactor Network Synthesis Vinyl Chloride ProcesDocument17 pagesReactor Network Synthesis Vinyl Chloride Procespolikopil0No ratings yet
- Biomass GasificationDocument25 pagesBiomass GasificationlucabenedettiNo ratings yet
- DY-1-20 Scrap Tires Recycling Pyrolysis Oil Plant of Doing GroupDocument3 pagesDY-1-20 Scrap Tires Recycling Pyrolysis Oil Plant of Doing GrouppyrolysisoilNo ratings yet
- Iso 6964 2019Document9 pagesIso 6964 2019Dilipan DiliNo ratings yet
- HE MeltingDocument11 pagesHE MeltingDanilo AmorimNo ratings yet
- Journal of Analytical and Applied Pyrolysis: Guan Jie, Li Ying-Shun, Lu Mai-XiDocument5 pagesJournal of Analytical and Applied Pyrolysis: Guan Jie, Li Ying-Shun, Lu Mai-XiPaulo SantosNo ratings yet
- DrillingDocument21 pagesDrillingNabil RajNo ratings yet
- Novel and Innovative Pyrolysis and Gasification Technologies For Energy Efficient and Environmentally Sound MSW DisposalDocument27 pagesNovel and Innovative Pyrolysis and Gasification Technologies For Energy Efficient and Environmentally Sound MSW Disposalvitor_alberto_7100% (1)
- 100KW Biomass Syngas Power Plant SA-2021Document14 pages100KW Biomass Syngas Power Plant SA-2021Shih Chieh HuangNo ratings yet
- Waste Paper Biochar Project Final ReportDocument31 pagesWaste Paper Biochar Project Final ReportFAIZANNo ratings yet
- INEC 2022 Paper - Vaporise After Reading - Pyrolysis and Waste Management On Queen Elizabeth Class Carriers - Post Comments - 27.09.22Document11 pagesINEC 2022 Paper - Vaporise After Reading - Pyrolysis and Waste Management On Queen Elizabeth Class Carriers - Post Comments - 27.09.22Hayden C100% (1)
- Biochar From Oil Palm Empty Fruit Bunches and Oil Palm Shells Via Slow PyrolysisDocument44 pagesBiochar From Oil Palm Empty Fruit Bunches and Oil Palm Shells Via Slow Pyrolysispebrian sahputraNo ratings yet