Professional Documents
Culture Documents
Molecular Model
Uploaded by
Pedro SuyuOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Molecular Model
Uploaded by
Pedro SuyuCopyright:
Available Formats
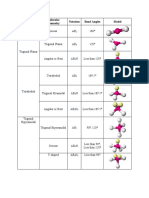
# of lone # of Hybridi
pair bonding
electrons groups (pair zation
Electron-pair Molecular Bond
on electrons)
Geometry Geometry Angle
'central' on 'central'
atom atom
0 2 linear linear 180 sp
trigonal sp2
0 3 trigonal planar 120
planar
less sp2
1 2 trigonal planar bent
than 120
0 4 tetrahedral tetrahedral 109.5 Sp3
less sp3
trigonal
1 3 tetrahedral than
pyramidal
109.5
less sp3
2 2 tetrahedral bent than
109.5
trigonal trigonal 90, 120 sp3d
0 5
bipyramidal bipyramidal and 180
trigonal 90, 120 sp3d
1 4 seesaw
bipyramidal and 180
trigonal 90 and sp3d
2 3 T-shaped
bipyramidal 180
trigonal sp3d
3 2 linear 180
bipyramidal
90 and sp3d2
0 6 octahedral octrahedral
180
square 90 and sp3d2
1 5 octahedral
pyramidal 180
square 90 and sp3d2
2 4 octahedral
planar 180
You might also like
- MathsTraks: Geometry: A Collection of Blackline Masters for ages 11-14From EverandMathsTraks: Geometry: A Collection of Blackline Masters for ages 11-14No ratings yet
- Geometry SheetDocument1 pageGeometry Sheetapi-3697114100% (1)
- Vsepr Table PDFDocument1 pageVsepr Table PDFlucasNo ratings yet
- 3.10 Molecular CompoundsDocument3 pages3.10 Molecular CompoundsIBRAHIM ABOU EL NAAJNo ratings yet
- Nota VSEPR PDFDocument1 pageNota VSEPR PDFMarlene GazconNo ratings yet
- Bondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eDocument3 pagesBondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eaadhyaNo ratings yet
- Molecular ShapeDocument1 pageMolecular ShapeNUR DEENA KHALID KM-PensyarahNo ratings yet
- Lone Pairs of Central AtomDocument2 pagesLone Pairs of Central AtomChup KarNo ratings yet
- Tips & Tricks Inorganic Chemistry FlashCardsDocument25 pagesTips & Tricks Inorganic Chemistry FlashCardsseemagoyal0206No ratings yet
- Vsepr ChartDocument1 pageVsepr ChartpankajNo ratings yet
- 3 AB Trigonal Planar Trigonal Planar 120 Between All BondsDocument5 pages3 AB Trigonal Planar Trigonal Planar 120 Between All BondsVedantNo ratings yet
- VSEPR TableDocument1 pageVSEPR TableAudrey HizonNo ratings yet
- Electron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles ExampleDocument2 pagesElectron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles ExampleRichamille Ann RicaforteNo ratings yet
- Lewis StructureDocument1 pageLewis Structureits aryamNo ratings yet
- 3 1 3 As Shapes of Molecules ChemsheetsDocument27 pages3 1 3 As Shapes of Molecules ChemsheetsAkshar PatelNo ratings yet
- Lesson 1 - Angles and Their MeasuresDocument42 pagesLesson 1 - Angles and Their MeasuresAna Marie ValenzuelaNo ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryIsraClarkeNo ratings yet
- Molecular Orbitals PDFDocument6 pagesMolecular Orbitals PDFGeraldNo ratings yet
- Lecture Statics of Rigid BodiesDocument5 pagesLecture Statics of Rigid BodiesalainxanoricoNo ratings yet
- Worktable For Shape and PolarityDocument2 pagesWorktable For Shape and PolarityDestinee LegendsNo ratings yet
- Brayton Cycle: Avellana, OcceñaDocument54 pagesBrayton Cycle: Avellana, OcceñaMarcial Jr. MilitanteNo ratings yet
- Properties of 2D Shapes - AnswersDocument1 pageProperties of 2D Shapes - Answerscloud scapeNo ratings yet
- LAS Physical-Science Week2Document11 pagesLAS Physical-Science Week2Shekaina Faith Cuizon LozadaNo ratings yet
- Vsepr-HlDocument25 pagesVsepr-HlRyan BoukaaNo ratings yet
- Compound Hybridization Lone Pair Bond Angle (°) Shape Becl Co BF CH NH H O PCL SF Xef Xef Xef NH BF CoclDocument1 pageCompound Hybridization Lone Pair Bond Angle (°) Shape Becl Co BF CH NH H O PCL SF Xef Xef Xef NH BF CoclSakib KhanNo ratings yet
- Steric No. Form Shape Angle HybridizationDocument1 pageSteric No. Form Shape Angle Hybridizationmica_tsukadaNo ratings yet
- VSEPR GeometriesDocument1 pageVSEPR GeometriesJason JacksonNo ratings yet
- Geometry of Molecules ChartDocument6 pagesGeometry of Molecules ChartShamsiNo ratings yet
- Solution Tutorial 2Document1 pageSolution Tutorial 2ibrahimelsahharNo ratings yet
- PolygonsDocument5 pagesPolygonsmkhantareen78No ratings yet
- Circle TheoremsDocument21 pagesCircle TheoremsAngella Reece IsraelNo ratings yet
- Circle TheoremsDocument21 pagesCircle Theoremsananya bagade100% (4)
- Soporte Cub 003 Sc716 (Nature Isle Steel Bending)Document9 pagesSoporte Cub 003 Sc716 (Nature Isle Steel Bending)Vladimir DovalNo ratings yet
- Electron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageDocument2 pagesElectron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageBianca GuillermoNo ratings yet
- 09 - Molecular Structure and Covalent Bonding TheoriesDocument10 pages09 - Molecular Structure and Covalent Bonding TheoriesReiVanNo ratings yet
- Quarter3 - Module - Week5Document1 pageQuarter3 - Module - Week5JAMAICA PAULA REGALANo ratings yet
- Geometry Formulas: Triangle FormulaDocument4 pagesGeometry Formulas: Triangle FormulaSubramanian MuthukrishnanNo ratings yet
- Geometry Formulas: Triangle FormulaDocument4 pagesGeometry Formulas: Triangle FormulaDivya MohataNo ratings yet
- GMAT Quant Powered Geometry FormulasDocument4 pagesGMAT Quant Powered Geometry FormulasSubramanian MuthukrishnanNo ratings yet
- Geometry FormulasDocument4 pagesGeometry FormulashuntNo ratings yet
- Geometry Formulas: Triangle FormulaDocument4 pagesGeometry Formulas: Triangle FormulaBrian P. JohnsonNo ratings yet
- 4.1 Radians and DegreesDocument1 page4.1 Radians and DegreesmsbakermathNo ratings yet
- T02-Sol 71 PDFDocument1 pageT02-Sol 71 PDFASIYA KAZINo ratings yet
- Chemistry-Molecular GeometryDocument2 pagesChemistry-Molecular GeometryBubbles Bubbles100% (1)
- Trigonometry ReviewDocument13 pagesTrigonometry ReviewAntonio DionisioNo ratings yet
- Chapter 3 Polygons2Document26 pagesChapter 3 Polygons2Jonard G. TrajanoNo ratings yet
- Wring For The 2856670 Pyrometer G3512Document13 pagesWring For The 2856670 Pyrometer G3512PLANTAS ELECTRICAS H&BNo ratings yet
- ACTIVITY SHEET Geometry of Simple CompoundsDocument4 pagesACTIVITY SHEET Geometry of Simple CompoundsUy, Jhavelaine Cassandra F.No ratings yet
- Surveying Angles and TraversingDocument46 pagesSurveying Angles and Traversingblackleunamme1427No ratings yet
- General AnglesDocument2 pagesGeneral AnglesJovian AlvinoNo ratings yet
- FSC Trigonometric Handout PDFDocument3 pagesFSC Trigonometric Handout PDFFaisal KhawarNo ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryAryanna CamachoNo ratings yet
- Penentuan Klas Simetri Menurut Herman Mauguin Dan SchoenflishDocument1 pagePenentuan Klas Simetri Menurut Herman Mauguin Dan SchoenflishKhalalhitaNo ratings yet
- Shapes of Molecules & Ions: Name . . FormDocument2 pagesShapes of Molecules & Ions: Name . . FormjnfjngsdjNo ratings yet
- Common Mensuration Formulae: Drawing SR - No Object Surface AreaDocument4 pagesCommon Mensuration Formulae: Drawing SR - No Object Surface AreamariaLazarus75% (4)
- Parallel Lines & Transversal and The Triangle Sum TheoremDocument24 pagesParallel Lines & Transversal and The Triangle Sum TheoremJenifer Obatay Dana100% (1)
- Molecular Structure Part 02Document1 pageMolecular Structure Part 02MahaNo ratings yet
- Sum of The Interior Angles of A Convex PolygonDocument15 pagesSum of The Interior Angles of A Convex PolygonMaricel Balbuena100% (1)
- Trig Graphs in RadiansDocument21 pagesTrig Graphs in Radiansjerryvincent129No ratings yet
- 4.1 Radian and Degree MeasureDocument21 pages4.1 Radian and Degree MeasureJet EstefaniNo ratings yet
- STS ReviewerDocument7 pagesSTS ReviewerPedro SuyuNo ratings yet
- Branches of BiologyDocument3 pagesBranches of BiologyShadam BonoNo ratings yet
- Cell Structure and FunctionDocument44 pagesCell Structure and FunctionPedro SuyuNo ratings yet
- Cell Structure and FunctionDocument44 pagesCell Structure and FunctionPedro SuyuNo ratings yet
- Encarta EncyclopediaDocument11 pagesEncarta EncyclopediaPedro SuyuNo ratings yet
- Naming Coordination CompoundsDocument18 pagesNaming Coordination CompoundsPedro SuyuNo ratings yet
- Early Ideas On: Origins of The Cell Theory AnaximanderDocument22 pagesEarly Ideas On: Origins of The Cell Theory AnaximanderPedro SuyuNo ratings yet
- What Is A Cell?: Cells: Size & ShapeDocument7 pagesWhat Is A Cell?: Cells: Size & ShapePedro SuyuNo ratings yet
- Quiz On Molecular ModelDocument2 pagesQuiz On Molecular ModelPedro SuyuNo ratings yet
- Structure of WaterDocument25 pagesStructure of WaterPedro SuyuNo ratings yet
- Quiz On Elements in The Periodic TableDocument1 pageQuiz On Elements in The Periodic TablePedro SuyuNo ratings yet
- Quiz On Elements in The Periodic TableDocument1 pageQuiz On Elements in The Periodic TablePedro SuyuNo ratings yet
- Molecular ModelDocument1 pageMolecular ModelPedro SuyuNo ratings yet
- Quiz On Molecular ModelDocument2 pagesQuiz On Molecular ModelPedro SuyuNo ratings yet
- Alcohols 7Document14 pagesAlcohols 7Pedro SuyuNo ratings yet
- 5 Things Teachers To LearnDocument29 pages5 Things Teachers To LearnPedro SuyuNo ratings yet
- Naming EthersDocument11 pagesNaming EthersPedro SuyuNo ratings yet
- Worksheets On Naming EthersDocument2 pagesWorksheets On Naming EthersPedro Suyu100% (1)
- Worksheets On Naming EthersDocument2 pagesWorksheets On Naming EthersPedro Suyu100% (1)
- Naming EthersDocument11 pagesNaming EthersPedro SuyuNo ratings yet
- N A R I P S Answer: - : SprainDocument2 pagesN A R I P S Answer: - : SprainPedro SuyuNo ratings yet
- Steve Front PageDocument1 pageSteve Front PagePedro SuyuNo ratings yet
- Dislocation: T I O N L O C Dis AnswerDocument2 pagesDislocation: T I O N L O C Dis AnswerPedro SuyuNo ratings yet
- Let Reviewe Prof EdDocument12 pagesLet Reviewe Prof EdPedro SuyuNo ratings yet
- Test Bank 4Document10 pagesTest Bank 4Pedro SuyuNo ratings yet
- T U R E C R F A Answer: - : FractureDocument2 pagesT U R E C R F A Answer: - : FracturePedro SuyuNo ratings yet
- Theconeofexperience 2Document25 pagesTheconeofexperience 2Pedro SuyuNo ratings yet
- Let0317 PosDocument114 pagesLet0317 PosPRC Board90% (29)
- ChemDocument37 pagesChemPedro SuyuNo ratings yet
- Naming EthersDocument11 pagesNaming EthersPedro SuyuNo ratings yet
- Lab 11Document5 pagesLab 11derickNo ratings yet
- Em Wave Quest IIDocument3 pagesEm Wave Quest IIapi-260335088No ratings yet
- World Scientific Handbook of Metamaterials Properties PDFDocument551 pagesWorld Scientific Handbook of Metamaterials Properties PDFRajib ChowdhuryNo ratings yet
- G10 SummativeDocument2 pagesG10 SummativeMelmar ReverenteNo ratings yet
- RSTL 1804 0001Document16 pagesRSTL 1804 0001Lane LopesNo ratings yet
- AP Quantum Numbers WorksheetDocument2 pagesAP Quantum Numbers WorksheetSoumi VesaliNo ratings yet
- Vray For 3ds MaxDocument1 pageVray For 3ds MaxharikumarindiaNo ratings yet
- Optical Mineralogy: Minerals in Plane Polarised LightDocument29 pagesOptical Mineralogy: Minerals in Plane Polarised LightRidho FirdausmanNo ratings yet
- Science8 q3 Mod3 Week5-6 Subatomic-Particles v5Document28 pagesScience8 q3 Mod3 Week5-6 Subatomic-Particles v5Lawrence Bianes100% (1)
- Chapter 2 BTHDocument19 pagesChapter 2 BTHPHƯƠNG ĐẶNG YẾNNo ratings yet
- IAOC2018Document632 pagesIAOC2018lamjwNo ratings yet
- Unit 4 - MCQDocument12 pagesUnit 4 - MCQrohitsalave29No ratings yet
- Calibration of UV SpectrophotometerDocument27 pagesCalibration of UV SpectrophotometerAmit ArkadNo ratings yet
- Q2 Types of Bonding and Their PropertiesDocument35 pagesQ2 Types of Bonding and Their PropertiesTosee istoseeNo ratings yet
- Additional Slides On Latent Heat PhenomenaDocument2 pagesAdditional Slides On Latent Heat PhenomenaChester FengNo ratings yet
- Session-3 (Color Codind and Splicing)Document17 pagesSession-3 (Color Codind and Splicing)Muhammad Ameer SabriNo ratings yet
- ESQ 03 QuantumDocument11 pagesESQ 03 QuantumJack BornNo ratings yet
- Price List For FTTH-Jan 2019Document1 pagePrice List For FTTH-Jan 2019ScalperNo ratings yet
- 1 Atomic StructureDocument16 pages1 Atomic StructureMr TanNo ratings yet
- Obt751 PPT 2Document13 pagesObt751 PPT 2Ryan Miller100% (1)
- The Panasonic Lumix™ DMC-FZ1 and DMC-FZ2 Beta FAQDocument28 pagesThe Panasonic Lumix™ DMC-FZ1 and DMC-FZ2 Beta FAQdasxaxNo ratings yet
- Inorganic Chemistry: Serial No. NoDocument160 pagesInorganic Chemistry: Serial No. NoRekha BhasinNo ratings yet
- Esm Infrared SpectrophotometerDocument11 pagesEsm Infrared SpectrophotometerNuralia Radiani RustamNo ratings yet
- 4.3-VSEPR - Shapes of MoleculesDocument1 page4.3-VSEPR - Shapes of MoleculesStephan MinhNo ratings yet
- (Walls D.F., G.j.milburn.) Quantum OpticsDocument370 pages(Walls D.F., G.j.milburn.) Quantum OpticsFranklin RiosNo ratings yet
- Jan 20 U2 QP - Merged PDFDocument224 pagesJan 20 U2 QP - Merged PDFHalal BoiNo ratings yet
- Atomic Absorption Spectroscopy (AAS) : Dr. Chotimah, M.SiDocument60 pagesAtomic Absorption Spectroscopy (AAS) : Dr. Chotimah, M.Sisari wahyuniNo ratings yet
- ElecComTomasi Chapter 13Document2 pagesElecComTomasi Chapter 13jerson eyas0% (1)
- Las-Shs Gen - Chem Melc 1 q2 Week-1Document11 pagesLas-Shs Gen - Chem Melc 1 q2 Week-1Carl Baytola RatesNo ratings yet
- GENERALBIOLOGY1Q1MOD1Document12 pagesGENERALBIOLOGY1Q1MOD1Shaira C. QuiselNo ratings yet